Abstract
Introduction. Transmission of cytomegalovirus (CMV) via breast milk can lead to severe acute illness in very low-birth-weight (VLBW) preterm infants. Although the majority of CMV-seropositive women shed CMV in milk, symptomatic postnatal infection of VLBW infants occurs infrequently, suggesting that virologic or immunologic factors in milk may be associated with the risk and severity of postnatal CMV infection.
Methods. We investigated the magnitude of CMV-specific cellular and humoral immune responses in milk of 30 seropositive mothers of VLWB preterm infants and assessed their relationship to milk CMV load and symptomatic CMV transmission.
Results. Milk immunoglobulin G (IgG) avidity was inversely correlated to milk CMV load (r = −0.47; P = .009). However, milk CMV load and CMV-specific cellular and humoral immune responses were similar in mothers of VLBW infants with and those without symptomatic postnatal CMV infection.
Conclusions. Similar immunologic parameters in milk of CMV-seropositive mothers of VLBW infants with and without symptomatic postnatal CMV infection indicate that screening milk by these parameters may not predict disease risk. However, the inverse correlation between milk CMV IgG avidity and CMV load may suggest that enhancement of maternal CMV-specific IgG responses could aid in reduction of CMV shedding into breast milk.
Cytomegalovirus (CMV) is a common congenital infection that can result in sensorineural hearing loss and other neurologic sequelae [1–4]. Postnatal transmission of CMV is mainly asymptomatic in full-term infants [5]. However, in preterm very low-birth-weight (VLBW) infants, postnatal infection can result in a sepsis-like illness characterized by thrombocytopenia, hepatitis, and pneumonitis [5–10]. The specific impact of postnatal CMV infection on the neurodevelopmental outcome of preterm infants has not been established in large-scale studies [7, 11, 12]. However, the detrimental effects of both culture-proven bacterial sepsis and “culture-negative” clinical sepsis on the growth and neurodevelopment of extremely low-birth-weight infants [13] suggests that symptomatic postnatal CMV infection would also negatively impact long-term outcomes in these most vulnerable infants. With the use of CMV-seronegative blood products in preterm infants, breast milk is the primary source of postnatal CMV transmission [14]. Although the majority of CMV-seropositive lactating women have polymerase chain reaction (PCR)–detectable viral shedding in milk [6–8, 15], the rate of postnatal CMV transmission ranges from 4% to 69%, with variation in reported incidence rates attributable to study design [5–9, 16, 17]. However, the incidence of symptomatic postnatal CMV infection is low and restricted to the most premature infants [17, 18]. Identified risk factors for postnatal CMV transmission include the magnitude of the milk CMV load, early shedding of CMV in milk, duration of breast-feeding, and lower infant gestational age and birth weight [5–8, 17]. Although virus-specific cellular immune responses are required for control of systemic CMV replication [19–21] and CMV-specific humoral responses play a role in protection against congenital CMV transmission [22, 23], the role of maternal CMV-specific immune responses in postnatal CMV transmission is unclear.
Premature infants receive significant nutritional and immunologic benefits from breast milk, because breast milk feeding is associated with lower incidence of necrotizing enterocolitis and late-onset sepsis [24–28]. Although freezing milk can reduce CMV load [18, 29], it does not eliminate transmission [30, 31], and this treatment likely decreases its positive effects [27, 32]. Therefore, it is important to evaluate the relative benefits of breast milk for preterm infants of CMV-seropositive mothers and determine whether milk can be screened to assess the risk of CMV transmission. Identification of maternal virologic or immunologic correlates of protection against symptomatic postnatal CMV infection could guide immunologic or antiviral prophylaxis strategies.
In this study, we characterized CMV-specific cellular and humoral immune responses in milk of seropositive mothers of preterm infants. In addition, we compared CMV-specific immune responses in blood and milk of CMV-seropositive mothers whose VLBW infants either did or did not develop symptomatic postnatal CMV.
MATERIALS AND METHODS
Subjects
Mothers of full-term infants (n = 53) and VLBW (<1500 g) or <32-week gestational age preterm infants (n = 85) were recruited over a 2-year period from neonatal intensive care units (NICUs) at 3 tertiary hospitals in Boston, Massachusetts. All mothers provided written consent. Approximately 1 ounce of fresh milk and 10 mL of blood was collected at >2 weeks post partum. CMV serostatus was determined by CMV immunoglobulin (Ig) G (Trinity Biotech) and IgM enzyme-linked immunosorbent assay (ELISA) (Diamedix). Thirty of 85 mothers of preterm infants were CMV IgG seropositive and were assessed for CMV-specific immune responses. Twelve of 53 mothers of full-term infants were CMV IgG seropositive and were used for comparison. None of the mothers of preterm infants were CMV IgM seropositive; only 1 mother of a full-term infant was CMV IgM seropositive. Clinical data were collected by maternal questionnaire and chart review at infant discharge. Because no universal CMV testing is performed in the NICUs in this study, CMV testing of infants (urine or saliva CMV shell vial culture) was ordered at the provider’s discretion. Mothers of infants with suspected symptomatic postnatal CMV infection (acute sepsislike symptoms and positive CMV shell vial culture performed at >2 weeks of age) were specifically recruited into the study and comprise the “symptomatic” group in this study; thus, the incidence of postnatal CMV infection in this cohort does not reflect that of the entire NICU population. The study was approved by each of the participating hospitals by ceded review to the Committee on Clinical Investigation at Children’s Hospital Boston.
Plasma and Milk CMV Load
DNA was extracted from plasma and milk supernatant (QIAgen) and concentrated to a volume of 70 μL in ultracentrifugation columns (Millipore) to decrease the limit of detection in the assay, as determined in preliminary experiments. Quantitative polymerase chain reaction (PCR) was performed using conditions, primers, and probes described elsewhere [33], using the 7300 Real Time PCR System (Applied Biosystems). Serial dilutions of quantitated CMV AD169 stock (Advanced Biotechnologies, Inc) were used as a standard. The limit of quantification was 175 copies/mL. If virus amplification was detected but was below the level of the minimum virus standard, a value of half the lowest virus standard was assigned. If no virus was amplified, it was considered nondetectable.
