Abstract
Zebrafish has been gaining popularity in behavioral genetics and behavioral neuroscience as this species offers an excellent compromise between system complexity and practical simplicity for mechanistic analyses of brain and behavior function. Recently, a number of studies started to investigate methods with which fear responses may be induced reliably in zebrafish. The ultimate goal of these studies has been to develop zebrafish models of pathological processes and to investigate the mechanisms of fear and to eventually translate the findings to the human clinic. Previously, animated image of a sympatric predator of zebrafish was shown to induce fear responses. Here we expand on this recently gained knowledge and investigate whether other moving images may induce more robust fear responses. The images investigated include the original sympatric predator, the Indian leaf fish, another sympatric predator, the needle fish, a bird silhouette moved on the side or above the tank, an expanding dot mimicking rapid approach of an object shown on the side and from above the tank, as well as non-fear inducing images including a single and a group of zebrafish. Our results indicate that although the sympatric predators do induce some fear responses, the other images, particularly the expanding dot but also the bird silhouette shown from above are more effective. The results also reveal a stimulus dependent motor pattern response repertoire of zebrafish demonstrating that perhaps univariate quantification methods may not be appropriate for uncovering the complexity of fear or anxiety related phenotypical changes in this species.
Keywords: anti-predatory behavior, anxiety, fear, zebrafish
INTRODUCTION
Throughout this paper we use the term “fear” to refer to an internal state or behavioral response that is elicited by negative or painful stimuli or stimuli that predict imminent danger. In many respects anxiety is similar to fear but here we emphasize an important distinguishing feature. Fear is directly induced by stimuli, however, anxiety is more diffuse as it is not associated with such specific stimuli and can manifest without their presence (for more discussion on the definition of fear and anxiety as it pertains to laboratory animal models see recent review by Gerlai, 2010). Anxiety disorders are often regarded as pathologically exaggerated or misdirected forms of fear (e.g. Weisberg, 2009). Irrespective of the definitions, however, most agree that fear and anxiety are the outputs of the brain, an organ that evolved in response to the demands of the natural environment (e.g. Blanchard et al., 2009). Therefore, some also argue that fear is a natural response that is adaptive (Denver, 2009; Gerlai, 2010). Consequently, pathologically altered fear responses and/or anxiety disorders are likely to be the result of abnormalities of mechanisms that (used to) serve adaptive functions.
Anxiety in the 21st century human society represents a large unmet medical need (Weisberg, 2009). Despite concerted efforts and numerous pharmaceutical and other therapeutic applications, a large number of patients still suffer from anxiety related problems. The answer to the question of why this is so is complex and manifold but perhaps two important points may be made here. One, the mechanisms of anxiety related disorders are not fully understood and as a result appropriate treatment could not be developed (Mathew et al., 2008). Two, there is substantial variability in the genotype of anxiety patients as well as in the environmental causes of anxiety disorders (Nandi et al., 2009). One way to limit such complexity and reduce the uncontrolled (environmental and genetic) variation is to use laboratory animals (Hohoff, 2009; Gerlai, 2009). The zebrafish has been proposed as a possibly appropriate laboratory species for this purpose (Speedie & Gerlai, 2008; Parra et al., 2009; Bass & Gerlai, 2008; Hall & Suboski, 1995; Serra et al., 1999; Egan et al., 2009; Levin et al., 2007).
The zebrafish is believed to represent an optimal compromise between system complexity and practical simplicity (Gerlai, 2010). While it is a complex organism with brain structure and function typical of vertebrates, it is small, prolific and easy and cheap to maintain in the laboratory (Chatterjee & Gerlai, 2009; Mueller et al., 2004; Alsop & Vijayan, 2008; Tropepe & Sive, 2003). However, the zebrafish suffers from one major disadvantage compared to more traditional laboratory vertebrates, such as the mouse and the rat. It is quite new in behavioral brain research, and as a result the amount of information on its behavior and the number of behavioral tasks available for it is relatively small (Sison et al., 2006). Given that behavior is the ultimate output of the brain, a phenotype that can be successfully utilized to detect functional alterations in the brain, it is crucial that one properly understands the behavioral characteristics of the laboratory organism of choice and has access to appropriate behavioral test methods that allow such analyses (Gerlai, 2002). Most recently there has been an upsurge of empirical studies using zebrafish that started to address this important goal, the development of behavioral phenotyping tools for zebrafish.
A successful method to induce fear responses in zebrafish was developed with use of the alarm substance several decades ago (Schutz, 1956; Pfeiffer, 1963). The problem with this approach has been that the substance had to be extracted from the skin of the fish and the exact concentration of the substance could not be determined. Recently, this issue was resolved by Parra et al. (2009) who showed that a synthetic substance, hypoxanthine 3-N-oxide (or H3NO) that contains a chemical structure common to osteriophysan alarm substances, elicited alarm (fear) reactions indistinguishable from those induced by the species-specific natural alarm substance in zebrafish. The problem with these studies, however, has been that alarm substances, as well as other olfactory cues, are difficult to work with because the cue is cumbersome to turn on and off repeatedly. For instance, its removal from the experimental tanks requires labor intensive cleaning efforts. To address this issue, and because zebrafish is a diurnal species with excellent visual acuity, we have been advocating the use of visual stimuli.
In the belief that naturalistic behavioral approaches have the highest chance of success in the biological analysis of brain function (Gerlai & Clayton, 1999), we have investigated the effect of predators on zebrafish. First we studied whether sympatric or allopatric predators or harmless fish species induced different reactions and found that indeed a sympatric predator, the Indian leaf fish, induced the most robust fear responses (Bass & Gerlai, 2008). Interestingly, we found the strength of these responses independent of whether the test zebrafish had access only to visual cues of the predator or olfactory and lateral line (low frequency vibration) cues as well (Bass & Gerlai, 2008). Thus, we concluded that visual cues, i.e. the sight of the sympatric predator alone, are sufficient to induce a robust fear response in zebrafish. An important drawback associated with the presentation of live stimulus fish is that their behavior may vary across multiple test sessions, which may introduce unwanted error variation. Thus we proposed, and later demonstrated, that computer animated (moving) images may be appropriate (Gerlai et al., 2009). We found the moving image of the Indian leaf fish to induce significant fear responses similar to those elicited by the live predator (Gerlai et al., 2009).
It is notable, however, that we have not systematically analyzed whether other types of animated images could also induce fear responses and whether these responses would be more robust. To investigate these questions, here we employ three new animated images along with the already established Indian leaf fish image. One of these images was of another predator sympatric with zebrafish, the needle fish (Xenentodon cancila, Engeszer et al., 2007; Spence et al., 2006). The other two images were also chosen with the natural history of zebrafish in mind. One image was of a black bird silhouette. Although actual capture of zebrafish by fishing birds in their natural habitat has not been observed, it is likely that birds are some of the most frequent predators of zebrafish. For example, the Indian pond heron (Ardeola grayii) and the common kingfisher (Alcedo atthis) were found ubiquitous in the habitats of zebrafish (Spence et al., 2006). Furthermore, both the mouth structure (opening upward) and foraging behavior of zebrafish (they eat insects that fall into the water) as well as observations in the laboratory (e.g. Egan et al., 2010) and in nature (Spence et al., 2006) suggest that this species occupies the upper water column and thus must be highly exposed to fishing birds. The other new stimulus we employed was a simple black dot that increased its size mimicking a rapidly approaching object (piscivorous fish from the side or a bird from above). Last, to contrast aversive (fear inducing) and attractive stimuli, we employed images of conspecifics. We showed an image of a single zebrafish (which may induce shoaling, reproductive, and/or agonistic responses, all found to be attractive, e.g. Gerlai & Hogan, 1992). We also presented images of multiple zebrafish, an artificial shoal (which is expected to be also attractive, Al Imari & Gerlai, 2008). In the current paper we investigate whether these animated images induce more robust or different fear (antipredatory) responses compared to the previously employed image of the Indian leaf fish.
METHODS
Animals and Housing
Two-hundred and fifteen zebrafish (Danio rerio) of the AB strain (approximately 50-50% male-female) were used in the experiments. The fish originated from progenitors obtained from the Zebrafish International Research Centre (ZIRC) (Eugene, Oregon) and were bred, raised and housed in the same holding room of the University of Toronto Mississauga (UTM) Vivarium as deribed efore (Fernandes & Gerlai, 2009). Briefly, 5 days post-fertilization (dpf) the free swimming zebrafish fry were fed twice daily with Larval Artificial Plankton 100 (particle size below 100 μm, ZeiglerBros, Inc., Gardners, PA, USA) and after their age of 15dpf they continued on nauplii of brine shrimp (Artemia salina) until they were four weeks old. Subsequently, all fish were given a mixture of flake food (Tetramin Tropical fish flake food, Tetra Co, Melle, Germany) and powered spirulina (1 part, Jehmco Inc., Lambertville, NJ, USA). Zebrafish were housed in 2.8 L Plexi-glass tanks (approximately 15 fish per tank) that were part of a recirculating system (Aquaneering Inc., San Diego, CA, USA) with multi-stage filtration including a mechanical filter, a fluidized glass bed biological filter, activated carbon filter, and a UV light sterilizing unit. Ten percent of the water was replaced with fresh system water (de-ionized oxygenated water supplemented with 60 mg/L Instant Ocean Sea Salt, Big Al’s Pet Store, Mississauga, Ontario, Canada) each day. The water temperature was controlled by a thermostat and was kept at 27 C. The light cycle was also controlled with fluorescent lights on the ceiling turned on at 07:00 h and off at 19:00 h. Five month old, young adults were tested in our study.
