Abstract
The tight junction forms the paracellular permeability barrier in all epithelia, including the renal tubule. Claudins are a family of tight junction membrane proteins with four transmembrane domains that form the paracellular pore and barrier. Their first extracellular domain appears to be important for determining selectivity. A number of claudin isoforms have been found to be important in renal tubule function, both in adults and in neonates. Familial hypomagnesemic hypercalciuria with nephrocalcinosis is an autosomal recessive syndrome characterized by impaired reabsorption of Mg and Ca in the thick ascending limb of Henle's loop. Mutations in claudin-16 and 19 can both cause this syndrome, but the pathophysiological mechanism remains controversial.
Keywords: Tight junction, Claudin, Renal tubule, Paracellular, Transport, Calcium, Hypomagnesemia
Introduction
The renal tubule plays a major role in water and electrolyte homeostasis by mediating transepithelial reabsorption and secretion into the tubular fluid and hence regulating the final composition of urine. The route of transepithelial transport can be either transcellular (passing through cells and mediated by transmembrane channels, transporters and pumps) or paracellular (passing between adjacent epithelial cells). In contrast to transcellular transport, paracellular transport is always passive and so must be driven by an electrical or chemical gradient.
Paracellular permeability of the renal tubule
The magnitude and selectivity of paracellular permeability varies along the nephron. The proximal tubule is responsible for bulk reabsorption of salt and water. In the early proximal tubule, Na+ is reabsorbed transcellularly together with glucose, amino acids, and bicarbonate. This generates luminal fluid that is high in chloride and low in bicarbonate relative to the peritubular space. Because the late proximal tubule has a high paracellular permeability to both Na+ and Cl- but not to bicarbonate, Cl- diffuses paracellularly down its concentration gradient, generating a lumen-positive transepithelial voltage that then drives concomitant paracellular reabsorption of Na+ [1, 2]. Interestingly, the neonatal proximal straight tubule has a lower paracellular Cl- permeability than the adult tubule [3] and hence a lower rate of fluid reabsorption [4], which may predispose neonates to dehydration.
In the thick ascending limb of Henle, Na+ is reabsorbed predominantly transcellularly via an apical Na-K-2Cl cotransporter and basolateral Na-K-ATPase. This generates a lumen-positive voltage by two mechanisms: (a) K+ reabsorbed across the apical membrane by the Na-K-2Cl cotransporter is recycled to the lumen through the K+ channel, ROMK; and (b) Na+ accumulated in the peritubular fluid back-leaks into the lumen via a paracellular pathway [5]. The paracellular route is also highly permeable to divalent cations and so this lumen-positive potential provides an electrical driving force for reabsorption of Ca2+ and Mg2+ paracellularly.
The distal tubule and collecting duct fine-tune urinary composition at the end of the nephron. Active, transcellular Na+ reabsorption and K+ and H+ secretion generate steep transtubular concentration gradients for these ions. In these segments, the paracellular pathway is relatively impermeable and acts as a barrier to solutes, so that these ion gradients are not dissipated.
Tight junctions regulate paracellular permeability of epithelia
Epithelial cells are connected via multiple junctional complexes. The tight junction separates the apical and basolateral membrane domains and acts as the paracellular barrier while remaining selectively permeable to ions and water. By electron microscopy, the tight junction appears as a band of parallel fibril strands in the subapical compartment.
The tight junction has been shown to be the site of paracellular permeability in epithelia. Ussing and Windhager [6] incubated frog skin cells apically with Ba2+ and basolaterally with SO2-4 and showed that, under the condition of hyperosmotic solution on the apical side, BaSO4 precipitated in junctions. Machen [7] demonstrated that La3+ permeates the junctional complex in rabbit gallbladder and ileum epithelium and proposed the concept that some tight junctions are permeable to ions and small molecules like water and mannitol.
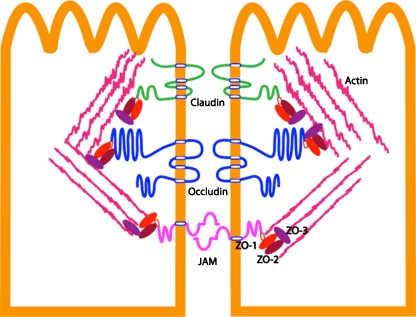
Studies on the biochemical composition of the tight junction have helped to elucidate the regulation of tight junction permeability. The tight junction is constituted of several groups of proteins (Fig. 1). The first group consists of membrane-spanning proteins (claudin, occudin, and junctional adhesion molecules), which are of particular interest because their extracellular domains face the paracellular space and are thought to regulate paracellular transport directly. The second group consists of scaffolding proteins (e.g., zona occludens family), which link the membrane-spanning proteins to the actin cytoskeleton. The third group consists of signaling molecules, including transcription factors and kinases/phosphatases that regulate tight junction protein transcription and expression (for a more detailed review, see [8]).
Fig. 1.