CMV-Specific Interferon γ Enzyme-Linked Immunospot Assay
Peripheral blood mononuclear cells (PBMCs) and milk cells were isolated as described elsewhere [34], and mononuclear cells were quantitated by manual hemocytometer assessment. Cells were incubated with a pool of 15-mer peptides overlapping by 11 amino acids spanning the product of the CMV AD169 pp65 gene (2 μg/mL) in a multiscreen 96-well plate coated with anti–interferon (IFN) γ antibody. Cells were also incubated with Staphylococcus aureus enterotoxin B and media only. In total, 2.5 × 105 PBMCs and 1.5 × 104 to 2 × 105 milk cells were incubated with each condition in duplicate or triplicate, as cell number allowed. Results from milk samples with insufficient cell number (<1.5 × 104 cells per well in duplicate for each condition, determined as the limit of detection) were not included. Spots were counted by automated enzyme-linked immunospot assay reader (Hitech Instruments). CMV-specific cellular responses were calculated by subtracting the average spot number in the media-only wells from that of the experimental wells and reported as spot-forming units (SFUs) per 106 cells. A response was considered detectable if the average spot number in the experimental wells was at least twice that of media-only wells.
CMV-Binding Antibody Responses
CMV-specific IgG and IgA binding was measured by CMV AD169 (American Type Culture Collection [ATCC]) whole-lysate ELISA. Plates were incubated overnight with lysate from CMV-infected human foreskin fibroblasts (ATCC), washed, and then blocked with phosphate-buffered saline (PBS) with 5% nonfat dried milk, 5% fetal bovine serum, and 0.05% Tween 20 for 2 hours. Serially diluted plasma and milk supernatant samples were incubated in the wells for 2 hours. Plates were then washed 6 times and incubated for 1 hour with 1 or 2 μg/mL of goat anti-human horseradish peroxidase–conjugated IgG (Millipore) or IgA (Pierce), respectively. Finally, plates were developed with tetramethylbenzidine substrate (KPL) and read at 450 nm. Titer was defined as the inverse of the lowest sample dilution that resulted in an optical density at least twice that of the background.
Total IgG and IgA was quantitated by commercial ELISA per protocol (Immunology Consultants Laboratory). The normalized CMV-specific IgG or IgA titer was calculated by dividing the CMV-binding antibody titer by the total IgG or IgA content (in milligrams per milliliter) in each sample.
CMV-specific IgG and IgA avidity was measured by a modification to the CMV-binding ELISAs described above [35]. After the incubation of diluted plasma (1:10) or milk (1:3) with the CMV lysate, duplicate wells were incubated for 10 minutes with either 9 mol/L urea or PBS. After washing, CMV binding was detected as above. The avidity index was calculated by dividing the mean absorbance of urea-treated wells by that of untreated wells.
CMV-Neutralizing Antibody Responses
CMV plaque neutralization assay was performed by plating 3.2 × 105 human foreskin fibroblasts in each well of 16-well Lab-Tek Chamber Slides (Thermo). The next day, duplicate serial dilutions of milk supernatant (1:3–1:3000) and plasma (1:3–1:30 000) were incubated with 2 × 105 plaque-forming units/mL of CMV AD169 for 1 hour; 50 μL of the serum/virus dilutions was added to each well and incubated at 37°C overnight. On the third day, cells were fixed with 100% ethanol for 5 minutes and rehydrated with PBS. Slides were then blocked with PBS containing 7.5% normal goat serum and 7.5% Casein blocker and stained with 100 μL of 20 μg/mL of Alexa Fluor 488–conjugated anti-CMV immediate early antigen monoclonal antibody clone 8B1.2 (Millipore). After a 2-hour incubation, wells were washed and counterstained with DAPI nuclear stain (Invitrogen). The number of infected cells was manually counted using a fluorescent microscope (Olympus BX40). The 50% inhibitory dose was calculated as the sample dilution that caused a 50% reduction in the number of infected cells compared with virus-only control wells.
Assessment of Mastitis
Sodium and potassium concentration of milk supernatant was measured using the Gen2 Ion Selective Electrode on the Roche Cobias c501 platform (Roche Diagnostics). A sodium-potassium ratio >1 indicates mastitis [36–38].
Statistical Analysis
All comparisons of continuous values were performed using the Mann-Whitney U test, and categorical values were compared using the Fisher exact test. When comparing parameters in paired blood and milk samples, the Wilcoxon signed-rank test was employed. Correlations were determined with the Spearman rank coefficient. Prism 5 software was used for all analyses. Significance was defined as P < .05, and trends toward significance were defined as P = .05–0.1.
RESULTS
Clinical Characteristics of Study Population
Mean maternal age, weeks post partum to enrollment, milk CMV load, and infant gestational age and birth weight were similar between the groups of infants who did or did not develop symptomatic CMV infections (Table 1). There were also no significant differences in maternal history of sexually transmitted infections, previous maternal live births [39, 40], and delivery by cesarean section between these 2 groups. However, infants of nonwhite mothers were more likely to develop symptomatic CMV infections (P = .05; Table 1).
Table 1.
Demographics of the Study Cohort of Very Low-Birth-Weight (VLBW) Preterm Infants and Their Cytomegalovirus (CMV)–Seropositive, Lactating Mothersa
| Demographic | Entire cohort (30 mothers, 35 infants) | Symptomatic postnatal CMV infection (5 mothers, 6 infants) | Symptomatic postnatal CMV infection (25 mothers, 29 infants) | P |
| Maternal age, years | 30.5 (18–41) | 26.5 (22–41) | 31 (18–38) | .13 |
| Weeks postpartum at enrollment | 6 (3–11) | 5 (5–14) | 4 (2–11) | 1.0 |
| Milk CMV load, copies/mL | 215 (ND to 53731) | 87.5 (87.525182) | 222 (ND to 53731) | .74 |
| Infant gestational age, weeks | 27 (24–32) | 26 (24–28) | 27 (24–32) | .33 |
| Infant birth weight, g | 900 (400–2100) | 900 (400–1070) | 830 (590–2100) | .12 |
| Maternal characteristic, No. of mothersb | ||||
| History of sexually transmitted infections | 9/29 | 3/5 | 6/24 | .29 |
| Nonwhite race | 17/30 | 5/5 | 12/25 | .05 |
| Previous live births | 9/30 | 2/5 | 7/25 | .62 |
| Delivery via cesarean section | 19/30 | 5/5 | 14/25 | .13 |
Except for maternal history characteristics given as proportions, values represent means (ranges). ND, not detectable.
Numbers are given as proportions of total group.
