Abstract
(See the editorial commentary by Williams, on pages 1651–3.)
Background. Heterozygous states of hemoglobin (Hb) A and HbS (HbAS, sickle-cell trait) or HbC (HbAC) protect against Plasmodium falciparum malaria by unclear mechanisms. Several studies suggest that HbAS and HbAC accelerate the acquisition of immunity to malaria, possibly by enhancing P. falciparum–specific antibody responses.
Methods. We used a protein microarray representing 491 P. falciparum proteins expressed during exoerythrocytic and erythrocytic stages of the life cycle to test the hypothesis that HbAS and HbAC enhance the P. falciparum–specific IgG response compared with normal HbAA. Plasma samples were collected from Malian children aged 2–10 years before and after a 6-month malaria season and were probed against the microarray. Immunoglobulin G (IgG) profiles of children with HbAA (n = 106), HbAS (n = 15), and HbAC (n = 20) were compared.
Results. Although the magnitude and breadth of P. falciparum–specific IgG responses increased with age and from before to after the malaria season in each antigen category, Hb type did not independently predict significant differences in P. falciparum–specific IgG profiles.
Conclusions. These data do not support the hypothesis that HbAS and HbAC protect against malaria by enhancing P. falciparum–specific antibody responses. It remains possible that HbAS and HbAC protect against malaria by enhancing antibody responses to antigens not studied here or through other immune mechanisms.
Heterozygous states of normal hemoglobin (Hb) A and HbS (HbAS, sickle-cell trait) or HbC (HbAC) protect against Plasmodium falciparum malaria by unclear mechanisms, although several possibilities have been suggested [1–8]. These include poor growth of P. falciparum in HbAS red blood cells (RBCs) under conditions of low oxygen tension [9, 10], accelerated removal of P. falciparum–infected HbAS RBCs (iRBCs) by splenic macrophages [11], and abnormal display of P. falciparum erythrocyte membrane protein 1 (PfEMP1), the parasite’s cytoadherence ligand and principal virulence factor, on the surface of HbAS and HbAC iRBCs [12, 13].
Another not mutually exclusive hypothesis is that HbAS and HbAC ‘enhance’ naturally acquired immunity to malaria [14, 15]. Children with HbAS and HbAC acquire the same level of antimalarial immunity as HbAA children, but at a younger age. In support of this hypothesis, Williams et al [16] found that the incidence of uncomplicated malaria in HbAS children decreases more rapidly with age, a surrogate for naturally acquired immunity in endemic areas, than in HbAA children. That purified immunoglobulin G (IgG) from malaria-immune adults can clear high-density parasitemias in children with malaria strongly indicates that P. falciparum–specific IgG is a central effector of antimalarial immunity [17]. In light of these observations, early seroepidemiologic studies compared total serum IgG levels between HbAA and HbAS children. These studies produced inconsistent results, with some reporting similar [18] or higher [19] IgG levels in HbAS children. The significance of these findings is unclear, however, because P. falciparum–specific IgG responses either were not measured [19] or were found to be lower in HbAS children [18].
More recently, seroepidemiologic studies have explored the relationship between HbAS and P. falciparum–specific IgG levels. Most of these studies have tested 1 of 2 hypotheses: (i) HbAS and HbAC enhance immunity to malaria by ‘generally’ stimulating IgG responses to P. falciparum proteins, independent of the parasite life-cycle stage at which they are expressed; and (ii) HbAS and HbAC enhance immunity to malaria by ‘specifically’ stimulating IgG responses to P. falciparum proteins expressed on the iRBC surface. Investigators believe these specific IgG responses are directed to variant surface antigens (VSAs) including PfEMP1, which is abnormally displayed on the surface of P. falciparum-infected HbAS and HbAC RBCs [12, 13]. Studies testing the first hypothesis have produced mixed results, with some finding higher, similar, or lower P. falciparum–specific IgG levels in HbAS children [20–25]. These studies are inherently biased, however, in that IgG specific for relatively few proteins (eg, CSP, EBA-175, GLURP, MSP1, MSP2, MSP3, or Pf155/RESA) or fragments thereof has been measured (<0.5% of the >5000 predicted P. falciparum proteins [26]). Studies testing the second hypothesis have shown higher IgG titers to VSAs in HbAS versus HbAA children [25, 27, 28], and more recently in HbAC versus HbAA children [25]. However, this approach has not produced consistent findings in different epidemiological settings [25] and lacks antigen specificity because it assays IgG reactivity to multiple uncharacterized antigens on the iRBC surface.