Test Apparatus and Experimental Procedure
Each experimental fish was tested once and individually in a 40 liter experimental tank (51cm×30cm×25cm, width × depth × height). A dark green corrugated plastic sheet was placed on the back side and bottom of the test tank to mimic the natural habitat of zebrafish and to increase visibility and contrast for video-recording. The tank was illuminated from above by a 15 W fluorescent lamp. The tests were conducted between 11:00 and 17:00 h. A digital hard disk video-camera (JVC Everio GZ-MG37U) was placed in front of the tank to record the subject’s behavior. The recordings were later replayed and analyzed using the Observer (version 5.0) software application (Noldus Information Technologies, Wageningen, The Netherlands). In some tests two computer monitors (Samsung Syncmaster 732N) covered the two opposite sides of the test tank and in other tests a computer monitor was placed above the test tank facing downward. Each monitor was connected to a laptop computer (Dell Vostro 1000) that ran a custom software application (first described in Saverino & Gerlai, 2008), which allowed the presentation of computer-animated images.
Zebrafish were randomly assigned to eight stimulus groups. The fish were presented with one of the eight animated (moving) images, a between subject experimental design. The experimental fish was placed in the test tank and were allowed to habituate to it for 5 minutes (pre-stimulus period). The pre-stimulus period was followed by a 5 min stimulus presentation period during which one of the eight stimuli was shown as described below. This period was followed by a post-stimulus period, a 4 minute long interval during which no stimulus was presented. The length of these periods were selected based upon prior studies (e.g. Gerlai et al., 2009; Gerlai & Fernandes, 2009; also see Gerlai, 2010 for a recent review) but it is notable that systematic analysis of what would constitute an optimal habituation and presentation period has not been conducted. It is also notable that significant error variation (or individual differences) may arise as a result of handling of the subjects and thus the experimental fish were handled in a manner so as to minimize stress (Gerlai, 2010).
The following 8 stimuli were employed: “bird on top” (n = 15), “bird on the side” (n = 31), “dot on the side” (n = 20), “dot on top” (n = 22), “Indian leaf fish” (n = 31), “needle fish” (n = 35), “one zebrafish” (n = 33), “six zebrafish” (n = 28). The overall rationale for these 8 stimuli was as follows. Although a sympatric predator was found previously to be more effective than an allopatric predator or harmless fish species, we do not know whether this finding can be generalized to all sympatric predators, i.e. whether zebrafish exhibit fear responses only to this particular stimulus (the Indian leaf fish) or whether they would show signs of fear to other stimuli too. It is also not known whether all aspects of antipredatory behavior would be induced by threatening or dangerous stimuli, or whether only a subset of the behavioral repertoire would be shown by zebrafish and in a stimulus-type dependent manner. Last, to aid the interpretation of responses to putative fear inducing stimuli, we wanted to contrast and compare the responses induced by these stimuli with those known not to induce fear, hence the presentation of the images of conspecifics.
It is likely that fishing birds pose significant danger to zebrafish in their natural habitat (Spence et al., 2006) because zebrafish live and forage near the surface of the water (Spence et al., 2006; Engeszer et al., 2007) and thus are expected to be exposed to such predators. In the first condition, a black bird silhouette (5 cm from beak to tail and 10 cm wingspan) moved across a white illuminated background on the computer monitor placed above the test tank. The speed of movement of the bird was set to 14 cm/sec, i.e. the stimulus traversed the entire 50 cm long screen within 3.5 seconds. This bird stimulus was presented multiple times with 5 sec inter-stimulus intervals during the stimulus period and each time the direction of movement of the bird silhouette was changing randomly between left to right or right to left. The second stimulus condition was identical to the first one except that the bird silhouette was shown on one of the side monitors and not from above. Each experimental fish was shown the stimulus from one of the side monitors but the presentation side varied randomly across the experimental subjects. In the third stimulus condition a black dot increasing in diameter on a white background was shown in the middle of the computer monitor placed above the tank during the stimulus presentation period. The dot increased its size with a linear acceleration from 1 cm to 5 cm in diameter within a period of 3 seconds to mimic the forward motion of the rapid frontal approach of an object. Similarly to the bird silhouette the dot presentation periods were interspersed with 5 sec inter-stimulus intervals during which the dot was not shown. The fourth stimulus condition was identical to the third one except that the dot was presented on one of the side monitors. The side on which the stimulus was presented was randomly chosen for a given test subject but varied between left and right side randomly across the different test subjects. In the fifth stimulus condition the image of the Indian leaf fish (Nandus nandus) was shown as described before (Gerlai et al., 2009). This species is a piscivore that is sympatric with zebrafish and has been shown to be particularly effective in eliciting fear responses as compared to an allopatric piscivore or allopatric and sympatric harmless fish species (Bass & Gerlai, 2008). The image of the Indian leaf fish moved with a horizontal speed of 0.3 cm/sec, a slow movement that was meant to mimic the natural behavior of this ambush predator. The image also had small vertical movements but stayed in the middle horizontal layer of the computer screen. The length of the image was 10 cm, a size at which a live leaf fish could easily devour a fully grown zebrafish. In the sixth condition the image of another piscivore, the needle fish (Xenentodon cancila) was presented. This predator was observed in the same microhabitat where zebrafish live in nature (Engeszer et al., 2007; Spence et al., 2006). The movement pattern and location of this image was identical to that of the Indian leaf fish’s. In the seventh and eight stimulus conditions the subjects were shown conspecifics, i.e. images of zebrafish of size, shape and color pattern similar to those of the test subjects. In both conditions the image (or images) of the very same zebrafish, a female was (were) shown. The rationale for this has been explained elsewhere (e.g. Fernandes & Gerlai, 2009) but briefly, females have been found to be attractive to both males and females whereas males have been found to be only attractive to females in zebrafish social encounters (Ruhl & McRobert, 2005). Briefly, a single female or all female shoals is/are expected to elicit a more uniform social attraction thus reducing gender dependent variation. In the seventh condition a single zebrafish image was shown. This image moved in the middle horizontal layer of one of the side monitors with a horizontal speed randomly varying between 1.5 and 4 cm/sec, a range of swim speeds that is in line with how live zebrafish swim under normal conditions. This situation is expected to induce social behavior ranging potentially from shoaling (group cohesion) to aggression (agonistic behavior induced attraction) and courtship (reproductive behavior induced attraction). In the eighth stimulus condition, the test fish was shown six moving images of zebrafish but other than the larger number of images, the presentation of the stimulus was identical to that employed in the seventh stimulus condition. This stimulus condition is expected to induce group cohesion related social attraction, i.e. shoaling behavior. The order in which the experimental fish belonging to the 8 different stimulus conditions was tested followed a random sequence. At the time of behavioral quantification (replay of the recorded videos) the experimenter was blind to the stimulus condition.