Low-Magnitude Milk CMV-Specific Immune Responses Compared With Those in Blood
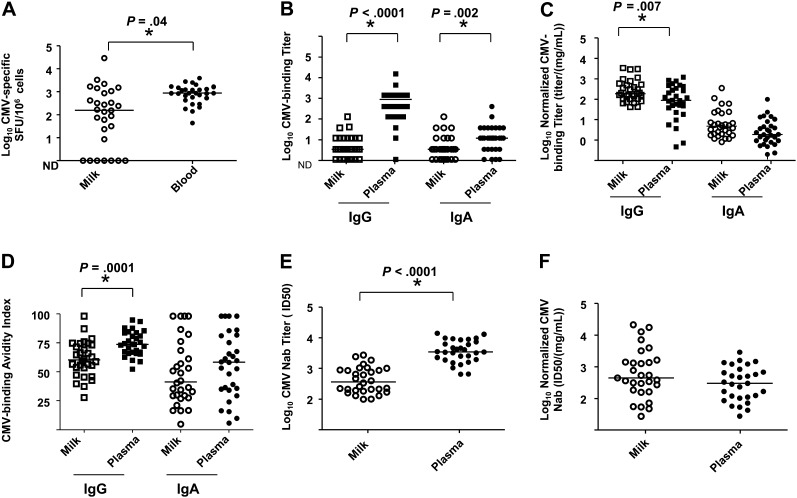
We first compared CMV-specific immune responses in milk and blood of CMV-seropositive mothers of VLBW preterm infants. The magnitude of the CMV-specific cellular responses was significantly higher in blood than milk (Figure 1A). Similarly, CMV-binding IgG and IgA responses were of significantly higher magnitude in plasma than milk (Figure 1B). However, when CMV-binding IgG responses were normalized by total IgG content, milk responses were of significantly higher magnitude than those in plasma. Similarly, normalized CMV-binding IgA responses trended toward being more robust in milk than plasma (Figure 1C).
Figure 1.
Low-magnitude cytomegalovirus (CMV)–specific cellular and humoral immune responses in milk compared with that in blood of CMV-seropositive mothers of very low-birth-weight (VLBW) infants including CMV-specific cellular responses (A), CMV-binding immunoglobulin (Ig) G and IgA titers (B), normalized CMV-binding IgG and IgA titers (C), CMV IgG and IgA avidity (D), CMV-neutralizing titers (E), and normalized CMV-neutralizing titers (F) in milk (open symbols) and blood (filled symbols) of CMV-seropositive mothers of VLBW preterm infants. Lines indicate median value; *P < .05. Abbreviation: ND, not detectable.
Despite higher normalized CMV-binding antibody responses in milk than plasma, CMV-binding IgG avidity was significantly higher in plasma than milk. CMV-binding IgA avidity also trended toward being greater in plasma than milk (Figure 1D). Similarly, the magnitude of plasma-neutralizing antibody responses was approximately 1 log higher than that in milk (Figure 1E). However, when the neutralizing responses were normalized by total immunoglobulin content, the responses were comparable (Figure 1F).
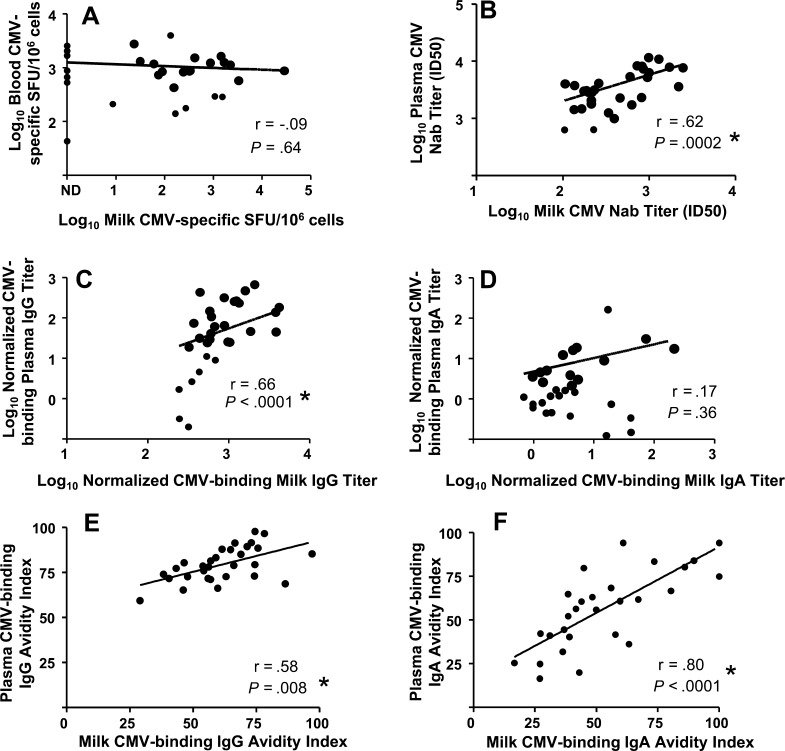
To investigate whether milk CMV responses are compartmentalized from those in blood, we examined whether the magnitude of the blood and milk CMV-specific immune responses were correlated. The magnitude of the CMV-specific cellular responses in milk did not correlate with that in blood (Figure 2A). However, the magnitudes of both the neutralization (Figure 2B) and normalized CMV-binding IgG (Figure 2C) responses were strongly correlated in milk and plasma, suggesting a lack of compartmentalization in these responses. In contrast, the magnitude of the normalized CMV-binding IgA responses in milk and plasma did not correlate (Figure 2D). We also observed correlations between nonnormalized CMV-binding milk and plasma IgG (r = 0.65; P < .0001) and IgA responses (r = 0.47; P = .0008; data not shown). Milk and plasma CMV-specific IgG and IgA avidity (Figure 2E and 2F) were also strongly correlated.
Figure 2.
Direct correlation of cytomegalovirus (CMV)–neutralizing and CMV-binding immunoglobulin (Ig) G responses in milk and plasma of CMV-seropositive mothers of very low–birth-weight (VLBW) infants, including CMV-specific cellular responses (A), neutralizing antibody responses (B), normalized CMV-binding IgG (C) and IgA (D) titers, and CMV-binding IgG (E) and IgA (F) avidity. Antibody titers were normalized by total IgG or IgA content in milk or plasma. *P < .05. Abbreviation: ND, not detectable.