To explore whether HbAS and HbAC enhance the P. falciparum–specific IgG response, we used a protein microarray representing ∼23% of the P. falciparum proteome (1204 known and hypothetical proteins) to compare IgG profiles of HbAA, HbAS, and HbAC children with lifelong exposure to intense seasonal P. falciparum transmission. Specifically, we tested whether HbAS and HbAC enhance the IgG response to: (i) all P. falciparum proteins on the microarray irrespective of the life-cycle stage at which they are expressed; (ii) P. falciparum proteins that are maximally expressed during the erythrocytic stage; (iii) P. falciparum proteins known or predicted to be expressed on the iRBC surface; and (iv) P. falciparum VSAs known to be expressed on the iRBC surface. In testing each hypothesis, we compared the magnitude (level) and breadth (proportion of proteins recognized) of IgG responses as surrogates for their ability to confer protection against malaria.
MATERIALS AND METHODS
Ethics Statement
The Ethics Committee of the Faculty of Medicine, Pharmacy, and Odontostomatology at the University of Bamako, and the National Institute of Allergy and Infectious Diseases Institutional Review Board approved this study. Written, informed consent was obtained from parents or guardians of participating children.
Study Site
This study was conducted in Kambila, Mali, a small (1 km2) rural village with a population of 1500 persons where P. falciparum transmission is seasonal and intense [29, 30]. In May 2006, during a 2-week period just prior to the malaria season, 176 children aged 2–10 years were enrolled in the study after random selection from an age-stratified census of the entire village population. Enrollment exclusion criteria were Hb level <7 g/dL, axillary temperature ≥37.5°C, acute illness discernable on examination, or use of antimalarial or immunosuppressive medications in the past 30 days. Blood smears and venous blood samples were collected during the 2-week enrollment period and during a 2-week period at the end of the 6-month malaria season. A detailed description of the study site is reported elsewhere [29].
Blood Samples
We drew venous blood into sodium citrate–containing tubes (BD Biosciences, Vacutainer CPT) and transported it 20 km to the laboratory for processing within 3 hours of collection. We isolated the plasma and stored it at −80°C.
Hemoglobin Type
We used high-performance liquid chromatography (d-10 instrument; Bio-Rad) to identify Hb type.
Blood Smears
We stained thick blood smears with Giemsa and counted them against 300 leukocytes. We recorded P. falciparum densities as the number of asexual parasites/μL blood, based on a mean leukocyte count of 7500 cells/μL. Two microscopists evaluated each smear; a third resolved discrepancies.
Microarray Construction and Antibody Profiling
As described elsewhere [31, 32], we constructed protein microarrays in 4 steps: (1) polymerase chain reaction (PCR) amplification of complete or partial P. falciparum open-reading frames, (2) in vivo recombination cloning, (3) in vitro transcription/translation, and (4) microarray chip printing. Fluorescently labeled anti-IgG was used to detect IgG in plasma samples that reacted to proteins on the microarray.
Data Management and Analysis
We used STATA (StataCorp, release 10.0) to analyze data. We used Fisher exact and Wilcoxon rank–sum tests to compare binary and continuous variables, respectively. We used the Wilcoxon rank–sum test identify differential IgG reactivity between groups. We used a linear regression model to assess the effect of age, gender, and Hb type on the magnitude and breadth of IgG responses. We considered 2-tailed P values significant if ≤.05, unless correcting for false-discovery rate (FDR), in which case the Dunn–Bonferroni method was used, setting the level of significance at P = .0001 (β = α/n, where α = .05 and n = 491 tests).
RESULTS
Study Population
This study was conducted in Kambila, Mali, because of the sharp demarcation between the 6-month malaria season, during which there is intense P. falciparum transmission, and the 6-month dry season, during which there is little to no P. falciparum transmission [29, 30]. In May 2006, during a 2-week period at the end of the dry season, we enrolled 176 children aged 2–10 years in the study after random selection from an age-stratified census of the village population. At enrollment, 13 children (7.4%) had asymptomatic P. falciparum infection; we excluded them to focus on antibodies that persist in the absence of P. falciparum infection at the end of the dry season. We also excluded children with HbCC (n = 1) or whose Hb type was not identified (n = 6). Of the remaining 156 children, microarray data were available for 141: 106 with HbAA, 15 with HbAS, and 20 with HbAC. The proportions of children with HbAA (75%), HbAS (11%), and HbAC (14%) reflect the prevalence of these Hb variants in this region (Fairhurst et al, unpublished data). The average age, weight, and gender ratio did not differ by Hb type (Table 1). Nearly all children irrespective of Hb type had ≥1 P. falciparum infection during the study (Table 1). As previously reported, age and HbAS were independently associated with a decreased risk of malaria in this cohort [29].