Biochemical components of tight junction: transmembrane protein: claudin, occludin, junctional adhesion molecule (JAM) seal the paracellular space between adjacent epithelial cells, separating the cell into apical compartment and basolateral compartment. Claudin constitutes the paracellular barrier and pore by homogenic and heterogenic interaction. C-termini of claudin, occludin, and JAM have PDZ binding domain linking to scaffold ZO protein. ZO protein can bind directly [60] to cytoskeleton actin filament. Protein kinase, protein phosphatase, and transcription factors (not shown in the figure) can interact with cytosolic part of claudin, occludin and JAM and ZO protein to regulate tight junction assembly
Claudins are tight junction proteins that constitute the paracellular barrier and pore
General properties of claudins
Twenty-four claudin genes have so far been identified in mammalian cells. Claudins are 4-transmembrane domain proteins that have two extracellular loops facing the paracellular space. Among the membrane-spanning proteins at the tight junction, claudins are by far the most variable in terms of number of genes/isoforms. These isoforms are expressed variably in epithelia of different organs in a tissue-specific manner, which probably correlates with the variability in permeability of these epithelia. The initial discovery of the pathogenic linkage of claudin-16 to familial hypomagnesemia hypercalciuria nephrocalcinosis (FHHNC) first suggested that the claudins might be general regulators of paracellular permeability at the tight junction. At the tight junction, claudins work in concert with the other components of the tight junction, which makes it difficult to control for and define the specific roles in permeability of any individual claudin. Nevertheless, a variety of experimental approaches have been used to answer three key questions: what are the permeability properties of a specific claudin? What part of the claudin determines the permeability? What amino acids are the active sites of paracellular transport? These studies are summarized in the following section. Note that we focus here on renal epithelial physiology, but claudins have a broader role in many other aspects of biology, e.g., barrier function in gastrointestinal epithelia, retina and cochlea, cancer, etc.
Expression studies in epithelial cell lines and knockout studies in mice
The function of claudins has been addressed by numerous overexpression studies in epithelial cell lines (for a detailed review, see [9]). To briefly summarize, claudin-1 [10], 4 [11], 5 [12], 8 [13], 9 [14], 11 [11], 15 [11], and 19 [15] are considered to be barrier claudins because they increase transepithelial resistance (TER) when overexpressed in leaky cell lines. Claudin-2 [16] and 16 [17] are considered to be pore claudins because they decrease the TER in most cell lines. Because epithelial cells express endogenous claudins, the change of TER and ion selectivity reflects the overall effect of exogenous and endogenous claudins interaction rather than the properties of the expressed claudin alone.
The function of claudins has also been addressed by knockout studies in mice. This study design minimizes the interaction effects and may better reveal the function of the target claudin of interest. Knockout of claudins-1 and -5 increases paracellular permeability in the skin and blood–brain barrier, respectively, while knockout of claudin-2 decreases permeability in the proximal renal tubule [18]. Taken together, these findings confirm that claudins regulate paracellular permeability at the tight junction.
Chimera and mutagenesis studies
While the previous studies showed that claudins regulate the paracellular permeability, evidence that claudins form the paracellular pore/barrier itself came from chimera and mutagenesis studies. As mentioned above, claudins have two extracellular loops. Colegio et al. [19] swapped the extracellular loops between claudin-2 (‘pore claudin’) and claudin-4 (‘barrier claudin’) and showed that the first extracellular loop (ECL) of claudin-2 with the second ECL of claudin-4 still functioned as a pore, whereas the first ECL of claudin-4 with the second ECL of claudin-2 functioned as a barrier. Thus, it directly supports the theory that claudins form either a paracellular pore or a barrier and that this is determined by the first ECL. Taking this a step further, mutagenesis studies of the first ECL of claudins revealed the existence of sites conferring permeability and selectivity. Using charge-reversing mutations, Colegio [20] first showed that K65D (positive charge mutated to negative charge) in claudin-4 increased Na+ permeability, while D55R, E64K (negative charge mutated to positive charge) both individually and synergistically reversed the claudin-15 ion preference from Na+ to Cl-. However, the effect of reversing the charge at a site may not necessarily reveal the normal physiological role of the residue at that site but could instead reflect non-physiological effects of introducing the opposite charge. Yu et al. [21] therefore used charge-neutralizing mutations in claudin 2. They found that D65 appears to be the major contributor to an intra pore Na+ binding site. In aggregate, these data support a model in which the first ECL of claudins forms the lining of the paracellular pore and determines the magnitude and selectivity of its permeability to small ions.
Properties of the claudin pore
In general, the claudin pore is a narrow, fluid-filled, charge- and size-selective pore formed by the first extracellular loops of adjacent claudin. Ions are transported passively, parallel to the cell membrane within the paracellular space, driven by the chemical and electrical gradient. Van Itallie [22] applied molecules of different sizes to the apical side of the epithelial cell layer and measured the concentration of the small molecules from the basolateral site. They reported that the radius of claudin pores was ∼4 Å in MDCK cell lines. Similarly, Yu et al. [21] measured the permeability of organic cations and estimated the pore size of claudin 2 as 6.5 Å in diameter. The molecular basis of the charge selectivity of the claudin pore is electrostatic interaction of partially dehydrated permeating ions with an oppositely charged site within the pore (e.g., D65 in cation-selective claudin-2) [21]. More recently, the structure of the first ECL fold has begun to be explored by cysteine mutagenesis [23]. By mutating several amino acids of the first ECL of claudin-2 to cysteine, it was possible to study the accessibility of the mutated residue to extracellularly applied chemical probes that can bind to the –SH group of cysteine. The function and role in human disease of selected claudin genes are summarized in Table 1.
Table 1.