Similar CMV-Specific Immune Responses in Milk of CMV-Seropositive Mothers of Preterm and Full-term Infants
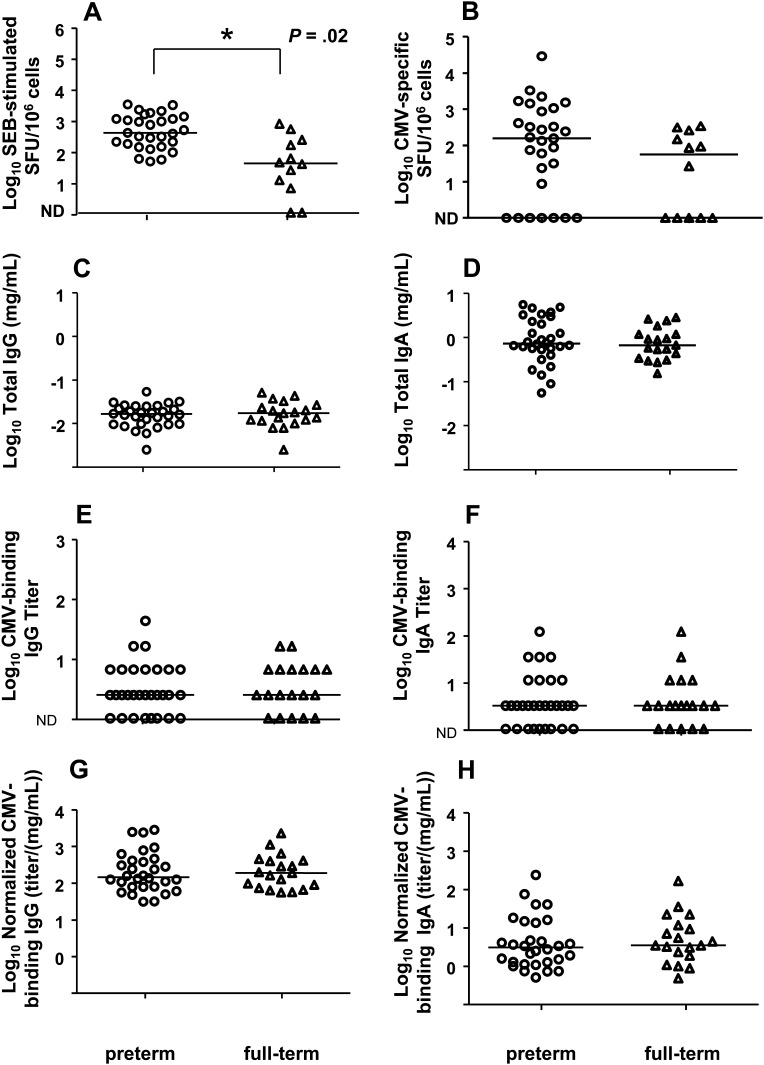
We investigated whether suboptimal milk immune responses could contribute to the pathogenicity of the virus in preterm infants by comparing the magnitude of the milk total and CMV-specific immune responses in mothers of preterm and full-term infants. Mononuclear cell content was very similar in milk of CMV-seropositive mothers of preterm infants (median, 7000 cells/mL; range, 615–126000 cells/mL) and full-term infants (median, 8733 cells/mL; range, 1067–33333 cells/mL; P = .71; data not shown). Interestingly, the global T-lymphocyte responses in milk as measured by IFN-γ response to a superantigen, were of higher magnitude in mothers of preterm infants than in mothers of full-term infants (Figure 3A). However, CMV-specific cellular responses in milk were similar for mothers of preterm and mothers of full-term infants (Figure 3B).
Figure 3.
Cytomegalovirus (CMV)–specific cellular and humoral immune responses are similar in milk of seropositive mothers of preterm and full-term infants. Superantigen (staphylococcal enterotoxin B) (A) and CMV-specific cellular responses (B) in milk are shown for mothers of preterm (circles) and full-term (triangles), along with total immunoglobulin (Ig) G (C) and IgA (D) concentrations and CMV-binding IgG (E) and IgA (F) titers normalized for total IgG (G) or IgA (H) content in milk. Lines indicate median values. Abbreviation: ND, not detectable.
Total IgG and IgA content in milk was comparable for mothers of preterm and full-term infants (Figure 3C and 3D). Moreover, the milk CMV-binding IgG and IgA responses were also similar in mothers of preterm and full-term infants, whether assessed without (Figure 3E and 3F) or with (Figure 3G and 3H) normalization for total IgG or IgA content.
Inverse Correlation of Milk CMV Load and Milk CMV-Binding IgG Avidity
We next examined the relationship between the CMV-specific immune responses and the magnitude of milk CMV shedding in mothers of preterm infants. Milk CMV load did not correlate with milk or blood CMV-specific cellular responses, normalized milk CMV-binding IgG titer, normalized milk and plasma CMV-binding IgA titers, milk and plasma neutralizing antibody titers, plasma CMV-binding IgG avidity, or milk and plasma CMV-binding IgA avidity. There was a trend toward a correlation of the magnitude of the milk CMV load and normalized plasma CMV-binding IgG titers. Importantly, there was an inverse correlation between milk CMV-binding IgG avidity and milk CMV load (r = 0.47; P = .009, Table 2), which approached significance even after the Bonferroni correction was applied (P = .004).
Table 2.
Correlation of Breast Milk Cytomegalovirus (CMV) Load and CMV-Specific Immune Responses in Milk and Blood of CMV-Seropositive Mothers of Very Low-Birth-Weight Preterm Infants
| CMV-specific response | r | P |
| Milk CMV-specific IFN-γ response (SFUs) | −0.06 | .76 |
| Blood CMV-specific IFN-γ response (SFUs) | 0.12 | .54 |
| Normalized milk CMV-binding IgG titer | 0.19 | .31 |
| Normalized plasma CMV-binding IgG titer | 0.36 | .05 |
| Normalized milk CMV-binding IgA titer | 0.19 | .32 |
| Normalized plasma CMV-binding IgA titer | −0.07 | .73 |
| Milk neutralizing antibody titer | −0.05 | .80 |
| Plasma neutralizing antibody titer | −0.04 | .83 |
| Milk CMV-binding IgG avidity | −0.47 | .009 |
| Plasma CMV-binding IgG avidity | −0.12 | .53 |
| Milk CMV-binding IgA avidity | 0.12 | .54 |
| Plasma CMV-binding IgA avidity | 0.26 | .17 |
| Milk sodium-potassium ratio | −0.18 | .35 |
| Weeks postpartum | −0.06 | .77 |
Abbreviations: IFN interferon; Ig, immunoglobulin; SFUs, spot-forming units.
In assessing the effect of breast inflammation on milk CMV shedding, we observed an inverse correlation between elevated milk sodium-potassium ratio, a marker of mastitis [36–38], and milk CMV load. Finally, we investigated the relationship between the postpartum interval and milk CMV load and found no association between the weeks postpartum and milk viral load in our cohort (Table 2).