Table 1.
Characteristics of 141 Aparasitemic Malian Children Aged 2–10 Years by Hemoglobin Type
| Characteristic | HbAA (n = 106) | HbAS (n = 15) | P valuea | HbAC (n = 20) | P valuea |
| Age, y (mean ± SD) | 5.7 ± 2.2 | 5.2 ± 2.2 | .51 | 6.4 ± 2.8 | .28 |
| Female sex, no. (%) | 53 (50.0) | 11 (73.3) | .11 | 9 (45.0) | .43 |
| Weight, kg (mean ± SD) | 18.0 ± 5.4 | 18.8 ± 8.2 | .95 | 19.3 ± 6.6 | .40 |
| Plasmodium falciparum smear positive at least once during the 6-mo malaria season (May–Dec), no. (%)b | 104 (98.1) | 13 (86.7) | .08 | 20 (100) | 1.00 |
Abbreviations: HbAA, hemoglobin type AA; HbAS, hemoglobin type AS; HbAC, hemoglobin type AC; SD, standard deviation.
The characteristics of HbAS and HbAC children were compared with those of HbAA children using Fisher exact tests and Wilcoxon rank–sum tests for binary and continuous variables, respectively.
P. falciparum smear positivity detected either during passive surveillance for malaria episodes over the 6-mo malaria season, or by active surveillance during 3 cross-sectional surveys conducted every 2 mo, as previously described [29].
The Magnitude and Breadth of the P. falciparum–Specific IgG Response Increases With Age and P. falciparum Transmission
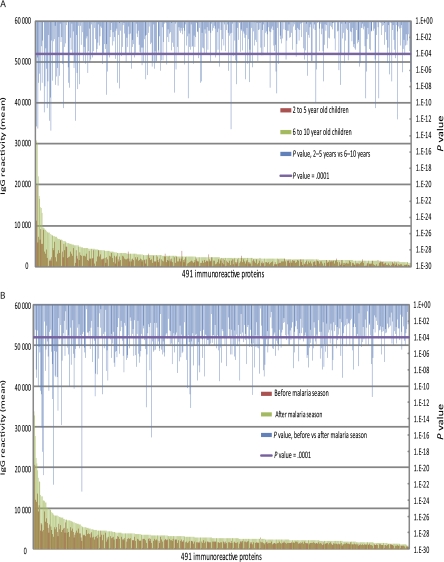
We first examined the IgG response with age, a surrogate for cumulative P. falciparum exposure, and from before to after the 6-month malaria season, irrespective of Hb type. Analyses pertain to the 491 P. falciparum proteins to which IgG reactivity exceeded 2 standard deviations (SDs) above the negative control. These proteins (Table 1; online only) represent a broad range of exoerythrocytic- and erythrocytic-stage P. falciparum proteins and are referred to here as ‘immunoreactive.’ Using plasma collected before the malaria season, we compared the mean level of IgG reactivity specific for each of the 491 proteins in children aged 2–5 years with that in children aged 6–10 years (Figure 1A). IgG reactivity specific for 478 of 491 proteins was higher in children aged 6–10 years; IgG reactivity to 126 of these remained significantly higher after correcting for the FDR (significance cutoff P < .0001), a statistical method used to correct for multiple comparisons. The average number of P. falciparum proteins to which IgG was detected increased with age (2–5 y: 138 proteins [95% CI, 128–148 proteins]; 6–10 y: 234 proteins [95% CI, 221–247 proteins]; P < .0001). We compared the mean level of IgG reactivity specific for each of the 491 proteins before and after the 6-month malaria season (Figure 1B). IgG reactivity specific for 490 of the 491 proteins was higher after the malaria season, and reactivity to 254 of these remained significantly higher after correcting for the FDR (significance cutoff P < .0001). Likewise, the average number of P. falciparum proteins to which IgG was detected increased from before to after the malaria season (before: 183 proteins [95% CI, 174–192 proteins]; after: 292 proteins [95% CI, 283–301 proteins]; P < .0001). As previously reported, IgG reactivity specific for Epstein–Barr nuclear antigen 1 (EBNA-1) printed on the same microarray did not change from before to after the malaria season [31]. Taken together, these data provide evidence for the P. falciparum–specificity of IgG reactivity measured on the microarray.
Figure 1.