Selected claudin isoforms of known function
| Claudin isoforms | Localization in the kidney [63] | Permeability properties | Phenotypes of knockout/transgenic mouse model | Biological role in human disease |
|---|---|---|---|---|
| Claudin-1 | Bowman’s capsule | Cation barrier [64] | Impaired epidermal barrier leading to dehydration and death [68] | HCV entry into hepatocyte [66, 67] |
| Claudin-2 | Proximal tubule and early thin descending limb | Cation selective pore [21] | Impaired proximal tubule Na reabsorption [18] | Upregulated in inflammatory bowel disease [68] |
| Claudin-3 | Thin ascending limb to collecting duct | Unknown | Pro-neoplastic in many malignancies [69] | |
| Claudin-4 | Thin ascending limb and collecting duct | Cation barrier [72] and anion selective pore [73] | Receptor for Clostridium perfringens enterotoxin [70, 71] | |
| Claudin-5 | N/A | Unknown | Normal development and morphology of blood vessel, no cerebral bleeding or edema. Loosen blood–brain barrier [74] | Maintains integrity of endothelial blood-brain barrier [75] |
| Claudin-6 | Glomerulus, proximal tubule, thick ascending limb, distal tubule, collecting duct | Unknown | HCV entry into hepatocyte [66, 67] | |
| Claudin-7 | Distal thin descending limb, macular densa, distal tubule, collecting duct | Anion barrier [55] | Renal NaCl wasting, chronic dehydration, secondary hyperaldosteronism and growth retardation [57] | |
| Claudin-8 | Distal thin descending limb, distal tubule, collecting duct | Cation barrier [13] | ||
| Claudin-10 | From proximal tubule to collecting duct | Variable splicing, either Anion pore or cation pore [76] | ||
| Claudin-11 | Proximal tubule, thick ascending limb | Cation barrier [77] | Severe neurological and reproductive deficit [78] | CNS myelin and Sertoli cell tight junction [78] |
| Claudin-12 | N/A | Unknown | Vitamin-D dependent intestinal Ca2+ absorption [79] | |
| Claudin-14 | Collecting duct | Cation barrier [48] | Deafness [48]. Normal renal salt handling [49] | Mutated in autosomal recessive deafness [80] |
| Claudin-16 | Thin ascending limb and thick ascending limb | Cation selective pore [39] | Chronic renal wasting of Mg and Ca, nephrocalcinosis [38] | Mutated in FHHNC [36, 37] |
| Claudin-19 | Thin ascending limb and thick ascending limb | Cation barrier [15], anion barrier [39] | Chronic renal wasting of Mg and Ca, nephrocalcinosis [81] | Mutated in FHHNC with ocular involvement [36, 37] |
Familial hypomagnesemic hypercalciuria with nephrocalcinosis
Familial hypomagnesemia hypercalciuria nephrocalcinosis (FHHNC) is the only monogenic renal syndrome so far ascribed to claudin gene mutations. It is a rare, autosomal recessive disorder characterized by renal Mg wasting, hypercalciuria, nephrocalcinosis, a trend toward renal insufficiency and occasionally ocular abnormalities [24]. FHHNC was first reported in 1972 followed by several other reports from different parts of the world [24–32]. The onset of this syndrome is in early childhood [24–31]. Kari et al. reported that the mean age at first presentation ranged 0.1–3 years; however, the mean age at diagnosis ranged 0.5–12 years [26]. End-stage renal disease usually follows in the second to third decade of life [24]. Approximately 30–75% of patients reported in case series have required hemodialysis within a decade of diagnosis [31, 33]. Other types of primary renal Mg wasting condition are also recognized, including isolated renal Mg wasting, mitochondrial cytopathy associated with renal Mg wasting, Gitelman disease and classic Bartter syndrome. Unlike FHHNC, however, these conditions frequently present with hypokalemia, metabolic alkalosis, and hypocalciuria, which are distinguishable from FHHNC [34].
Presentation and clinical course
The classic form of FHHNC (OMIM #248250) most commonly presents with kidney stones, convulsions, carpopedal spasm, polydipsia, polyuria, rickets, and recurrent urinary tract infections [24]. A subset of patients with the syndrome of FHHNC present in addition with ocular abnormalities including macular colobomata, nystagmus, severe myopia, corneal calcifications, and chorioretinitis (OMIM #248190, which turns out to be caused by claudin-19 mutations, see below) [24, 31, 35]. Since the primary defect is related to impaired tubular reabsorption of Mg, patients almost always have a high fractional excretion of urinary Mg, while serum Mg is inappropriately low [24–31]. Family members with heterozygous mutation may have only hypercalciuria and nephrolithiasis without having hypomagnesemia, suggesting a milder phenotype [31]. Nephrocalcinosis, which is believed due to increased urinary Ca excretion, Mg deficiency and urinary acidification disturbances, has been shown to be associated with the progression rate of renal insufficiency [24–31]. The pathogenesis of the renal failure remains unexplained. It was believed that the nephrocalcinosis and resultant tubulointerstitial nephropathy was also the cause of chronic renal failure.
Unfortunately, neither Mg supplement nor thiazide diuretics normalize serum Mg levels or urinary Ca excretion, or delay the progression of the disease [27, 31, 33]. Kidney transplantation, however, corrects the abnormalities of renal Mg and Ca handling [31, 34].
Etiology of FHHNC
It is now known that mutations in the claudin-16 gene [36], which encodes the tight junction protein, claudin-16 (previously known as paracellin-1), and in the claudin-19 gene [37] are responsible for FHHNC (Fig. 2). This is supported by the phenotype in mice with knockdown of these two genes. Hou et al. [38] used lentiviral transgenesis of shRNA to knock down claudin-16 expression by >99% in mouse kidneys. Claudin-16 knockdown mice had hypomagnesemia with fourfold increase in the fractional excretions of both Ca and Mg. Likewise claudin-19 knockdown mice clearly demonstrated a higher fraction excretion of Mg and Ca and the development of nephrocalcinosis, compared to wild-type mice [39]. The fact that knockdown of claudin-16 and claudin-19 reproduce the FHHNC phenotype confirms that the human disease is due to loss-of-function mutations in these genes. Interestingly, the clinical presentation of patients with mutations of claudin-16 and claudin-19 is different: only those with mutations of claudin-19 present with ocular abnormalities. This is in part because only claudin-19 is expressed in the tight junction of the retina [37].
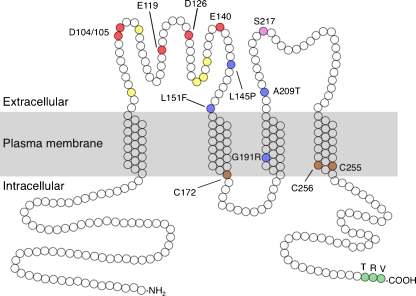
Fig. 2.