Quantification of behavior
The video-recordings were replayed and analyzed using the Observer software application (version 5, Noldus Information Technologies, Wageningen, the Netherlands). The following behavioral parameters were quantified. The experimental tank was divided into three equal imaginary horizontal layers and we measured the percent of time the test fish spent in the bottom third of the tank (percent of time on bottom). This behavior was chosen to be quantified because zebrafish may respond to predatory threat by hiding on the bottom, a fear response that has also been found to be induced by novelty itself in the laboratory (Levin et al., 2007; Egan et al., 2009). Zebrafish has been shown to respond to fear inducing stimuli including the sight of predators or the smell of the alarm substance with erratic movement (Gerlai et al., 2009; Parra et al., 2009). This movement is a characteristic fast zig-zagging response associated with rapid direction changes as described in detail elsewhere (e.g. Gerlai et al., 2009; Blaser & Gerlai, 2006). We measured the percent of time the fish performed this behavior. Another behavior that was found induced by the alarm substance or the sight of predators was jumping, a single fast jump with the use of the caudal fin (e.g. Bass & Gerlai, 2008; Gerlai et al., 2006). The number of times fish jumped was recorded. Occasionally, we observed that our test fish attempted to perform a forceful and fast swim similar in appearance to jumping but performed against the glass. We term this behavior “leaping”. We quantified the percent of time fish performed this behavior. Freezing, or immobility, has been shown to be elicited by fear inducing stimuli in zebrafish (e.g. Bass & Gerlai, 2008; Gerlai et al., 2000). We distinguished freezing from “floating” before, another form of immobility as the former, according to our definition, occurs only on the bottom of the tank or while the fish is in direct physical contact with an object (e.g. the corner of the tank). The latter, i.e. floating, can occur anywhere in the tank and is also associated with lack of locomotion. In the current paper we treat these two behaviors as the same and call it immobility because we have found them to be highly positively correlated (data not shown) in the current set of experimental results. Thus, immobility is defined as complete lack of locomotion. The fish is stationary and only its eyes, the opercula, and occasionally its pectoral fins may move. General activity levels may change in response to both threatening and attractive stimuli (Fernandes & Gerlai, 2009; Gerlai et al., 2009). We measured general activity by quantifying the percent of time fish swam actively. Active swimming is straight line locomotion with a speed ranging between 1.5 – 4 cm/sec. Although our observation based method did not precisely quantify the exact swim speed, a trained observer could easily distinguish this behavior from other motor/posture and activity patterns. We quantified the percent of time fish swam. Another form of active swimming is thrashing. Thrashing is defined and described elsewhere in detail (Bass & Gerlai, 2008). Briefly, this behavior is similar to swimming except that the fish is in direct physical contact with the glass of the tank while performing it. It is a forceful swim that appears as if the subject was trying to swim through the glass of the tank and manifests as circular motion directed towards the glass. In the current paper we quantified the percent of time thrashing towards the stimulus, a response that is expected to be induced by attractive but not by aversive stimuli. To further quantify the effect of aversive vs. attractive stimuli, we divided the tank into three equal segments with two imaginary vertical lines and measured the percent of time in the segment nearest to the stimulus. Last, in order to quantify activity, we divided the tank into 9 equal imaginary compartments (three horizontal layers × three vertical segments) and measured the number of times fish crossed into a new compartment from a previous one. The ambulation score quantified this way estimates the total distance travelled by the fish.
Statistical analysis
Data were analyzed using SPSS version 14 written for the PC. First, repeated measure two factorial ANOVA’s were conducted with Interval, the repeated measure factor, (3 levels: pre-stimulus, stimulus and post-stimulus interval) and Stimulus condition, the between subject factor (with 8 levels, the 8 different stimuli explained above). In addition, the effect of sex as well as side of stimulus presentation was also analyzed. However, these main effects and the interaction between them and other main factors turned out to be non-significant therefore data were pooled for these factors. In case of significant Interval, Stimulus, and/or Interval × Stimulus interaction effects, repeated measure ANOVA was conducted for each stimulus condition separately to examine the interval effects. The null hypothesis of no stimulus or interval effect, and/or no stimulus × interval interaction, was rejected when its probability was less then 0.05. The significant effects were followed up with post hoc analyses. Multiple comparison post hoc tests are not appropriate for repeated measure designs. Therefore, instead, we investigated whether presentation of the stimulus significantly altered the response of the experimental zebrafish by calculating the difference between the performance of the fish during the stimulus period and the habituation period (DB = Bstim − Bhab) and conducting one sample t-tests (comparing the difference value to zero) with Bonferroni correction to reduce type one error.
In addition, we conducted analyses based upon Pearson product moment bivariate correlation coefficients between the behavioral measures, a Principal Component Analysis (PCA). The Principal Component solution was obtained after Varimax rotation with Kaiser normalization and 25 maximum number of iterations leading to convergence. This rotation procedure leads to orthogonal (non-correlating) component solution. The criterion for inclusion of principal components was set at eigenvalues greater than 1. The analysis was performed using data from all fish, i.e. all stimulus conditions included. First, a PCA was conducted with the 9 behavioral measures of all 3 intervals (pre-stimulus or habituation, stimulus, and post-stimulus periods). In this analysis the behavioral variables of each period were treated as unique to that period and thus the PCA contained 27 behavioral variables. This analysis allowed us to investigate how identical motor patterns across multiple intervals may or may not correlate with each other. Subsequently, we also conducted a separate PCA for the stimulus interval, an analysis that allowed us to investigate stimulus induced response clusters that are independent of each other.
RESULTS
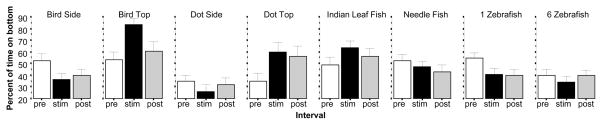
Bottom dwell time has been shown to be a characteristic indicator of fear, i.e. its amount has been found increased by aversive stimuli (for review see Gerlai, 2010). Zebrafish spent highly different amounts of time on the bottom depending on which stimulus they were shown (figure 1). ANOVA confirmed this observation and found the effect of Stimulus significant (F(7, 207) = 3.562, p = 0.001), and it also revealed a significant Interval × Stimulus interaction (F(14, 414) = 5.534, p < 0.001). The main effect of Interval was non-significant (F(2, 414) = 1.180, p > 0.30). Further analysis of the results showed that the percent of time on the bottom significantly decreased in response to the bird silhouette shown from the side (Interval F(2, 60) = 6.377, p < 0.01), and also when shown from the top (Interval F(2, 28) = 10.118, p < 0.001. This conclusion was confirmed by the post hoc one sample t-test which found the difference between the stimulus and habituation period to significantly deviate from zero (bird side t = −3.21, df = 30, p < 0.05; bird top t = 5.22, df = 14, p < 0.01). The dot presented from the side was ineffective (Interval F(2, 38) = 2.336, p > 0.10) but when it was shown from above it did lead to zebrafish spending significantly increased time on the bottom (Interval F(2, 42) = 6.792, p < 0.01) a conclusion confirmed by the post hoc t-test that showed the difference between the stimulus and habituation period to significantly deviate from zero (t = 3.23, df = 21, p < 0.05). The presentation of the Indian leaf fish although apparently less effective, also led to a significant increase in the percent of time on the bottom (Interval F(2, 60) = 3.684, p < 0.05), a conclusion confirmed by the post hoc t-test that showed a significantly higher than zero value for the difference between stimulus and habituation period performance (t = 2.95, df = 30, p < 0.05). However, the needle fish did not induce such a significant response (Interval F(2, 68) = 2.082, p > 0.10). A single zebrafish image induced decreased bottom dwell time (F(2, 64) = 6.649, p < 0.01; post hoc t test for the difference between stimulus and habituation period performance compared to zero t = −2.87, df = 32, p < 0.05), but when 6 zebrafish images were shown, their effect was non-significant (Interval F(2, 54) = 0.647, p > 0.50). In summary, bottom dwell time increased in response to some fear inducing stimuli but not in response to all of them.
Figure 1.
The percent of time zebrafish spent in the bottom third layer of the test tank is significantly altered (decreased or increased) in a stimulus dependent manner. Mean ± S.E.M. are shown. Sample sizes (n) are indicated in the text. White bars represent performance during the pre-stimulus (habituation) period, black bars represent the stimulus period performance and grey bars the post-stimulus period performance. The stimulus type employed is indicated above the bar graphs. Note the robust increase of time on bottom in response to the bird shown from above and the dot shown from above the test tank.
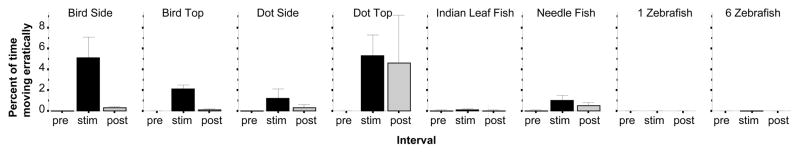
Erratic movement has been shown to be induced by a variety of fear stimuli (for review see Gerlai, 2010). In the current series of tasks, erratic movement was observed only in response to certain aversive stimuli(figure 2). ANOVA revealed a significant Interval effect (F(2, 414) = 8.809, p < 0.001) a significant Stimulus effect (F(7, 207) = 2.559, p < 0.05) and also a significant Interval × Stimulus interaction (F(14, 414) = 2.348, p < 0.01). Subsequent analyses showed that the bird silhouette shown on the side (Interval F(2, 60) = 6.645, p < 0.01, post hoc t test t = 2.59, df = 30, p < 0.05) or from the top (Interval F(2, 28) = 24.114, p < 0.001; post hoc t test t = 4.96, df = 14, p < 0.01) as well as the Dot shown from the top (Interval F(2, 42) = 2.710, p < 0.05; post hoc t-test t = 2.668, df = 21, p < 0.05) induced significant increase of erratic movement duration but the other stimuli did not (Interval effect for the other stimulus conditions was non-significant, F(2, 68) < 1.770, p > 0.15).
Figure 2.