The magnitude of the Plasmodium falciparum–specific immunoglobulin G (IgG) response increases with age and from before to after the malaria season. A, Shown is the average IgG reactivity specific for each of the 491 immunoreactive P. falciparum proteins measured in plasma collected before the malaria season from P. falciparum–uninfected children aged 2–5 y (n = 73) and 6–10 y (n = 68). IgG reactivity specific for 478 of the 491 proteins was higher among children aged 6–10 y, and reactivity to 126 of these remained significantly higher after correcting for the false-discovery rate. B, Shown is the average IgG reactivity specific for each of the 491 immunoreactive P. falciparum proteins measured in plasma collected from all children (n = 141) before and after the 6-month malaria season. IgG reactivity specific for 490 of the 491 proteins was higher after the malaria season, and reactivity to 254 of these remained significantly higher after correcting for the false-discovery rate. The Dunn–Bonferroni method was used to correct for the false discovery rate by setting the level of statistical significance at P = .0001 (β = α/n, where α = .05 and n = 491 tests performed).
HbAS and HbAC Are Not Associated With Significant Differences in the Magnitude or Breadth of the P. falciparum–Specific IgG Response
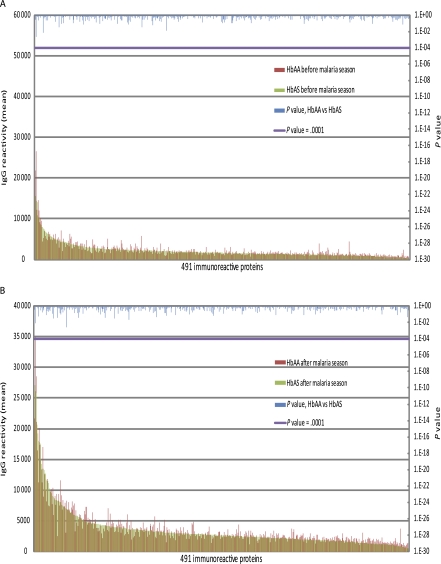
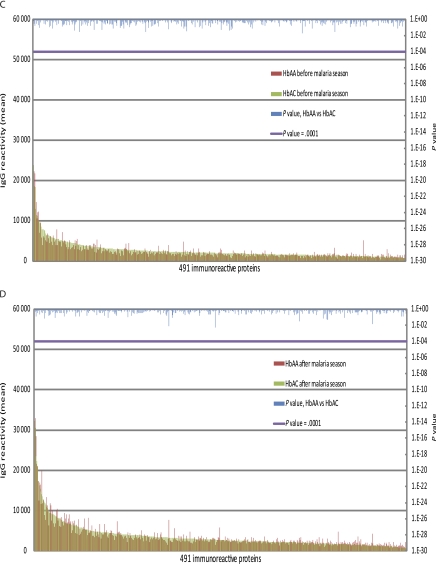
Next we tested the hypothesis that HbAS and HbAC generally enhance P. falciparum–specific IgG responses against a broad range of exoerythrocytic- and erythrocytic-stage proteins. We compared the magnitude and breadth of IgG reactivity specific for the 491 proteins among children with HbAA, HbAS, and HbAC before and after the malaria season. In comparing 2- to 10-year-old children with HbAA versus those with HbAS, we found no significant differences in IgG levels specific for any of the 491 proteins before (Figure 2A) or after (Figure 2B) the malaria season. Similarly, the average number of P. falciparum proteins to which IgG was detected did not differ significantly between HbAA and HbAS children before (HbAA: 184 proteins [95% CI, 174–194 proteins]; HbAS: 169 proteins [95% CI: 138–200 proteins]; P = .44) or after the malaria season (HbAA: 291 proteins [95% CI, 281–301 proteins]; HbAS: 284 proteins [95% CI, 257–311 proteins]; P = .83). In comparing 2–10 year-old children with HbAA versus those with HbAC, we found no significant differences in IgG levels specific for any of the 491 proteins before (Figure 2C) or after (Figure 2D) the malaria season. Likewise, the average number of P. falciparum proteins to which IgG was detected did not differ significantly between HbAA and HbAC children before (HbAA: 184 proteins [95% CI:174–194]; HbAC: 201 proteins [95% CI:170–232]; P = .98) or after the malaria season (HbAA: 291 proteins [95% CI:281–301]; HbAC: 303 proteins [95% CI:274–332]; P = .91). Taken together, these data do not support the hypothesis that HbAS and HbAC generally enhance the magnitude or breadth of the P. falciparum–specific IgG response. Retrospective power analysis indicated that the minimum difference in antibody reactivity likely to be detected against the 491 antigens ranges from 28% to 53% for the HbAA versus HbAC comparison and 31%–60% for the HbAA versus HbAS comparison at the .05 significance level with 80% power.