Molecular model of paracellin-1/claudin-16. Claudin-16 has four predicted transmembrane domains with intracellular N- and C-termini. Acidic residues in the first extracellular domain that participate in cation permeation (as evidenced by neutralizing mutations that preserve normal expression and trafficking but reduce paracellular Na permeability [17]), probably by affecting pore electronegativity [20], are shaded red; those that do not appear to be important for permeation are shaded yellow. FHHNC mutations that preserve paracellin-1 expression and trafficking but impair permeability are shaded blue [17]; those that abolish expression or cause miss-trafficking are mostly uninformative of paracellin-1 function and are not shown. Serine-217 (purple) is phosphorylated by PKA and thereby facilitates trafficking to the tight junction [45]. Cysteines located at the intracellular end of the second and fourth transmembrane domains and shaded brown are homologous to those that are palmitoylated and participate in tight junction trafficking in other claudins [61]. A C-terminal PDZ-binding motif (TRV) that is required for binding to ZO1 and tight junction trafficking is shaded green [62]
Hypotheses regarding the pathogenesis of FHHNC
Rodriguez-Soriano et al. first proposed that FHHNC might be due to a defect in tubular reabsorption in the thick ascending limb (TAL) of loop of Henle [40]. This was based on the magnitude of the observed increase in fractional excretion of magnesium, which could only be accounted for by a defect in the TAL, and on the fact that linked transport of Ca and Mg is characteristic of the TAL. Blanchard et al. [41] subsequently demonstrated that FHHNC patients are unable to further increase their fractional excretion of Mg and Ca in response to the loop diuretic, furosemide, while having a preserved natriuretic response, thus confirming that there is a selective defect in divalent cation reabsorption in the TAL [41].
In the TAL, NaCl is actively reabsorbed against an uphill concentration gradient via a transcellular pathway involving the apical Na-K-2Cl cotransporter, NKCC2, and basolateral Na-K-ATPase. Ca and Mg reabsorption occurs by passive diffusion via the paracellular pathway, which is driven by a lumen-positive voltage. This transepithelial voltage is generated by two mechanisms: (a) Na, K, and Cl reabsorption through NKCC2 occurs simultaneously with recycling of K back into the tubule lumen via the apical K channel, ROMK; (b) NaCl that has been reabsorbed actively by the transcellular pathway establishes a concentration gradient of NaCl that favors back-diffusion into the lumen. Because the paracellular pathway is more permeable to Na than Cl (Na-to-Cl permeability ratio, PNa/PCl, has been found to vary from 2 to 6 [42, 43, 44]), this generates a dilution potential that is lumen-positive.
Two independent hypotheses have been advanced as to how mutations in the TAL tight junction proteins, claudin-16 and -19, can impair TAL Ca and Mg reabsorption. The first hypothesis is that claudin-16 and/or -19 might function as divalent cation-selective paracellular pores, so that in FHHNC the paracellular Ca and Mg permeability of the TALH would be expected to be impaired. However, the in vitro studies of claudin-16 permeability properties have yielded conflicting results, and the idea that claudin-16 by itself forms a Mg pore is not well supported by the available evidence [17, 45, 46]. Likewise, when claudin-19 is expressed alone or together with claudin-16, it does not seem to reconstitute a functioning Mg pore [39].
The second hypothesis, put forward by Goodenough, Hou and colleagues, is that claudin-16 and -19 function synergistically to form highly Na-selective paracellular pores. Overexpression of claudin-16 in vitro increases Na permeability [17], while overexpression of claudin-19 decreases Cl permeability [39]. Furthermore, claudin-16 and -19 physically interact to form heteromultimers, and coexpression of both has additive effects to increase PNa/PCl [39]. Also consistent with this, in in vitro perfused TALH tubules from claudin-16 knockdown mice, PNa/PCl was found to be decreased twofold with no change in PNa/PMg [38]. Since a high TAL PNa/PCl is necessary to generate the transepithelial dilution potential, loss-of-function mutations in claudin-16 and -19 would be predicted to abrogate the electrical driving force for Ca and Mg reabsorption in the TAL.
Claudin mutations and kidney stones
Recently, Thorleifsson et al. reported an association between common synonymous variants in the claudin-14 gene and both increased risk of kidney stones and lower bone mineral density [47]. These variants were also associated with increased urine calcium excretion and decreased serum total CO2, suggesting that the effects on both kidney stones and bone mineral density are mediated by hypercalciuria and metabolic acidosis. Claudin-14 was initially reported to be expressed in the collecting duct [48], but subsequently localized by immunohistochemistry to the thin descending and thick ascending limbs of the loop of Henle, and the proximal convoluted tubule [49]. In vitro, it seems to behave as a paracellular cation barrier [48]. However, exactly how claudin-14 mutations cause hypercalciuria or kidney stones remains to be determined.
Role of other Claudins in renal tubule function
Knowledge of the role of claudins other than 16 and 19 in renal function is limited, and comes mainly from inferences from localization studies, and analysis of knockout mice (Table 1).
Claudin-2
Claudin-2 is expressed in the proximal tubule (with highest levels in the late proximal tubule) and the early thin descending limb [50, 51]. In vitro studies have shown that it functions as a cation-selective paracellular pore (Na:Cl permeability ratio of 8:1) [21, 52]. Late proximal tubule fluid has a relatively high chloride concentration (due to transcellular reabsorption of Na with bicarbonate and organic solutes). This drives passive reabsorption of chloride and generates a lumen-positive electrical potential. The latter then acts as the driving force for paracellular reabsorption of Na, which thereby accounts for one-third of Na reabsorption in this segment [2]. The localization and properties of claudin-2 suggest that it is the pore protein that mediates this process. Consistent with this, the S2 segments of claudin-2 knockout mice proximal tubules exhibit decreased Na and Cl reabsorption [18]. Moreover, the mice have higher fractional excretions of Na and Cl after NaCl infusion.