The percent of time zebrafish moved erratically is significantly increased by the bird silhouette (shown from the side or the top) as well as the Dot shown from the top. Mean ± S.E.M. are shown. Sample sizes (n) are indicated in the text. White bars represent performance during the pre-stimulus (habituation) period, black bars represent the stimulus period performance and grey bars the post-stimulus period performance. The stimulus type employed is indicated above the bar graphs. Note that erratic movement was absent in response to the attractive stimuli (the zebrafish images) and also was very rarely observed in response to the needle fish.
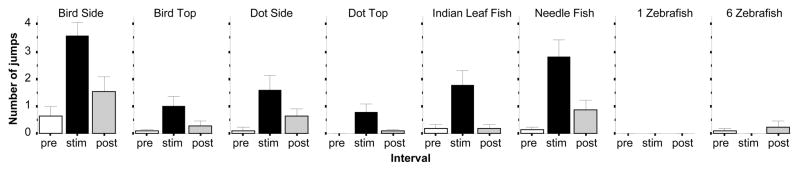
The number of times experimental zebrafish jumped was increased by all stimuli employed except in the two zebrafish image conditions (figure 3). The stimulus dependent increase in the frequency of jumps was confirmed by ANOVA, which showed a significant Interval effect (F(2, 414) = 39.960, p < 0.001) a significant Stimulus effect (F(7, 207) = 9.117, p < 0.001) and a significant Interval × Stimulus interaction (F(14, 414) = 4.788, p < 0.001). Subsequent analyses demonstrated that all aversive stimuli, including the bird silhouette shown from the side (Interval F(2, 60) = 15.185, p < 0.001; post hoc t test t = 6.33, df = 30, p < 0.01), or from the top (Interval F(2, 28) = 5.408, p < 0.01; post hoc t test t = 2.61, df = 14, p < 0.05), the dot shown from the side (Interval F(2, 38) = 4.846, p < 0.05; post hoc t test t = 2.71, df = 19, p < 0.05), or from the top (Interval F(2, 42) = 5.997, p < 0.01; post hoc t test t = 2.63, df = 21, p < 0.05), the Indian leaf fish (Interval F(2, 60) = 8.137, p < 0.001; post hoc t test t = 2.85, df = 30, p < 0.05), as well as the needle fish (Interval F(2, 68) = 15.019, p < 0.001; post hoc t test t = 4.57, df = 34, p < 0.01) were effective in inducing significantly increased number of jumps. However, the single zebrafish (no jumps detected, values for all three intervals were zero) and the six zebrafish (Interval F(2, 54) = 0.929, p > 0.40) did not significantly increase the number of jumps. In summary, jumping appears to be a fairly uniform, albeit not very frequent, response to aversive visual stimuli.
Figure 3.
The number of jumps zebrafish performed is significantly increased by all aversive stimuli. Mean ± S.E.M. are shown. Sample sizes (n) are indicated in the text. White bars represent performance during the pre-stimulus (habituation) period, black bars represent the stimulus period performance and grey bars the post-stimulus period performance. The stimulus type employed is indicated above the bar graphs. Note jumping was practically absent in response to the attractive stimuli (the zebrafish images).
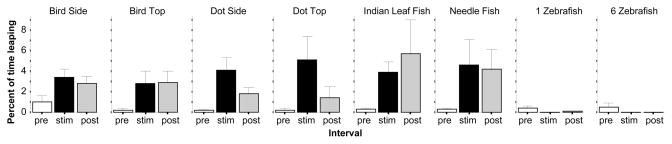
Leaping is another response that is often seen under aversive conditions. The results were very similar to the above for the measure, percent of time leaping (figure 4). ANOVA found a significant Interval effect (F(2, 414) = 11.440, p < 0.001). The Interval × Stimulus interaction was found to border significance (F(14, 414) = 1.622, p = 0.07) but the Stimulus effect was non-significant (F(7, 207) = 1.763, p > 0.05). Subsequent repeated measure ANOVAs conducted for each stimulus condition separately showed the Bird shown from the side (Interval F(2, 60) = 5.182, p < 0.01) or from the Top (Interval F(2, 28) = 4.216, p < 0.05) to significantly increase leap. Similarly the dot shown from the side (Interval F(2, 38) = 9.052, p < 0.001) and from the top (Interval F(2, 42) = 4.823, p < 0.05) also significantly increased the leap response. But despite the apparent effect seen in figure 4, the results were non-significant for the Indian leaf fish (Interval F(2, 60) = 2.395, p > 0.05) and also for the needle fish (Interval F(2, 68) = 2.699, p > 0.05). Although not apparent from figure 4, ANOVA found the effect of stimulus presentation in the 1 zebrafish stimulus condition significant (Interval F(2, 64) = 3.308, p < 0.05) but not in the 6 zebrafish condition (Interval F(2, 54) = 2.213, p > 0.10). These results were confirmed by the post hoc one sample t-tests, which analyzed whether the difference between the behavior recorded during the stimulus period and during the habituation period deviates from zero or not for Bird Side (t = 3.37, df = 30, p = 0.01) and (Dot Side t = 3.68, df = 19, p = 0.01) but not for the Bird Top (t = 2.33, df = 14, p > 0.05), Dot Top (t = 2.15, df = 21, p > 0.05) and 1 Zebrafish stimulus conditions (t = −1.93, df = 32, p > 0.05).
Figure 4.
The percent of time leaping is significantly increased by all aversive stimuli. Mean ± S.E.M. are shown. Sample sizes (n) are indicated in the text. White bars represent performance during the pre-stimulus (habituation) period, black bars represent the stimulus period performance and grey bars the post-stimulus period performance. The stimulus type employed is indicated above the bar graphs. Note lrsping was practically absent in response to the attractive stimuli (the zebrafish images).
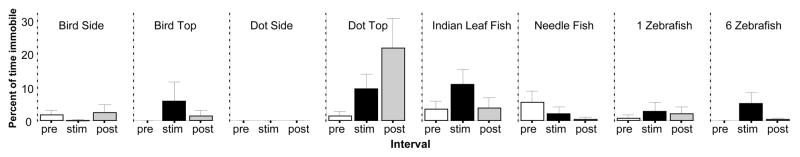
Freezing, or immobility, is also often seen under aversive conditions. However, in the set of tasks employed here this behavioral response was only sporadic. The results obtained for the variable, percent of time Immobile are shown in figure 5. ANOVA detected a significant Interval effect (F(2, 414) = 3.010, p = 0.05) a significant Stimulus effect (F(7, 207) = 2.501, p < 0.05) and also a significant Interval × Stimulus interaction (F(14, 414) = 2.701, p < 0.001). Subsequent ANOVAs conducted for each stimulus condition separately found a significant interval effect for the Dot Top condition only (F(2, 42) = 4.809, p < 0.05), but not for the other stimuli (F(2, 28–60) < 2.076, p > 0.10). A post hoc one sample t-test to investigate whether the difference between stimulus presentation and the habituation period deviates from zero could not confirm this significant effect (Dot Top t = 1.71, df = 21, p > 0.05). It is likely that the homogeneous test environment (unstructured open tank) biased zebrafish behavior against immobility and this was why our subjects chose active escape strategies in response to the fear inducing stimuli instead of immobility.
Figure 5.
The percent of time for which zebrafish were immobile is significantly increased only by the dot shown from the top. Mean ± S.E.M. are shown. Sample sizes (n) are indicated in the text. White bars represent performance during the pre-stimulus (habituation) period, black bars represent the stimulus period performance and grey bars the post-stimulus period performance. The stimulus type employed is indicated above the bar graphs.
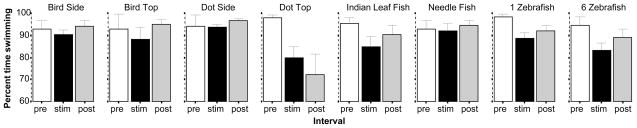
General activity, as measured by the percent of time swimming appeared affected by presentation of some of the stimuli (figure 6). ANOVA confirmed a significant Interval effect (F(2, 414) = 9.663, p < 0.001) and a significant Interval × Stimulus interaction (F(14, 414) = 2.078, p < 0.05) but found the Stimulus effect non-significant (F(7, 207) = 1.824, p > 0.05). Subsequent ANOVAs conducted separately for each stimulus condition confirmed these findings and showed significant interval effect only for the stimulus Dot Top (F(2, 42) = 6.906, p < 0.01) and the 1 Zebrafish (F(2, 64) = 12.976, p < 0.001) and 6 Zebrafish conditions (F(2, 54) = 3.360, p < 0.05). Post hoc t-tests found the difference between stimulus period and habituation period performance to significantly deviate from zero in case of the Dot Top (t = −3.44, df = 21, p < 0.01), and 1 Zebrafish stimulus condition (t = −5.05, df = 32, p < 0.01) but the result was only marginally significant for the 6 Zebrafish condition (t = −2.25, df = 27, p < 0.10). It appears that reduction of swimming activity may be achieved not only by presenting aversive but also attractive stimuli, i.e. the response is not specific to fear.