CMV-Specific Immune Responses Are Similar in Mothers of Symptomatic and Asymptomatic VLBW Infants
In our cohort of 30 CMV-seropositive mothers of 35 VLBW infants, symptomatic postnatal CMV infection was diagnosed in 6 infants of 5 mothers . This diagnosis was defined as clinical symptoms and/or laboratory findings consistent with postnatal CMV infection [5–10] (Table 3) and a concurrent positive shell vial CMV culture occurring at >21 days of life. Importantly, all infants with this diagnosis were delivered via cesarean section (Table 1), and 3 of the 6 had a previous negative CMV urine culture before isolation of CMV from the infant’s urine (Table 3). All case infants received both fresh and frozen milk during hospitalization, whereas 10 control infants received only fresh milk. Case infants began receiving mother’s milk at a similar day of life to the control infants (Table 1) and continued to receive milk throughout hospitalization.
Table 3.
Presenting Clinical Signs and Symptoms of Cytomegalovirus (CMV)–Infected Infants
| Subject no. | Day of life at positive urine CMV shell vial culture (previous negative culture) | Clinical symptomsa | Laboratory findingsa |
| 023 | 64 (yes) | Abdominal distention, dilated loops of bowel | Thrombocytopenia, transaminitis |
| 027 | 92 (yes) | Abdominal distention, dilated loops of bowel, increased respiratory support requirement | Thrombocytopenia, transaminitis, neutropenia |
| 041 | 62 (yes) | Vomiting, dilated bowel loops, increased respiratory support requirement, low blood pressure requiring dopamine | Thrombocytopenia, transaminitis, neutropenia |
| 032b | 50 (no) | Increased respiratory support requirement | Thrombocytopenia, transaminitis |
| 086.1c | 56 (no) | Abdominal distention, increased respiratory support requirement | Thrombocytopenia, transaminitis |
| 086.2c | 56 (no) | Bloody stools | None |
Clinical findings within 14 days before or after positive CMV shell vial culture.
Infant 032 had a twin, who remained uninfected.
Infants 086.1 and 086.2 were twins.
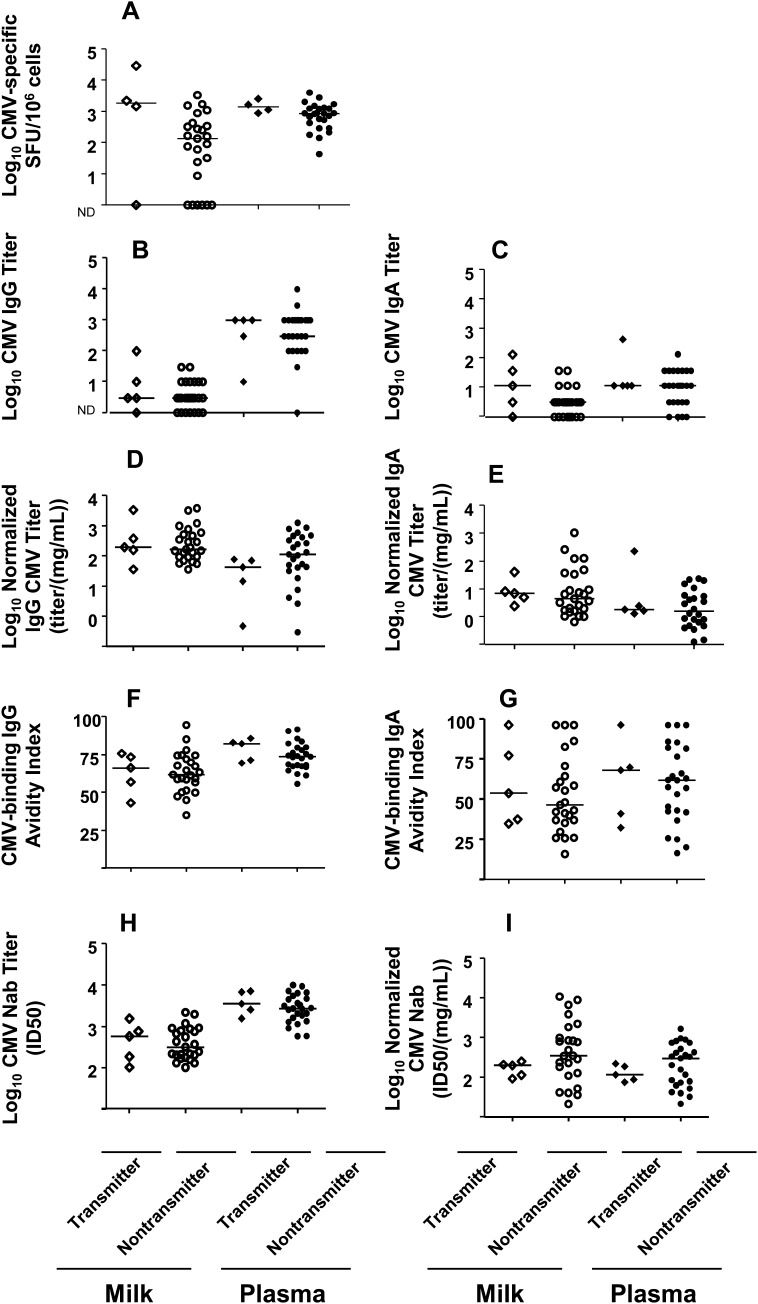
We defined the “symptomatic” group as seropositive mothers of VLBW infants with symptomatic postnatal CMV infection and the control or “asymptomatic” group as seropositive mothers of VLBW infants with no diagnosis of symptomatic postnatal CMV during hospitalization, despite milk exposure. Milk CMV load was comparable in the mothers of symptomatic and asymptomatic infants (Table 1). Notably, mothers of symptomatic infants had a median CMV-specific cellular response that was a log higher in magnitude than mothers of asymptomatic infants; however, this difference did not reach statistical significance (P = .13) (Figure 4A). Cellular responses in blood were similar in both groups (Figure 4A). Both normalized and nonnormalized milk and plasma CMV-binding IgG and IgA responses were comparable in mothers of symptomatic and asymptomatic infants (Figure 4B–E). CMV IgG and IgA avidity and neutralization, both before and after normalization for antibody content, were also similar in milk and plasma of each group (Figure 4F–I). Finally, we assessed CMV-specific immune responses in milk and blood at 3 consecutive weekly time points in a subset of CMV-seropositive mothers (3 mothers of symptomatic and 12 mothers of asymptomatic infants) but detected no distinct pattern of the kinetics of the viral load or CMV-specific immune responses in the mothers of symptomatic infants (data not shown).