There is also data to suggest that claudin-2 is permeable to other monovalent cations, including K+, and to a lesser extent to calcium [21]. A recent study also showed that it is permeable to water [53]. K+ and calcium are thought to be reabsorbed paracellularly in the proximal tubule. Whether water also permeates through the paracellular pathway has historically been controversial, especially since most water reabsorption is now known to pass transcellularly through aquaporin water channels. The in vitro data suggest that all of these substances can indeed be reabsorbed paracellularly and that claudin-2 is responsible.
Claudin-7
Claudin-7 is expressed in the distal convoluted tubule, connecting segment and collecting duct [54, 55]. In vitro data on its functional properties are somewhat contradictory. Overexpression studies in LLC-PK cells suggested that claudin-7 acts as a Cl barrier and a Na pore [55]. However, knockdown studies in MDCK cells suggested instead that claudin-7 acts as a Cl pore and a Na barrier [56]. Mice with knockout mice exhibit renal NaCl wasting and secondary hyperaldosteronism [57]. This suggests that claudin-7 is necessary in some way for maximum reabsorption of NaCl in the distal nephron.
Claudin-6 and -9
Neonatal proximal tubules have a lower capacity for fluid reabsorption than the adult proximal tubule. This is in part attributable to a lower passive paracellular permeability to chloride ions and higher resistance compared to that of adult proximal tubules [3]. Abuazza et al. showed that claudin-6, claudin-9, and claudin-13 were selectively expressed in the neonatal mouse kidney [58]. In adult kidneys, claudin-6 was expressed at relatively low levels while claudins-9 and -13 were absent. Claudin-6 and -9 were present in neonatal proximal convoluted tubules. Furthermore, when claudins-6 and -9 were transfected into Madin–Darby canine kidney II (MDCK II) cells, they each caused an increase in transepithelial resistance and decrease in Cl permeability, suggesting that they contribute to the limited reabsorption capacity of neonatal proximal tubules [59].
Conclusions
In conclusion, it is now well established that claudins form the paracellular barrier and pore in epithelia. The role of the first extracellular domain in lining the pore and determining the selectivity of paracellular permeation is also firmly established, and the roles of specific residues in this domain and the biophysical basis for selectivity are beginning to be elucidated. For a handful of claudin isoforms, there is now information about their physiological role in regulating tubule transport of fluid and electrolytes in the kidney, but there are still many isoforms for which no such information is available. The rare monogenic syndrome of FHHNC has provided an opportunity to understand the role of claudins in the function of the thick ascending limb of Henle. So far, however, the only polygenic renal disorder that has been associated with claudin gene variants is kidney stone disease. Importantly, there has been very little investigation into the mechanisms that regulate renal claudins, and their potential involvement in acquired kidney diseases, and we anticipate that this will be a particularly fertile area for future research.
Multiple choice questions (answers appear following the reference list)
- Which of the following best describes paracellular permeability and transport in the late proximal tubule?
- The proximal tubule has a high transepithelial resistance and acts as a barrier to small ions.
- NaCl is reabsorbed paracellularly, driven by the Cl concentration gradient.
- Organic anions are secreted across the paracellular pathway.
- Water is predominantly reabsorbed via the paracellular pathway, driven by the osmotic gradient.
- The proximal tubule is selectively permeable to bicarbonate which is reabsorbed paracellularly.
- Which domain of the claudin protein forms the lining of the paracellular pore?
- Amino terminal
- Carboxy terminal
- 1st transmembrane domain
- 1st extracellular domain
- Cytosolic loop between 2nd and 3rd transmembrane domain
- It has been shown that D65 (aspartate residue at position 65) in claudin-2 is a cation-binding site that is responsible for its cation-selectivity. Which of the following mutation of claudin-2 would be most likely to turn claudin-2 from a cation-selective pore to an anion-selective pore?
- D65K (mutation to lysine)
- D65C (mutation to cysteine)
- D65A (mutation to alanine)
- D65S (mutation to serine)
- D65L (mutation to leucine)
- All of the following are clinical features of familial hypomagnesemic hypercalciuria with nephrocalcinosis (FHHNC), EXCEPT:
- Increased fractional excretion of Mg
- Rickets
- Decreased GFR
- Ocular abnormalities
- Deafness
- Which of the following treatments ameliorate the urinary Mg and Ca wasting in FHHNC?
- Thiazide diuretics
- Magnesium supplementation
- Amiloride
- Oral phosphate binders
- Kidney transplantation
- Which of the following claudins are selectively expressed in the neonatal kidney but not in adult kidney?