Figure 6.
The percent of time zebrafish actively swam is significantly decreased by the dot shown from the top and also by the presentation of 1 or 6 zebrafish images. Mean ± S.E.M. are shown. Sample sizes (n) are indicated in the text. White bars represent performance during the pre-stimulus (habituation) period, black bars represent the stimulus period performance and grey bars the post-stimulus period performance. The stimulus type employed is indicated above the bar graphs.
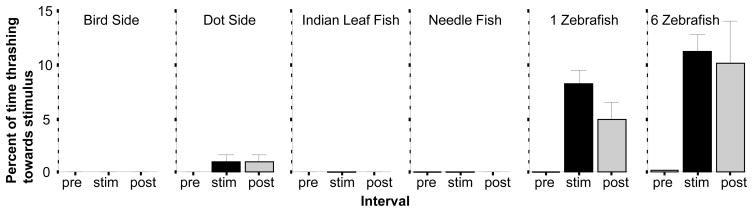
Analysis of the percent of time Thrashing towards the stimulus showed an entirely different set of results (figure 7). This behaviour was practically absent in all but two conditions, 1 Zebrafish and 6 Zebrafish. ANOVA found a significant Interval (F(2, 344) = 15.193, p <0.001) and Stimulus effects (F(5, 172) = 23. 294, p < 0.001) as well as significant Interval × Stimulus interaction (F(10, 344) = 5.973, p < 0.001). Subsequent separate ANOVAs for the stimulus conditions sowed a significant interval effect only for the 1 Zebrafish (F(2, 64) = 13.242, p < 0.001) and 6 Zebrafish conditions (F(2, 54) = 6.063, p < 0.01), effects that were confirmed by the post hoc one sample t-tests (1 Zebrafish t = 6.57, df = 32, p < 0.01; 6 Zebrafish t = 7.15, df = 27, p < 0.01).
Figure 7.
The percent of time thrashing towards the image is significantly increased by the presentation of 1 or 6 zebrafish images. Mean ± S.E.M. are shown. Sample sizes (n) are indicated in the text. White bars represent performance during the pre-stimulus (habituation) period, black bars represent the stimulus period performance and grey bars the post-stimulus period performance. The stimulus type employed is indicated above the bar graphs. Note that this response was practically absent in response to all aversive stimuli employed. Also note that the Dot top and Bird top conditions are not shown as this response is not relevant in these conditions.
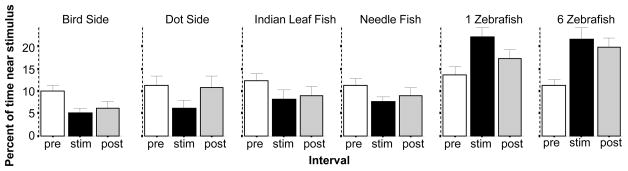
The location of experimental fish relative to that of the stimulus intuitively appears to be an obvious behavioral parameter that should allow the detection of the effects of aversive vs. attractive stimuli (but see Gerlai et al., 2009 vs. Ahmed et al., 2011). The percent of time experimental fish spent in the third of the tank nearest to the stimulus presentation side is shown in figure 8 (note that the Bird Top and Dot Top stimulus conditions were not evaluated or analyzed as these are not relevant with regard to left versus right side preference). ANOVA found no significant Interval main effect (F(2, 342) = 0.139, p > 0.85) but the Stimulus effect (F(5, 171) = 11.110, p < 0.001) and the Interval × Stimulus interaction was significant (F(10, 342) = 7.566, p < 0.001). Follow up ANOVAs conducted for each stimulus condition separately showed a significant interval effect for the Bird Side (F(2, 60) = 6.821, p < 0.01), 1 Zebrafish (F(2, 64) = 10.962, p < 0.001) and 6 Zebrafish stimulus conditions (F(2, 54) = 10.673, p < 0.001). The effect of interval did not reach significance in case of the Indian leaf fish although it was close to it (F(2, 60) = 2.706, p = 0.075). The other stimuli induced no significant interval dependent changes (F(2, 38–60) < 2.398, p > 0.10). The significant changes found were confirmed by the post hoc one sample t-tests that showed the difference between stimulus presentation period and habituation period performance to significantly deviate from zero for the Bird Side (t = −/379, df = 30, p < 0.01) the 1 Zebrafish (t = 5.06, df = 32, p < 0.01), the 6 Zebrafish (t = 3.72, df = 27, p < 0.01) and the Indian leaf fish stimulus conditions (t = −2.70, df = 30, p < 0.05).
Figure 8.
The percent of time near the stimulus (in the third of the tank closest to the image presentation side) is significantly decreased by the presentation of the bird silhouette on the side and significantly increased by the presentation of 1 or 6 zebrafish images. Mean ± S.E.M. are shown. Sample sizes (n) are indicated in the text. White bars represent performance during the pre-stimulus (habituation) period, black bars represent the stimulus period performance and grey bars the post-stimulus period performance. The stimulus type employed is indicated above the bar graphs. Note that the apparent changes induced by the Indian leaf fish and Dot presented on the side were not found significant by ANOVA although the former was found significant by a t-test. Also note that the Dot top and Bird top conditions are not shown as this response is not relevant in these conditions.
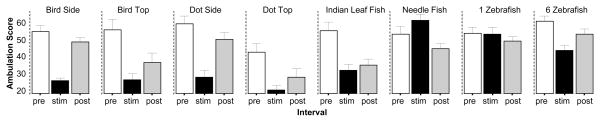
The last behavioral measure we quantified and analyzed is the Ambulation score, a measure of total distance swum by the experimental zebrafish (figure 9). We detected large stimulus induced differences across the different stimulus conditions employed. ANOVA confirmed this observation and detected significant Interval (F(2, 414) = 75.415, p < 0.001) and Stimulus effects (F(7, 207) = 6.681, p < 0.001) as well as a significant Interval × Stimulus interaction (F(14, 414) = 9.141, p < 0.001). Subsequent ANOVAs conducted separately for each stimulus condition found a significant interval effect for all but one condition, 1 Zebrafish (F(2, 64) = 1.094, p > 0.30). The Bird Side (F(2, 60) = 43.629, p < 0.001), Bird Top (F(2, 28) = 17.958, p < 0.001), Dot Side (F(2, 38) = 31.665, p > 0.001), Dot Top (F(2, 42) = 13.870, p < 0.001), Indian leaf fish (F(2, 60) = 14.140, p < 0.001), Needle fish (F(2, 68) = 8.512, p < 0.001) and 6 Zebrafish F(2, 54) = 8.730, p < 0.001) effects were all found significant. These results were confirm for all stimulus conditions except for the Needle fish (t = 1.854, df = 34, p > 0.05) by the one sample t tests whit which we analyzed whether the difference between stimulus period and habituation period performance deviated from zero (Bird Side t = −8.36, df = 30 p < 0.01; Bird Top t = −5.89, df = 14, p < 0.01; Dot Side t = −7.93, df = 19, p < 0.01; Dot Top t = −5.00, df = 21, p < 0.01; Indian leaf fish t = −4.84, df = 30, p < 0.01; 6 Zebra t = −4.85, df = 27, p < 0.01).
Figure 9.
Ambulation score (the number of times fish moved from one imaginary segment to another on a 3×3 grid) is significantly affected by all stimuli except the presentation of the single zebrafish image. Mean ± S.E.M. are shown. Sample sizes (n) are indicated in the text. White bars represent performance during the pre-stimulus (habituation) period, black bars represent the stimulus period performance and grey bars the post-stimulus period performance. The stimulus type employed is indicated above the bar graphs. Note that among the stimuli that had significant effect on the ambulation score the needle fish was the only one that increased the response while the others all decreased it.