Figure 4.
Milk cytomegalovirus (CMV) load and CMV-specific cellular and humoral immune responses are similar in seropositive mothers of very low-birth-weight (VLBW) infants diagnosed with symptomatic postnatal CMV and mothers of control VLBW infants. CMV-specific cellular responses (A), CMV-binding immunoglobulin (Ig) G titers (B), CMV-binding IgA titers (C), normalized CMV-binding IgG titers (D), normalized CMV-binding IgA titers (E), CMV-binding IgG avidity (F), CMV-binding IgA avidity (G), CMV-neutralizing antibody 50% inhibitory dose (ID50) titers (H), and normalized CMV-neutralizing antibody ID50 titers (I) in milk and plasma are shown for mothers of CMV-symptomatic (triangles) and asymptomatic (circles) VLBW infants. Lines indicate median values. Abbreviation: ND, not detectable.
DISCUSSION
The global benefits of breast milk for preterm infants are well established, yet postnatal acquisition of CMV remains a risk to VLBW infants of CMV-seropositive lactating mothers. We sought to identify possible maternal immunologic correlates of protection against symptomatic postnatal CMV infection that could guide approaches to reduce the risk of this infection in preterm infants. Because the virus is shed into and transmitted via breast milk, characterization of the milk immune responses is critical to assessing these immune correlates.
Our investigations found little correlation between the magnitude of CMV-specific immune responses and milk CMV load. We did, however, identify an inverse correlation between milk CMV-specific IgG avidity and CMV load in mothers of preterm VLBW infants. Because high milk CMV load and the ability to culture virus from milk has been tied to increased risk of transmission [6, 7, 41, 42], interventions aimed at reducing milk CMV load may contribute to the protection of breast milk–fed preterm infants. This association of poor quality antibody response with the magnitude of milk CMV shedding is consistent with the observation that poor CMV avidity is associated with congenital CMV transmission following primary maternal CMV infection [23, 43]. Although low-avidity milk antibodies may simply indicate the mother was recently exposed to CMV, this association could suggest that the quality of the CMV-specific humoral immune response is important to the containment of local CMV shedding. It is also possible that the antigens to which CMV IgG responses are directed affects the ability of these responses to attenuate virus shedding. The ability of local antibody responses to control mucosal CMV replication requires further investigation.
CMV-specific cellular responses were lower in milk than in blood despite milk CMV shedding in the majority of subjects. A large proportion of the T lymphocytes in milk are memory phenotype [44]; therefore, the low magnitude CMV-specific cellular immune response is surprising. Although milk CMV-specific humoral immune responses were also low magnitude compared with that in plasma, the milk responses were equal or greater in magnitude after normalization for total antibody content. Milk IgG primarily traffics from the plasma via transudation, whereas milk IgA is mainly produced by local plasma cells [45]. The correlation of milk and blood CMV-specific IgG responses is consistent with this biology. The robust milk CMV IgA response is in contrast to IgA responses against human immunodeficiency virus (HIV), another virus shed into and transmitted via breast-feeding. HIV-specific IgA responses remain low in milk even after normalization for milk IgA content [34, 46, 47].
Importantly, we did not detect differences in systemic or milk CMV-specific cellular or humoral immune responses between mothers of VLBW infants with or without diagnoses of symptomatic postnatal CMV infection. We hypothesized that poor local CMV-specific cellular immune responses may predict high milk CMV load and transmission; however, this response did not correlate with either milk CMV load or symptomatic disease in the infant. Although the breadth of the CMV-specific cellular responses may be important for containing viral shedding, the low milk cell number limited our assessment of cellular responses to those directed against a single virus antigen. It is possible that the apparent lack of protection by maternal antibody responses reflects poor placental transfer of maternally derived antibody to VLBW infants due premature birth. Determining whether our findings reflect the inability of maternal-derived immune responses to prevent symptomatic CMV transmission, or simply reflect the absence of protective antibody in these infants, is critical to the development of interventions, such as maternal vaccination or infant prophylaxis with monoclonal CMV-specific antibodies.
Our investigations of the role of CMV-specific immune responses in symptomatic postnatal CMV infection were limited by a small number of symptomatically infected infants. We relied on the diagnosis of symptomatic postnatal CMV infection by providers and did not prospectively screen for infection. However, because a practical goal for reduction in postnatal CMV transmission is to protect against symptomatic disease, we thought it was appropriate to limit the case definition to symptomatically infected infants for the search for protective immune correlates. Moreover, owing to a lack of universal CMV screening in the NICUs included in this study, congenital or perinatal CMV acquisition was not completely excluded in 3 of the 6 CMV-infected infants. However, acute symptoms consistent with postnatal CMV infection and lack of exposure to the vaginal canal (cesarean section delivery) in all case infants make it likely that the CMV infections in this cohort occurred postnatally.
It is clear that complex issues must be considered in designing vaccine- or antibody-based strategies to prevent neonatal CMV infection, because the mechanisms protecting the majority of VLBW preterm infants from symptomatic CMV infection despite repeated exposure remain elusive. Additional study is needed to define the maternal and infant factors important to postnatal CMV pathogenesis in this most vulnerable infant population. Importantly, our finding of an inverse correlation of milk IgG avidity and CMV load suggests that augmentation of mucosal CMV-specific humoral immune responses may reduce milk virus shedding, thereby decreasing risk of CMV transmission. Because current maternal vaccine prototypes for the prevention of congenital CMV infection are designed to elicit CMV-specific humoral immune responses [43, 48], these antibody-based maternal vaccines might prove useful in protection against symptomatic postnatal CMV.
Notes
Acknowledgments.
We would like to thank Tatenda Mahlokozera for his technical assistance and Les Kalish for his statistical guidance.
Financial support.