- Claudin-2
- Claudin-7
- Claudin-9
- Claudin-16
- Claudin-19
Footnotes
Answers
1. B
2. D
3. A
4. E
5. E
6. C
References
- 1.Rector FC, Jr, Martinez-Maldonado M, Brunner FP, Seldin DW. Evidence for passive reabsorption of NaCl in proximal tubule of rat kidney. J Clin Invest. 1966;45:1060–1070. doi: 10.1172/JCI105373. [DOI] [Google Scholar]
- 2.Berry CA, Rector FC., Jr Mechanism of proximal NaCl reabsorption in the proximal tubule of the mammalian kidney. Semin Nephrol. 1991;11:86–97. [PubMed] [Google Scholar]
- 3.Quigley R, Baum M. Developmental changes in rabbit proximal straight tubule paracellular permeability. Am J Physiol Ren Physiol. 2002;283:F525–F531. doi: 10.1152/ajprenal.00005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah M, Quigley R, Baum M. Maturation of rabbit proximal straight tubule chloride/base exchange. Am J Physiol. 1998;274:F883–F888. doi: 10.1152/ajprenal.1998.274.5.F883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hebert SC, Andreoli TE. Control of NaCl transport in the thick ascending limb. Am J Physiol. 1984;246:F745–F756. doi: 10.1152/ajprenal.1984.246.6.F745. [DOI] [PubMed] [Google Scholar]
- 6.Ussing HH, Windhager EE (1964) Nature of shunt path and active solute transport path through frog skin epithelium. Acta Physiol Scand 61:484–504 [PubMed]
- 7.Machen TE, Erlij D, Wooding FB. Permeable junctional complexes. The movement of lanthanum across rabbit gallbladder and intestine. J Cell Biol. 1972;54:302–312. doi: 10.1083/jcb.54.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Angelow S, Yu AS. Claudins and paracellular transport: an update. Curr Opin Nephrol Hypertens. 2007;16:459–464. doi: 10.1097/MNH.0b013e32820ac97d. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Skare IB, Lynch RD, Schneeberger EE. Inducible expression of claudin-1-myc but not occludin-VSV-G results in aberrant tight junction strand formation in MDCK cells. J Cell Sci. 2000;113(Pt 19):3387–3398. doi: 10.1242/jcs.113.19.3387. [DOI] [PubMed] [Google Scholar]
- 11.Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Ren Physiol. 2003;285:F1078–F1084. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 12.Wen H, Watry DD, Marcondes MC, Fox HS. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol Cell Biol. 2004;24:8408–8417. doi: 10.1128/MCB.24.19.8408-8417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu AS, Enck AH, Lencer WI, Schneeberger EE. Claudin-8 expression in Madin–Darby canine kidney cells augments the paracellular barrier to cation permeation. J Biol Chem. 2003;278:17350–17359. doi: 10.1074/jbc.M213286200. [DOI] [PubMed] [Google Scholar]
- 14.Nakano Y, Kim SH, Kim HM, Sanneman JD, Zhang Y, Smith RJ, Marcus DC, Wangemann P, Nessler RA, Banfi B. A claudin-9-based ion permeability barrier is essential for hearing. PLoS Genet. 2009;5:e1000610. doi: 10.1371/journal.pgen.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angelow S, El-Husseini R, Kanzawa SA, Yu AS. Renal localization and function of the tight junction protein, claudin-19. Am J Physiol Ren Physiol. 2007;293:F166–F177. doi: 10.1152/ajprenal.00087.2007. [DOI] [PubMed] [Google Scholar]
- 16.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin–Darby canine kidney I cells. J Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci. 2005;118:5109–5118. doi: 10.1242/jcs.02631. [DOI] [PubMed] [Google Scholar]
- 18.Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, Furuse K, Sasaki H, Fujimura A, Imai M, Kusano E, Tsukita S, Furuse M. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci USA. 2010;107:8011–8016. doi: 10.1073/pnas.0912901107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colegio OR, Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol. 2003;284:C1346–C1354. doi: 10.1152/ajpcell.00547.2002. [DOI] [PubMed] [Google Scholar]
- 20.Colegio OR, Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol. 2002;283:C142–C147. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- 21.Yu AS, Cheng MH, Angelow S, Gunzel D, Kanzawa SA, Schneeberger EE, Fromm M, Coalson RD. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol. 2009;133:111–127. doi: 10.1085/jgp.200810154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121:298–305. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 23.Angelow S, Yu AS. Cysteine mutagenesis to study the structure of claudin-2 paracellular pores. Ann NY Acad Sci. 2009;1165:143–147. doi: 10.1111/j.1749-6632.2009.04038.x. [DOI] [PubMed] [Google Scholar]
- 24.Benigno V, Canonica CS, Bettinelli A, Vigier RO, Truttmann AC, Bianchetti MG. Hypomagnesaemia-hypercalciuria-nephrocalcinosis: a report of nine cases and a review. Nephrol Dial Transplant. 2000;15:605–610. doi: 10.1093/ndt/15.5.605. [DOI] [PubMed] [Google Scholar]
- 25.Manz F, Scharer K, Janka P, Lombeck J. Renal magnesium wasting, incomplete tubular acidosis, hypercalciuria and nephrocalcinosis in siblings. Eur J Pediatr. 1978;128:67–79. doi: 10.1007/BF00496992. [DOI] [PubMed] [Google Scholar]
- 26.Kari JA, Farouq M, Alshaya HO. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis. Pediatr Nephrol. 2003;18:506–510. doi: 10.1007/s00467-003-1139-8. [DOI] [PubMed] [Google Scholar]
- 27.Kuwertz-Broking E, Frund S, Bulla M, Kleta R, August C, Kisters K. Familial hypomagnesemia-hypercalciuria in 2 siblings. Clin Nephrol. 2001;56:155–161. [PubMed] [Google Scholar]
- 28.Martin Aguado M, Canals Baeza A, Sanguino Lopez L, Gavilan Martin C, Flores Serrano J. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis. An Esp Pediatr. 2001;54:174–177. [PubMed] [Google Scholar]