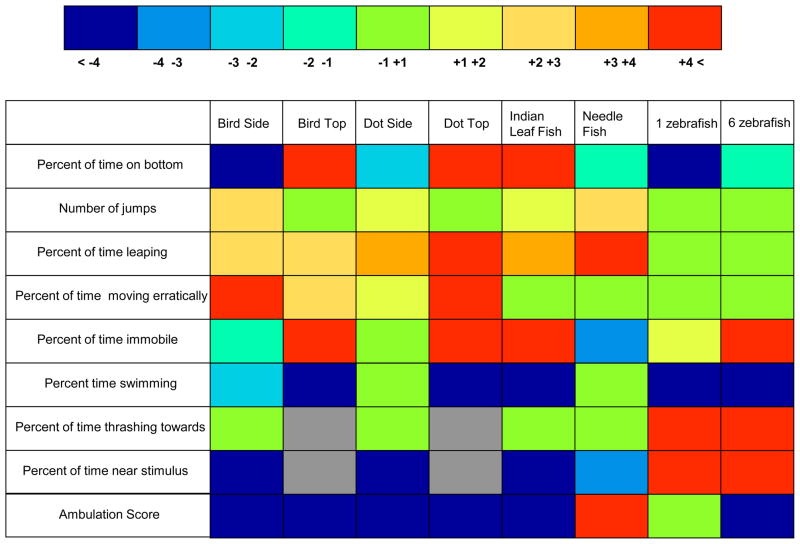
In figure 10 we summarize these results and show the size of the difference between the stimulus presentation and habituation period performance for each behavioral measure quantified (9 rows) and for each stimulus condition (8 columns) as a heat map with cooler (blue) colors representing a decrease in the value of the given behavior induced by the stimulus and warmer colors (red) an increase. The figure demonstrates induction of a stimulus-specific response repertoire that may differ widely across the different stimuli employed (e.g. Dot Top versus Dot Side, or Indian leaf fish versus Needle fish). It also shows that certain behaviors differentiate fear inducing stimuli and non-fear inducing stimuli well while others do not (e.g. Percent time escaping versus Ambulation score, or Percent time thrashing towards versus Percent time swimming).
Figure 10.
Relative changes between the pre-stimulus and stimulus periods in the quantified behaviors depend upon the stimulus employed. The change is calculated as a difference between the values obtained for the particular behavioral measure for the stimulus period and the pre-stimulus period. The figure shows a heat map of changes in which warmer colors represent changes in the positive direction (increase of response induced by the stimulus) and cooler colors represent changes in the negative direction (decrease of response induced by the stimulus). The heat map values and corresponding colors are shown above the heat map matrix. Note that the matrix can be interpreted in two different ways. One can compare the effect profile (the set of behavioral responses) induced by the different stimuli (comparison of columns) and one can also compare the behavioral variables as to how they responded to the different stimuli employed (comparison of rows).
To further investigate the relationships among the different behavioral measures we have conducted Principal Component Analyses (PCA’s), a correlation-based approach that allows one to reduce the large correlation matrix containing n*(n−1)/2 bivariate correlation coefficients (where n is the number of variables) to a simpler solution, the rotated principal component loading matrix. First we conducted the PCA using all 9 variables from the 3 intervals, i.e. analyzed the correlation structure of 27 variables. This PCA (table 1) extracted 11 components that explained 74.2 % of the total variance. The rotation was found to converge within 19 iterations. The first component contains major loadings of variables of the stimulus and post-stimulus periods thrashing towards the stimulus screen and staying near the stimulus screen, and thus represents approach of the stimulus screen. The second component again contains major loadings of variables of the stimulus and post-stimulus periods associated with immobility (negative loadings) or swimming activity (positive loadings) and thus represents activity versus passivity induced by stimulus presentation. The third component contains one motor response, percent of time on the bottom but from all three periods and thus appears to be independent of stimulus presentation. The fourth component is similar to the third in that again it contains variables, in this case representing activity (ambulation score with large positive loadings) from all three periods. Thus this component again appears independent of stimulus presentation. Component five has two variables with large loadings both for leaping, one for the stimulus and the other for the post-stimulus period. This component therefore we interpret as one which reflects stimulus induced leaping. The sixth component contains variables exclusively of the post-stimulus period with locomotory activity related variables showing negative loadings (percent of time swimming and ambulation score) versus immobility and thrashing towards the stimulus (showing positive loadings). The seventh component represents jumping from all three periods. The eighth component reflects activity (versus passivity) exclusively during the habituation period. The ninth component contains one behavioral measure erratic movement from the stimulus and post stimulus periods. The tenth and eleventh component both contain behaviors with major loadings exclusively of the habituation period.
Table 1.
Principal Component Analysis extracted a Rotated Component Matrix with 11 Components for the analysis that included behavioral variables from all three intervals of the recording session. The matrix contains the principal component loadings, which are essentially bivariate correlation coefficients representing correlation between the variable (row) and the component (column). Only major loadings (larger than 0.25) are shown. Note that variables quantified for the pre stimulus (pre), stimulus (stim), and post stimulus (post) intervals are considered separate variables here. Note that some components contain loadings for variables corresponding to particular intervals (e.g. the stimulus and/or post-stimulus intervals) whereas others contain loadings of variables corresponding to a particular behavior measured in all three intervals.
Rotated Component Matrix (Pre stimulus, Stimulus, Post stimulus intervals)
| Component
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| swimming_pre | .896 | ||||||||||
| leap_pre | .790 | ||||||||||
| erratic_pre | .275 | .715 | |||||||||
| thrashing towards_pre | .788 | ||||||||||
| swimming_stim | −.250 | ||||||||||
| leap_stim | .861 | ||||||||||
| erratic_stim | .852 | ||||||||||
| thrashing towards_stim | .722 | .307 | |||||||||
| swimming_post | .404 | −.814 | |||||||||
| leap_post | .873 | ||||||||||
| erratic_post | .863 | ||||||||||
| thrashing towards_post | .347 | .550 | −.282 | ||||||||
| bottom_pre | .749 | ||||||||||
| bottom_stim | .875 | ||||||||||
| bottom_post | .861 | ||||||||||
| jumping_pre | .657 | .274 | |||||||||
| jumping_stim | .702 | ||||||||||
| jumping_post | .767 | ||||||||||
| near stimulus_pre | .436 | −.310 | .563 | ||||||||
| near stimulus_stim | .903 | ||||||||||
| near stimulus_post | .778 | ||||||||||
| immobility_stim | −.876 | ||||||||||
| immobility_post | −.565 | −.251 | .585 | ||||||||
| immobility_pre | −.830 | ||||||||||
| ambulation score_pre | .720 | ||||||||||
| ambulation score_stim | .293 | .622 | |||||||||
| ambulation score_post | .708 | −.349 | |||||||||
Next, we examined the correlation structure of behavioral responses exhibited during the stimulus period. This is perhaps the most interesting period as examination of the correlation groups of behaviors may be expected to reveal stimulus induced independent behavioral response clusters (table 2). The PCA extracted four components that explained 69% of the total variance. The first component was found to contain behaviors with major loadings that reflect level of activity (swim duration and distance traveled versus immobility). The second component contained variables with major loadings that are associated with approach (thrashing towards and staying close to the stimulus screen) versus jumping. The third component confirmed our previous observation that erratic movement and bottom dwell time are often associated and negatively correlated with general activity levels. Finally, the fourth component showed that leap and bottom dwell time also represent a separate response cluster.
Table 2.
Principal Component Analysis extracted a Rotated Component Matrix with 4 Components for the analysis that included behavioral variables from the stimulus interval. The matrix contains the principal component loadings, which are essentially bivariate correlation coefficients representing correlation between the variable (row) and the component (column). Only major loadings (larger than 0.25) are shown.
Rotated Component Matrix (Stimulus interval)
| Component
|
||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| swimming | .928 | |||
| leap | .788 | |||
| erratic | .820 | |||
| thrashing towards | .827 | |||
| jumping | −.591 | |||
| near stimulus | .869 | |||
| bottom | .372 | .649 | ||
| immobility | −.941 | |||
| Ambulation score | .418 | −.521 | ||
Extraction Method: Principal Component Analysis.
Rotation Method: Varimax with Kaiser Normalization.
Rotation converged in 6 iterations.
DISCUSSION
We examined the effect of several stimuli presented on the computer screen that we expected to induce fear responses in zebrafish and we also examined the effect of two additional stimuli that we expected to be non-threatening. The results confirmed our expectations: the images mimicking some aspects of predators induced several fear responses while the sight of conspecifics, the non-threatening stimuli, was clearly attractive to the test subjects. Also importantly, we found that not all aversive “predator” stimuli elicited the same set of fear responses or the same level of fear responses. Perhaps the most robust responses were induced by the dot increasing in size shown from the top. This stimulus mimics an object rapidly approaching from above and as such may be similar to what zebrafish see when a fishing bird (e.g. the common kingfisher, Alcedo atthis, native to the geographical region where zebrafish live; Spence et al., 2006) attacks (birds pull their wings closer to their body before entering the water and thus may look circular from a head on view from below). This stimulus induced a robust increase of time on the bottom, erratic movement, leaping, a robust decrease of activity, and also a significant increase of number of jumps. The image of the Indian leaf fish also turned out to be effective confirming our previous results (Gerlai et al., 2009) but not as much as the dot “approaching” from above. Surprisingly, the needle fish was quite ineffective, although it too induced some fear responses, particularly jumps and leap episodes. Interestingly, this was the only stimulus among the fear inducing stimuli employed that did not reduce activity but rather increased it in our test zebrafish. Needle fish is a piscivore that has been found to coinhabit small streams and ponds with zebrafish in nature (Engeszer et al., 2007; Spence et al., 2006). Importantly, its body structure and mouth anatomy both suggest that this species, just like zebrafish, occupies the upper water layer. It is a predator that probably swims alongside zebrafish shoals in nature near the water surface and its hunting strategy may be highly different from that of the Indian leaf fish, which is a reclusive ambush predator. It is possible that in nature diving to the bottom and staying immobile there after exhibiting erratic movement to stir up debris is not an appropriate strategy against the needle fish, which probably encounters zebrafish far from the safety of the bottom. Instead, active leaping and performing jumps may be more effective, and this is why we observe such responses in the laboratory. It is also notable that the response repertoire induced by the needle fish is highly different from that induced by the presentation of 1 or 6 zebrafish images. Therefore, we conclude that it is unlikely the test subjects mistook the needle fish for a harmless conspecific.