This work was supported by a pilot grant from Harvard Catalyst: The Harvard Clinical and Translational Science Center (National Institutes of Health grant 1 UL1 RR 025758-01 and financial contributions from participating institutions) (S. R. P., K. M. P., and M. L. G.), the Children’s Hospital Boston Career Development Award (S. R. P.), the Pediatric Infectious Diseases Basic Science Research Award (S. R. P.), the Doris Duke Clinical Scientist Development Award (S. R. P.), and the International Lactation Consultants of America research grant (S. R. P. and K. H. B.).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Noyola DE, DemmLer GJ, Williamson WD, et al. Cytomegalovirus urinary excretion and long term outcome in children with congenital cytomegalovirus infection. Congenital CMV Longitudinal Study Group. Pediatr Infect Dis J. 2000;19:505–10. doi: 10.1097/00006454-200006000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Williamson WD, DemmLer GJ, Percy AK. Catlin FI. Progressive hearing loss in infants with asymptomatic congenital cytomegalovirus infection. Pediatrics. 1992;90:862–6. [PubMed] [Google Scholar]
- 3.Fowler KB, McCollister FP, Dahle AJ, Boppana S, Britt WJ, Pass RF. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr. 1997;130:624–30. doi: 10.1016/s0022-3476(97)70248-8. [DOI] [PubMed] [Google Scholar]
- 4.Yeager AS, Palumbo PE, Malachowski N, Ariagno RL, Stevenson DK. Sequelae of maternally derived cytomegalovirus infections in premature infants. J Pediatr. 1983;102:918–22. doi: 10.1016/s0022-3476(83)80025-0. [DOI] [PubMed] [Google Scholar]
- 5.Dworsky M, Yow M, Stagno S, Pass RF, Alford C. Cytomegalovirus infection of breast milk and transmission in infancy. Pediatrics. 1983;72:295–9. [PubMed] [Google Scholar]
- 6.Hamprecht K, Maschmann J, Vochem M, Dietz K, Speer CP, Jahn G. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet. 2001;357:513–8. doi: 10.1016/S0140-6736(00)04043-5. [DOI] [PubMed] [Google Scholar]
- 7.Jim WT, Shu CH, Chiu NC, et al. Transmission of cytomegalovirus from mothers to preterm infants by breast milk. Pediatr Infect Dis J. 2004;23:848–51. doi: 10.1097/01.inf.0000137571.35541.55. [DOI] [PubMed] [Google Scholar]
- 8.Vochem M, Hamprecht K, Jahn G, Speer CP. Transmission of cytomegalovirus to preterm infants through breast milk. Pediatr Infect Dis J. 1998;17:53–8. doi: 10.1097/00006454-199801000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Maschmann J, Hamprecht K, Dietz K, Jahn G, Speer CP. Cytomegalovirus infection of extremely low birth weight infants via breast milk. Clin Infect Dis. 2001;33:1998–2003. doi: 10.1086/324345. [DOI] [PubMed] [Google Scholar]
- 10.Hamele M, Flanagan R, Loomis CA, Stevens T, Fairchok MP. Severe morbidity and mortality with breast milk associated cytomegalovirus infection. Pediatr Infect Dis J. 2010;29:84–6. doi: 10.1097/INF.0b013e3181b6dbb5. [DOI] [PubMed] [Google Scholar]
- 11.Griesmaier E, Neubauer V, Blum S, Trawoger R, Keller M, Kiechl-Kohlendorfer U. Neurodevelopmental outcome following congenital cytomegalovirus infection in preterm infants with twin-to-twin transfusion syndrome: a case report. Klin Padiatr. 2010;222:312–4. doi: 10.1055/s-0030-1263130. [DOI] [PubMed] [Google Scholar]
- 12.Vollmer B, Seibold-Weiger K, Schmitz-Salue C, et al. Postnatally acquired cytomegalovirus infection via breast milk: effects on hearing and development in preterm infants. Pediatr Infect Dis J. 2004;23:322–7. doi: 10.1097/00006454-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–65. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 14.Stagno S, Reynolds DW, Pass RF, Alford CA. Breast milk and the risk of cytomegalovirus infection. N Engl J Med. 1980;302:1073–6. doi: 10.1056/NEJM198005083021908. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda A, Kimura H, Hayakawa M, et al. Evaluation of cytomegalovirus infections transmitted via breast milk in preterm infants with a real-time polymerase chain reaction assay. Pediatrics. 2003;111:1333–6. doi: 10.1542/peds.111.6.1333. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi S, Kimura H, Oshiro M, et al. Transmission of cytomegalovirus via breast milk in extremely premature infants. J Perinatol. 2011;31:440–5. doi: 10.1038/jp.2010.150. [DOI] [PubMed] [Google Scholar]
- 17.Capretti MG, Lanari M, Lazzarotto T, et al. Very low birth weight infants born to cytomegalovirus-seropositive mothers fed with their mother's milk: a prospective study. J Pediatr. 2009;154:842–8. doi: 10.1016/j.jpeds.2008.12.046. [DOI] [PubMed] [Google Scholar]
- 18.Chiavarini M, Bragetti P, Sensini A, et al. Breastfeeding and transmission of cytomegalovirus to preterm infants: case report and kinetic of CMV-DNA in breast milk. Ital J Pediatr. 2011;37:6. doi: 10.1186/1824-7288-37-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gratama JW, van Esser JW, Lamers CH, et al. Tetramer-based quantification of cytomegalovirus (CMV)-specific CD8+ T lymphocytes in T-cell-depleted stem cell grafts and after transplantation may identify patients at risk for progressive CMV infection. Blood. 2001;98:1358–64. doi: 10.1182/blood.v98.5.1358. [DOI] [PubMed] [Google Scholar]
- 20.Stone SF, Price P, French MA. Cytomegalovirus (CMV)-specific CD8+ T cells in individuals with HIV infection: correlation with protection from CMV disease. J Antimicrob Chemother. 2006;57:585–8. doi: 10.1093/jac/dkl049. [DOI] [PubMed] [Google Scholar]
- 21.Sylwester AW, Mitchell BL, Edgar JB, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–85. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nigro G, Adler SP, La Torre R, Best AM. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350–62. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 23.Boppana SB, Britt WJ. Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J Infect Dis. 1995;171:1115–21. doi: 10.1093/infdis/171.5.1115. [DOI] [PubMed] [Google Scholar]
- 24.Henderson G, Anthony MY, McGuire W. Formula milk versus maternal breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD002972.pub2. ; Oct 17:(4). CD002972. [DOI] [PubMed] [Google Scholar]