- 29.Mourani C, Khallouf E, Akkari V, Akatcherian C, Cochat P. Early hypomagnesemia, hypercalciuria and nephrocalcinosis: two cases in a family. Arch Pediatr. 1999;6:748–751. doi: 10.1016/S0929-693X(99)80357-1. [DOI] [PubMed] [Google Scholar]
- 30.Evans RA, Carter JN, George CR, Walls RS, Newland RC, McDonnell GD, Lawrence JR. The congenital “magnesium-losing kidney”. Report of two patients. Q J Med. 1981;50:39–52. [PubMed] [Google Scholar]
- 31.Praga M, Vara J, Gonzalez-Parra E, Andres A, Alamo C, Araque A, Ortiz A, Rodicio JL. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis. Kidney Int. 1995;47:1419–1425. doi: 10.1038/ki.1995.199. [DOI] [PubMed] [Google Scholar]
- 32.Michelis MF, Drash AL, Linarelli LG, Rubertis FR, Davis BB. Decreased bicarbonate threshold and renal magnesium wasting in a sibship with distal renal tubular acidosis. (Evaluation of the pathophysiological role of parathyroid hormone) Metabolism. 1972;21:905–920. doi: 10.1016/0026-0495(72)90025-X. [DOI] [PubMed] [Google Scholar]
- 33.Weber S, Schneider L, Peters M, Misselwitz J, Ronnefarth G, Boswald M, Bonzel KE, Seeman T, Sulakova T, Kuwertz-Broking E, Gregoric A, Palcoux JB, Tasic V, Manz F, Scharer K, Seyberth HW, Konrad M. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol. 2001;12:1872–1881. doi: 10.1681/ASN.V1291872. [DOI] [PubMed] [Google Scholar]
- 34.Cole DE, Quamme GA. Inherited disorders of renal magnesium handling. J Am Soc Nephrol. 2000;11:1937–1947. doi: 10.1681/ASN.V11101937. [DOI] [PubMed] [Google Scholar]
- 35.Torralbo A, Pina E, Portoles J, Sanchez-Fructuoso A, Barrientos A. Renal magnesium wasting with hypercalciuria, nephrocalcinosis and ocular disorders. Nephron. 1995;69:472–475. doi: 10.1159/000188522. [DOI] [PubMed] [Google Scholar]
- 36.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 37.Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, Schlingmann KP, Schmid M, Rodriguez-Soriano J, Ariceta G, Cano F, Enriquez R, Juppner H, Bakkaloglu SA, Hediger MA, Gallati S, Neuhauss SC, Nurnberg P, Weber S. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet. 2006;79:949–957. doi: 10.1086/508617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou J, Shan Q, Wang T, Gomes AS, Yan Q, Paul DL, Bleich M, Goodenough DA. Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. J Biol Chem. 2007;282:17114–17122. doi: 10.1074/jbc.M700632200. [DOI] [PubMed] [Google Scholar]
- 39.Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest. 2008;118:619–628. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez-Soriano J, Vallo A, Garcia-Fuentes M. Hypomagnesaemia of hereditary renal origin. Pediatr Nephrol. 1987;1:465–472. doi: 10.1007/BF00849255. [DOI] [PubMed] [Google Scholar]
- 41.Blanchard A, Jeunemaitre X, Coudol P, Dechaux M, Froissart M, May A, Demontis R, Fournier A, Paillard M, Houillier P. Paracellin-1 is critical for magnesium and calcium reabsorption in the human thick ascending limb of Henle. Kidney Int. 2001;59:2206–2215. doi: 10.1046/j.1523-1755.2001.00736.x. [DOI] [PubMed] [Google Scholar]
- 42.Burg MB, Green N. Function of the thick ascending limb of Henle's loop. Am J Physiol. 1973;224:659–668. doi: 10.1152/ajplegacy.1973.224.3.659. [DOI] [PubMed] [Google Scholar]
- 43.Greger R. Cation selectivity of the isolated perfused cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Arch. 1981;390:30–37. doi: 10.1007/BF00582707. [DOI] [PubMed] [Google Scholar]
- 44.Burg M, Good D. Sodium chloride coupled transport in mammalian nephrons. Annu Rev Physiol. 1983;45:533–547. doi: 10.1146/annurev.ph.45.030183.002533. [DOI] [PubMed] [Google Scholar]
- 45.Ikari A, Matsumoto S, Harada H, Takagi K, Hayashi H, Suzuki Y, Degawa M, Miwa M. Phosphorylation of paracellin-1 at Ser217 by protein kinase A is essential for localization in tight junctions. J Cell Sci. 2006;119:1781–1789. doi: 10.1242/jcs.02901. [DOI] [PubMed] [Google Scholar]
- 46.Kausalya PJ, Amasheh S, Gunzel D, Wurps H, Muller D, Fromm M, Hunziker W. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of Claudin-16. J Clin Invest. 2006;116:878–891. doi: 10.1172/JCI26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, Sulem P, Halldorsson BV, Vegt F, d'Ancona FC, Heijer M, Franzson L, Christiansen C, Alexandersen P, Rafnar T, Kristjansson K, Sigurdsson G, Kiemeney LA, Bodvarsson M, Indridason OS, Palsson R, Kong A, Thorsteinsdottir U, Stefansson K. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet. 2009;41:926–930. doi: 10.1038/ng.404. [DOI] [PubMed] [Google Scholar]
- 48.Ben-Yosef T, Belyantseva IA, Saunders TL, Hughes ED, Kawamoto K, Itallie CM, Beyer LA, Halsey K, Gardner DJ, Wilcox ER, Rasmussen J, Anderson JM, Dolan DF, Forge A, Raphael Y, Camper SA, Friedman TB. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet. 2003;12:2049–2061. doi: 10.1093/hmg/ddg210. [DOI] [PubMed] [Google Scholar]
- 49.Elkouby-Naor L, Abassi Z, Lagziel A, Gow A, Ben-Yosef T. Double gene deletion reveals lack of cooperation between claudin 11 and claudin 14 tight junction proteins. Cell Tissue Res. 2008;333:427–438. doi: 10.1007/s00441-008-0621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Enck AH, Berger UV, Yu AS. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am J Physiol Ren Physiol. 2001;281:F966–F974. doi: 10.1152/ajprenal.2001.281.5.F966. [DOI] [PubMed] [Google Scholar]
- 51.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13:875–886. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- 52.Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 53.Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, Gunzel D, Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010;123:1913–1921. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 54.Li WY, Huey CL, Yu AS. Expression of claudin-7 and -8 along the mouse nephron. Am J Physiol Ren Physiol. 2004;286:F1063–F1071. doi: 10.1152/ajprenal.00384.2003. [DOI] [PubMed] [Google Scholar]