Another observation one may make is that in addition to the visual parameters of the stimulus, the location of its presentation has also turned out to be important. For example, the dot presented on the side elicited much less robust responses compared to the dot presented from the top. Furthermore, the bird silhouette presented on the top induced a different set of fear responses compared to the same bird image presented on the side. Thus, it appears that the fear responses of zebrafish are stimulus type and presentation mode specific.
Fear responses have been some of the most well studied behavioral features of zebrafish (for a recent review see Gerlai, 2010). Yet, systematic analysis of what stimuli may induce fear and what forms of fear responses may be induced by these stimuli has not been conducted. Nevertheless, numerous independent studies suggest that zebrafish have a complex response repertoire. For example, erratic movement, jumping, swimming to the bottom, immobility or freezing, dynamic changes in interindividual distance in a shoal (e.g. temporary loosening and subsequent tightening of shoal cohesion) and escaping to and from differently illuminated areas of the test environment have all been described for zebrafish (e.g. Gerlai et al., 2000; Speedie & Gerlai, 2008; Parra et al., 2009; Bass & Gerlai, 2008; Hall & Suboski, 1995; Serra et al., 1999; Egan et al., 2009; Levin et al., 2007). These studies utilized a range of stimuli, from live predators and images of predators, to tapping on the glass, dropping objects into the water, or just simply placing zebrafish into novel tanks. Although not conducted as a randomized and systematic analysis, the above studies too suggest that the fear responses of zebrafish may be specific to particular stimuli or situations.
Numerous prey species have been studied with respect to their antipredatory behaviors and have been found to show predator specific or threat context dependent responses. Alarm calls of the chickadee differ depending on the size of the birds of prey the chickadee responds to (Templeton et al., 2005). Fish respond to approaching predators with fleeing, fin erection display, or can even ignore the predator depending on the distance between them and the predator, or on the level of satiation of the predator and many other factors including access to escape routes, just to mention but a few examples (e.g. Gerlai, 1993). Our current results are in line with these findings: zebrafish may also have unique, predator, or threat specific responses that do not fully generalize to all contexts or all fear inducing stimuli. This is an important notion not only as it presumes the evolution of a complex behavioral repertoire even in fish, but also because it has practical consequences in the induction and analysis of fear in the laboratory. Reliable induction of robust fear responses in zebrafish is crucial as it would allow the use of zebrafish in genetic as well as drug screens aimed at investigating the mechanisms of fear responses and the identification of efficacious anxiolytic drugs.
From the perspective of behavior quantification one may ask which behavior characterizes fear in zebrafish best, i.e. what should the experimenter quantify to capture drug or mutation induced changes in fear or anxiety in this experimental species. Our results suggest that it is likely that one will need to measure several behavioral parameters to properly characterize the fear response or changes in the fear response induced by the experimental manipulation. For example, we (Bass & Gerlai, 2008; Gerlai et al., 2009), and others too (Levin et al., 2007; Egan et al., 2009), have routinely quantified the level of activity or the amount of freezing as an index of fear, when in fact our results now show that reduction of activity can also be induced by a non-threatening stimulus, the presentation of an image of a shoal of zebrafish. Reduction of activity has been already documented in the literature for single experimental zebrafish in response to presentation of an image of a zebrafish shoal (Fernandes & Gerlai, 2009). Thus, reduction of activity measured alone will not be a good indicator of the level of fear. Also notably, even such seemingly trivial fear responses as increasing the distance to the predator (or reducing the time spent near the predator stimulus) may only be observable under particular conditions. For example, we found this response robustly elicited by the Indian leaf fish when we employed a longer tank (Ahmed et al., 2011) but practically absent in a shorter test tank (Gerlai et al., 2009). Therefore, it appears that the test environment, the manner of presentation (e.g. location) and the type of stimuli presented all influence what fear responses may be exhibited by zebrafish.
In summary, the above results are compatible with the suggestion that zebrafish can employ more than one antipredatory strategy, and these strategies may be dependent upon the predatory threat (type and level) as well as the environment, the context, in which they occur. One way to investigate such “strategies” is to analyze how individual behavioral responses may correlate with each other. In our current study we conducted Principal Component Analyses to investigate the existence of correlation clusters or groups of behavior. Ideally, one should conduct such correlation analysis separately for each stimulus condition and then compare the correlation structures (e.g. Gerlai & Csányi, 1990). However, such analysis was not possible in the current study because of low sample sizes. For example, in order for one to achieve a stable component solution with PCA it is recommended to have at least six times as many subjects as variables (Gerlai & Csányi, 1990 and references therein). In our study the sample size was smaller than 35 for the different stimulus conditions and we had 9 behavioral variables and thus we did not meet the above criterion. However, we could conduct an overall PCA in which data of all 215 subjects (from all stimulus conditions) were used. First such an analysis was conducted distinguishing behavioral variables according to the observation session, i.e. including all behaviors measured during the pre-stimulus, stimulus and post-stimulus periods. This analysis allows one to investigate not only how behavioral measures correlate with each other within a particular period but also across periods. Given that no stimulus is presented during the pre-stimulus interval, using the above analysis one, for example, can distinguish stimulus presentation induced correlations from non-stimulus presentation related correlations, a point we illustrate below.
The first component of table 1 represents a single behavioral response: attraction to the stimulus. This component contains major loadings of behaviors thrashing towards stimulus and percent of time near stimulus. But also importantly, these behaviors are listed under this component only for the stimulus and post-stimulus periods, i.e. they are likely the result of the presentation of the stimuli and not of other factors independent of stimulus presentation. The same is true for component two, which represents active locomotion versus passivity in response to stimulus presentation. The fact that the given behaviors are loaded on these two separate components also suggests that approach and activity are two unrelated strategies (the two components are orthogonal, i.e. not correlated). The third component differs from the first two in that it contains a single behavioral response, time spent on the bottom, but this variable now is listed under this component for all three periods. Based on this result, we conclude that there is an external factor that influences how much time zebrafish spend on the bottom of their tank and that this factor is independent of stimulus presentation. This result is not surprising. Although bottom dwell time has been shown to be a response associated with fear (e.g. Egan et al., 2009; Levin et al., 2007; Gerlai et al., 2009; Speedie & Gerlai, 2008) and although several of the stimuli presented in the current study are expected to induce fear, it is notable that the stimuli presented on the computer screen were not the only ones that could induce fear. Handling and the novel nature of the test tank itself is aversive and thus we argue that the fourth principal component reflects this non-stimulus presentation dependent fear response. A similar conclusion may be drawn on the basis of the component loading pattern obtained for principal component number five. This component contains activity related behavioral measures (e.g. the ambulation scores) from all three periods and as such represents activity changes independent of stimulus presentation (covariance not induced by the stimuli shown). Component six also loads behavioral measures related to activity level but only from the post-stimulus period and thus we argue it reflects the activity altering after effect of stimulus presentation. Component 7 again suggests that behavior (jumping in this case) was influenced by factors unrelated to stimulus presentation. The fact that jumping from all three periods is loaded on this factor suggests that some fish showed elevated and others reduced number of jumps consistently across the three periods (hence the positive correlation) and these differences were independent of stimulus presentation and lasted for the entire length of behavioral recording. Interestingly, component eight is again about activity but it lists only two behaviors with major loadings (swimming with positive and immobility with negative signs) from the pre-stimulus periods suggesting perhaps the existence of factors that only influence (lead to covariation in) activity during this period. Component nine with major loadings of erratic movement from the stimulus and post-stimulus periods represents stimulus presentation induced fear that is somewhat independent of other fear responses. Components 10 and 11 again suggest that there may be factors that induce and influence fear related responses before the stimulus period, a point we have discussed above.