- 25.Morgan JA, Young L, McGuire W. Pathogenesis and prevention of necrotizing enterocolitis. Curr Opin Infect Dis. 2011;24:183–9. doi: 10.1097/QCO.0b013e328345d5b5. [DOI] [PubMed] [Google Scholar]
- 26.Ronnestad A, Abrahamsen TG, Medbo S, et al. Late-onset septicemia in a Norwegian national cohort of extremely premature infants receiving very early full human milk feeding. Pediatrics. 2005;115:e269–76. doi: 10.1542/peds.2004-1833. [DOI] [PubMed] [Google Scholar]
- 27.Schanler RJ, Lau C, Hurst NM, Smith EO. Randomized trial of donor human milk versus preterm formula as substitutes for mothers' own milk in the feeding of extremely premature infants. Pediatrics. 2005;116:400–6. doi: 10.1542/peds.2004-1974. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan S, Schanler RJ, Kim JH, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatrics. 2010;156:562–7. doi: 10.1016/j.jpeds.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 29.Hamprecht K, Maschmann J, Muller D, et al. Cytomegalovirus (CMV) inactivation in breast milk: reassessment of pasteurization and freeze-thawing. Pediatr Res. 2004;56:529–35. doi: 10.1203/01.PDR.0000139483.35087.BE. [DOI] [PubMed] [Google Scholar]
- 30.Lee HC, Enright A, Benitz WE, Madan A. Postnatal cytomegalovirus infection from frozen breast milk in preterm, low birth weight infants. Pediatr Infect Dis J. 2007;26:276. doi: 10.1097/01.inf.0000254412.66944.3e. [DOI] [PubMed] [Google Scholar]
- 31.Maschmann J, Hamprecht K, Weissbrich B, Dietz K, Jahn G, Speer CP. Freeze-thawing of breast milk does not prevent cytomegalovirus transmission to a preterm infant. Arch Dis Child Fetal Neonatal Ed. 2006;91:F288–90. doi: 10.1136/adc.2004.050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersson Y, Savman K, Blackberg L, Hernell O. Pasteurization of mother's own milk reduces fat absorption and growth in preterm infants. Acta Paediatr. 2007;96:1445–9. doi: 10.1111/j.1651-2227.2007.00450.x. [DOI] [PubMed] [Google Scholar]
- 33.Kalpoe JS, Kroes AC, de Jong MD, et al. Validation of clinical application of cytomegalovirus plasma DNA load measurement and definition of treatment criteria by analysis of correlation to antigen detection. J Clin Microbiol. 2004;42:1498–504. doi: 10.1128/JCM.42.4.1498-1504.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Permar SR, Wilks AB, Ehlinger EP, et al. Limited contribution of mucosal IgA to Simian immunodeficiency virus (SIV)-specific neutralizing antibody response and virus envelope evolution in breast milk of SIV-infected, lactating rhesus monkeys. J Virol. 2010;84:8209–18. doi: 10.1128/JVI.00656-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prince HE, Leber AL. Validation of an in-house assay for cytomegalovirus immunoglobulin G (CMV IgG) avidity and relationship of avidity to CMV IgM levels. Clin Diagn Lab Immunol. 2002;9:824–7. doi: 10.1128/CDLI.9.4.824-827.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semba RD, Kumwenda N, Taha TE, et al. Mastitis and immunological factors in breast milk of lactating women in Malawi. Clin Diagn Lab Immunol. 1999;6:671–4. doi: 10.1128/cdli.6.5.671-674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willumsen JF, Filteau SM, Coutsoudis A, Uebel KE, Newell ML, Tomkins AM. Subclinical mastitis as a risk factor for mother-infant HIV transmission. Adv Exp Med Biol. 2000;478:211–23. doi: 10.1007/0-306-46830-1_19. [DOI] [PubMed] [Google Scholar]
- 38.Gomo E, Filteau SM, Tomkins AM, Ndhlovu P, Michaelsen KF, Friis H. Subclinical mastitis among HIV-infected and uninfected Zimbabwean women participating in a multimicronutrient supplementation trial. Trans R Soc Trop Med Hyg. 2003;97:212–6. doi: 10.1016/s0035-9203(03)90124-6. [DOI] [PubMed] [Google Scholar]
- 39.Fowler KB, Stagno S, Pass RF. Maternal age and congenital cytomegalovirus infection: screening of two diverse newborn populations, 1980–1990. J Infect Dis. 1993;168:552–6. doi: 10.1093/infdis/168.3.552. [DOI] [PubMed] [Google Scholar]
- 40.Fowler KB, Pass RF. Sexually transmitted diseases in mothers of neonates with congenital cytomegalovirus infection. J Infect Dis. 1991;164:259–64. doi: 10.1093/infdis/164.2.259. [DOI] [PubMed] [Google Scholar]
- 41.Doctor S, Friedman S, Dunn MS, et al. Cytomegalovirus transmission to extremely low-birthweight infants through breast milk. Acta Paediatr. 2005;94:53–8. doi: 10.1111/j.1651-2227.2005.tb01788.x. [DOI] [PubMed] [Google Scholar]
- 42.Jim WT, Shu CH, Chiu NC, et al. High cytomegalovirus load and prolonged virus excretion in breast milk increase risk for viral acquisition by very low birth weight infants. Pediatr Infect Dis J. 2009;28:891–4. doi: 10.1097/INF.0b013e3181a55c52. [DOI] [PubMed] [Google Scholar]
- 43.Nozawa N, Fang-Hoover J, Tabata T, Maidji E, Pereira L. Cytomegalovirus-specific, high-avidity IgG with neutralizing activity in maternal circulation enriched in the fetal bloodstream. J Clin Virol. 2009;46(Suppl 4):S58–63. doi: 10.1016/j.jcv.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eglinton BA, Roberton DM, Cummins AG. Phenotype of T cells, their soluble receptor levels, and cytokine profile of human breast milk. Immunol Cell Biol. 1994;72:306–13. doi: 10.1038/icb.1994.46. [DOI] [PubMed] [Google Scholar]
- 45.Mostov KE, Blobel G. Biosynthesis, processing, and function of secretory component. Methods Enzymol. 1983;98:458–66. doi: 10.1016/0076-6879(83)98173-9. [DOI] [PubMed] [Google Scholar]
- 46.Kuhn L, Trabattoni D, Kankasa C, et al. HIV-specific secretory IgA in breast milk of HIV-positive mothers is not associated with protection against HIV transmission among breast-fed infants. J Pediatr. 2006;149:611–6. doi: 10.1016/j.jpeds.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Becquart P, Hocini H, Levy M, Sepou A, Kazatchkine MD, Belec L. Secretory anti-human immunodeficiency virus (HIV) antibodies in colostrum and breast milk are not a major determinant of the protection of early postnatal transmission of HIV. J Infect Dis. 2000;181:532–9. doi: 10.1086/315255. [DOI] [PubMed] [Google Scholar]
- 48.Adler SP, Nigro G. The importance of cytomegalovirus-specific antibodies for the prevention of fetal cytomegalovirus infection or disease. Herpes. 2008;15:24–7. [PubMed] [Google Scholar]