- 55.Alexandre MD, Lu Q, Chen YH. Overexpression of claudin-7 decreases the paracellular Cl- conductance and increases the paracellular Na + conductance in LLC-PK1 cells. J Cell Sci. 2005;118:2683–2693. doi: 10.1242/jcs.02406. [DOI] [PubMed] [Google Scholar]
- 56.Hou J, Gomes AS, Paul DL, Goodenough DA. Study of claudin function by RNA interference. J Biol Chem. 2006;281:36117–36123. doi: 10.1074/jbc.M608853200. [DOI] [PubMed] [Google Scholar]
- 57.Tatum R, Zhang Y, Salleng K, Lu Z, Lin JJ, Lu Q, Jeansonne BG, Ding L, Chen YH. Renal salt wasting and chronic dehydration in claudin-7-deficient mice. Am J Physiol Ren Physiol. 2010;298:F24–F34. doi: 10.1152/ajprenal.00450.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abuazza G, Becker A, Williams SS, Chakravarty S, Truong HT, Lin F, Baum M. Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am J Physiol Ren Physiol. 2006;291:F1132–F1141. doi: 10.1152/ajprenal.00063.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sas D, Hu M, Moe OW, Baum M. Effect of claudins 6 and 9 on paracellular permeability in MDCK II cells. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1713–R1719. doi: 10.1152/ajpregu.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wittchen ES, Haskins J, Stevenson BR. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J Biol Chem. 1999;274:35179–35185. doi: 10.1074/jbc.274.49.35179. [DOI] [PubMed] [Google Scholar]
- 61.Itallie CM, Gambling TM, Carson JL, Anderson JM. Palmitoylation of claudins is required for efficient tight-junction localization. J Cell Sci. 2005;118:1427–1436. doi: 10.1242/jcs.01735. [DOI] [PubMed] [Google Scholar]
- 62.Muller D, Kausalya PJ, Claverie-Martin F, Meij IC, Eggert P, Garcia-Nieto V, Hunziker W. A novel claudin 16 mutation associated with childhood hypercalciuria abolishes binding to ZO-1 and results in lysosomal mistargeting. Am J Hum Genet. 2003;73:1293–1301. doi: 10.1086/380418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Ren Physiol. 2008;295:F867–F876. doi: 10.1152/ajprenal.90264.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Inai T, Kobayashi J, Shibata Y. Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur J Cell Biol. 1999;78:849–855. doi: 10.1016/S0171-9335(99)80086-7. [DOI] [PubMed] [Google Scholar]
- 65.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Evans MJ, Hahn T, Tscherne DM, Syder AJ, Panis M, Wolk B, Hatziioannou T, McKeating JA, Bieniasz PD, Rice CM. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- 67.Meertens L, Bertaux C, Cukierman L, Cormier E, Lavillette D, Cosset FL, Dragic T. The tight junction proteins claudin-1, -6, and -9 are entry cofactors for hepatitis C virus. J Virol. 2008;82:3555–3560. doi: 10.1128/JVI.01977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morin PJ. Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res. 2005;65:9603–9606. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- 70.Fujita K, Katahira J, Horiguchi Y, Sonoda N, Furuse M, Tsukita S. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett. 2000;476:258–261. doi: 10.1016/S0014-5793(00)01744-0. [DOI] [PubMed] [Google Scholar]
- 71.Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, Horiguchi Y, Tsukita S. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: evidence for direct involvement of claudins in tight junction barrier. J Cell Biol. 1999;147:195–204. doi: 10.1083/jcb.147.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107:1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci USA. 2010;107:18010–18015. doi: 10.1073/pnas.1009399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood–brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J, Piontek J, Wolburg H, Piehl C, Liss M, Otten C, Christ A, Willnow TE, Blasig IE, Abdelilah-Seyfried S. Establishment of a neuroepithelial barrier by Claudin5a is essential for zebrafish brain ventricular lumen expansion. Proc Natl Acad Sci USA. 2010;107:1425–1430. doi: 10.1073/pnas.0911996107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol Ren Physiol. 2006;291:F1288–F1299. doi: 10.1152/ajprenal.00138.2006. [DOI] [PubMed] [Google Scholar]
- 77.Itallie C, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion selective epithelial lines by expression of different claudins. Am J Physiol Cell Physiol. 2003;286:F1078–F1084. doi: 10.1152/ajpcell.00463.2003. [DOI] [PubMed] [Google Scholar]
- 78.Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE, Danias J, Bronstein JM, Kachar B, Lazzarini RA. CNS myelin and Sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 1999;99:649–659. doi: 10.1016/S0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- 79.Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, Yamashita T, Kato S, Sawada N, Chiba H. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell. 2008;19:1912–1921. doi: 10.1091/mbc.E07-09-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilcox ER, Burton QL, Naz S, Riazuddin S, Smith TN, Ploplis B, Belyantseva I, Ben-Yosef T, Liburd NA, Morell RJ, Kachar B, Wu DK, Griffith AJ, Friedman TB. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell. 2001;104:165–172. doi: 10.1016/S0092-8674(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 81.Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci USA. 2009;106:15350–15355. doi: 10.1073/pnas.0907724106. [DOI] [PMC free article] [PubMed] [Google Scholar]