The last result we consider is the Principal Component loading matrix extracted using behavioral measures quantified for the stimulus period. This matrix also suggest that certain fear responses, in this case likely induced by the presentation of the stimulus, represent independent response clusters we may interpret as separate antipredatory strategies. The first component represents passivity vs. active swimming. Not surprisingly the sign of the loadings is opposite for immobility and the active behaviors listed under this component but what is notable is that no other behaviors are listed with major loadings. This result suggests that activity vs. passivity is a response set that is independent of other behaviors shown by zebrafish during the stimulus period. Component two shows that active approach (thrashing towards and staying near the stimulus) negatively correlates with jumping and thus these behaviors form a response set that again is separate from other responses. Component three shows that high levels of erratic movement are associated with reduced activity and increased bottom dwell time, and vice versa. This response set is identical to what we have previously intuitively interpreted as potentially adaptive in nature (erratic movement is expected to stir up debris if performed on the bottom of the stream or pond and is especially effective as a predator avoidance reaction if followed by immobility). The last component shows that bottom dwell time is also involved in another behavioral response set, one which is associated with leaping.
In summary, our results suggest that zebrafish fear responses are not unitary. They may represent multiple unrelated response sets whose manifestation depends upon the type and location of the stimuli shown to the subjects. Our results also demonstrate that recording of a single behavioral response will be unlikely to be adequate for the evaluation of changes induced in fear by experimental manipulations including, for example, in drug or mutation screens.
Research Highlights.
Fear inducing as well as non-threatening computer animated images were presented to zebrafish.
Zebrafish responded with image-presentation specific behaviors that differentiated even the fear inducing images.
It is concluded that the fear response repertoire of zebrafish is stimulus and context dependent.
Acknowledgments
Supported by R01 grant to NIH/NIAAA to RG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed O, Seguin D, Gerlai R. An automated predator avoidance task in zebrafish. Behav Brain Res. 2011;216:166–171. doi: 10.1016/j.bbr.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Imari L, Gerlai R. Conspecifics as reward in associative learning tasks for zebrafish (Danio rerio) Behav Brain Res. 2008;189:216–219. doi: 10.1016/j.bbr.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Alsop D, Vijayan MM. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am J Physiol Regul Integr Comp Physiol. 2008;294:R711–719. doi: 10.1152/ajpregu.00671.2007. [DOI] [PubMed] [Google Scholar]
- Bass SLS, Gerlai R. Zebrafish (Danio rerio) responds differentially to stimulus fish: The effects of sympatric and allopatric predators and harmless fish. Behav Brain Res. 2008;186:107–117. doi: 10.1016/j.bbr.2007.07.037. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. Conditioning and residual emotionality effects of predator stimuli: some reflections on stress and emotion. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1177–1185. doi: 10.1016/j.pnpbp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Blaser R, Gerlai R. Behavioral phenotyping in Zebrafish: Comparison of three behavioral quantification methods. Beh Res Methods. 2006;38:456–469. doi: 10.3758/bf03192800. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Gerlai R. High Precision Liquid Chromatography Analysis of Dopaminergic and Serotoninergic Responses to Acute Alcohol Exposure in Zebrafish. Behav Brain Res. 2009;200:208–213. doi: 10.1016/j.bbr.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denver RJ. Structural and functional evolution of vertebrate neuroendocrine stress systems. Ann N Y Acad Sci. 2009;1163:1–16. doi: 10.1111/j.1749-6632.2009.04433.x. [DOI] [PubMed] [Google Scholar]
- Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien DH, Mohnot S, Beeson E, Glasgow E, Amri H, Zukowska Z, Kalueff AV. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeszer RE, Patterson LB, Rao AA, Parichy DM. Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish. 2007;4:21–40. doi: 10.1089/zeb.2006.9997. [DOI] [PubMed] [Google Scholar]
- Fernandes Y, Gerlai R. Long-term behavioral changes in response to early developmental exposure to ethanol in zebrafish. Alcohol: Clin Exp Res. 2009;33:601–609. doi: 10.1111/j.1530-0277.2008.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. Zebrafish antipredatory responses: A future for translational research? Behav Brain Res. 2010;207:223–231. doi: 10.1016/j.bbr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. Phenomics: Fiction or the future? Trends Neurosci. 2002;25:506–509. doi: 10.1016/s0166-2236(02)02250-6. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Can paradise fish (Macropodus opercularis) recognize its natural predator? An ethological analysis. Ethology. 1993;94:127–136. [Google Scholar]
- Gerlai R, Fernandes Y, Pereira T. Zebrafish (Danio rerio) responds to the animated image of a predator: Towards the development of an automated aversive task. Behav Brain Res. 2009;201:318–324. doi: 10.1016/j.bbr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R, Lee V, Blaser R. Effects of acute and chronic ethanol exposure on the behavior of adult zebrafish (Danio rerio) Pharmacol, Biochem Behav. 2006;85:752–761. doi: 10.1016/j.pbb.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R, Csányi V. Genotype-environment interaction and the correlation structure of behavioral elements in paradise fish (Macropodus opercularis) Physiol Behav. 1990;47:343–356. doi: 10.1016/0031-9384(90)90153-u. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Lahav M, Guo S, Rosenthal A. Drinks like a fish: Zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav. 2000;67:773–782. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Clayton NS. Analysing hippocampal function in transgenic mice: An ethological perspective. Trends Neurosci. 1999;22:47–51. doi: 10.1016/s0166-2236(98)01346-0. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Hogan JA. Learning to find the opponent: an ethological analysis of the behavior of paradise fish (Macropodus opercularis, Anabantidae) in intra- and inter-specific encounters. J Comp Psychol. 1992;106:306–315. doi: 10.1037/0735-7036.106.3.306. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiol Behav. 2007;90:54–58. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Hall D, Suboski MD. Visual and olfactory stimuli in learned release of alarm reactions by zebra danio fish (Brachydanio rerio) Neurobiol Learn Mem. 1995;63:229–240. doi: 10.1006/nlme.1995.1027. [DOI] [PubMed] [Google Scholar]
- Hohoff C. Anxiety in mice and men: a comparison. J Neural Transm. 2009;116:679–687. doi: 10.1007/s00702-009-0215-z. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Price RB, Charney DS. Recent advances in the neurobiology of anxiety disorders: implications for novel therapeutics. Am J Med Genet C Semin Med Genet. 2008;148C:89–98. doi: 10.1002/ajmg.c.30172. [DOI] [PubMed] [Google Scholar]
- Mueller T, Vernier P, Wullimann MF. The adult central nervous cholinergic system of a neurogenetic model animal, the zebrafish Danio rerio. Brain Res. 2004;1011:156–169. doi: 10.1016/j.brainres.2004.02.073. [DOI] [PubMed] [Google Scholar]
- Nandi A, Beard JR, Galea S. Epidemiologic heterogeneity of common mood and anxiety disorders over the lifecourse in the general population: a systematic review. BMC Psychiatry. 2009;9:31. doi: 10.1186/1471-244X-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra KV, Adrian JC, Jr, Gerlai R. The synthetic substance hypoxanthine 3-N-oxide elicits alarm reactions in zebrafish (Danio rerio) Behav Brain Res. 2009;205:336–341. doi: 10.1016/j.bbr.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer W. Alarm substances. Experientia. 1993;19:113–123. doi: 10.1007/BF02171582. [DOI] [PubMed] [Google Scholar]
- Ruhl N, McRobert SP. The effect of sex and shoal size on shoaling behaviour in Danio rerio. J Fish Biol. 2005;67:1318–1326. [Google Scholar]
- Saverino C, Gerlai R. The social zebrafish: Behavioral responses to conspecific, heterospecific, and computer animated fish. Behav Brain Res. 2008;191:77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz F. Vergleichende. Untersuchungen iiber die Schreckreaktion bei Fischen und deren Verbreitung Z. vergl. Physiol. 1996;38:84–135. [Google Scholar]
- Serra EL, Medalha CC, Mattioli R. Natural preference of zebrafish (Danio rerio) for a dark environment. Braz J Med Biol Res. 1999;32:1551–1553. doi: 10.1590/s0100-879x1999001200016. [DOI] [PubMed] [Google Scholar]
- Sison M, Cawker J, Buske C, Gerlai R. Fishing for genes of vertebrate behavior: Zebra fish as an upcoming model system. Lab Animal. 2006;35:33–39. doi: 10.1038/laban0506-33. [DOI] [PubMed] [Google Scholar]
- Speedie N, Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav Brain Res. 2008;188:168–177. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence R, Fatema MK, Reichard M, Huqk KA, Wahab MA, Ahmed ZF, Smith C. The distribution and habitat preferences of the zebrafish in Bangladesh. J Biol. 2006;69:1435–1448. [Google Scholar]
- Templeton CN, Greene E, Davis K. Allometry of Alarm Calls: Black-Capped Chickadees Encode Information About Predator Size. Science. 2005;308:1934–1937. doi: 10.1126/science.1108841. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Sive HL. Can zebrafish be used as a model to study the neurodevelopmental causes of autism? Genes Brain Behav. 2003;2:268–281. doi: 10.1034/j.1601-183x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- Weisberg RB. Overview of generalized anxiety disorder: epidemiology, presentation, and course. J Clin Psychiatry. 2009;(Suppl 2):4–9. [PubMed] [Google Scholar]