Figure 2.
Hemoglobin S trait (HbAS) and hemoglobulin C trait (HbAC) are not associated with significant differences in the magnitude of the Plasmodium falciparum–specific immunoglobulin G (IgG) response. Shown is the average IgG reactivity specific for each of the 491 immunoreactive P. falciparum proteins measured in plasma collected before (A) and after (B) the malaria season from P. falciparum–uninfected children aged 2–10 y with either HbAA (n = 106) or HbAS (n = 15). There was no statistically significant difference in the level of IgG reactivity specific for the 491 proteins between the 2 groups of children at either time point. Also shown is the average IgG reactivity specific for each of the 491 immunoreactive P. falciparum proteins measured in plasma collected before (C) and after (D) the malaria season from P. falciparum–uninfected children aged 2–10 y with HbAA (n = 106) or HbAC (n = 20). There was no statistically significant difference in the level of IgG reactivity specific for the 491 proteins between the 2 groups of children at either time point. The Dunn–Bonferroni method was used to correct for the false discovery rate by setting the level of statistical significance at P = .0001 (β = α/n, where α = .05 and n = 491 tests performed).
HbAS and HbAC Are Not Associated With an Enhanced IgG Response to P. falciparum Proteins Expressed During the Erythrocytic Stage
Next we tested the hypothesis that HbAS and HbAC selectively enhance the IgG response to P. falciparum proteins expressed during the erythrocytic stages of the P. falciparum life cycle (ring, trophozoite, schizont, gametocyte) relative to the response to proteins expressed during the exoerythrocytic stages (sporozoite, merozoite). Because age was a strong predictor of the P. falciparum–specific IgG response (Figure 1A), we performed age-adjusted regression analysis to examine the relationship among Hb type and IgG responses to P. falciparum proteins expressed at different life-cycle stages. We grouped the 491 immunoreactive P. falciparum proteins into life-cycle stages at which they have been found to be maximally expressed (Supplementary Table 1), either at the protein level by mass spectroscopy (sporozoite, merozoite, trophozoite, gametocyte; accessed on www.plasmodb.org) [33] or at the RNA transcript level by Affymatrix (merozoite, ring, trophozoite, schizont, gametocyte; accessed on www.plasmodb.org) [34]. Using mass-spectroscopic or transcriptional data, we found that Hb type did not predict the magnitude of IgG responses specific for different stages of the P. falciparum life cycle before or after the malaria season (Table 2). Similarly, the number of proteins recognized within each life-cycle stage before or after the malaria season (breadth of response) did not differ significantly by Hb type (Table 3). Of note, the magnitude and breadth of IgG responses specific for proteins expressed during each life-cycle stage increased significantly with age (Tables 2 and 3).
Table 2.
Linear Regression Results for the Magnitude of the Immunoglobulin G Response Specific for Plasmodium falciparum Antigen Categories Before and After the Malaria Season by Hemoglobin Type, Age, and Gender
|
Before malaria season (May) |
After malaria season (Dec) |
|||||||
| P. falciparum Protein Categories | Hb type (AC vs AA) | Hb type (AS vs AA) | Age | Gender | Hb type (AC vs AA) | Hb type (AS vs AA) | Age | Gender |
| Merozoite (RNA) | −41.9 (578.9)a P = .9422 | −140.8(659.1)a P = .8310 | 567.6 (87.0)a P < .0001 | 214.7 (407.0)a P = .5987 | 386.9 (704.2)a P = .5837 | 4.6 (661.8)a P = .9944 | 426.2 (89.4)a P < .0001 | −264.6(401.9)a P = .5114 |
| Ring (RNA) | 54.2 (444.8)a P = .9032 | −41.0(506.4)a P = .9355 | 315.8 (66.8)a P < .0001 | 259.1 (312.7)a P = .4088 | −6.2 (509.9)a P = .9901 | −80.7(479.2)a P = .8665 | 343.2 (64.7)a P < .0001 | 8.2 (291.0)a P = .9774 |
| Trophozoite (RNA) | 50.2 (400.6)a P = .9004 | 39.8 (456.2)a P = .9305 | 271.0 (60.2)a P < .0001 | 67.6 (281.7)a P = .8105 | 194.7 (460.2)a P = .6728 | −88.7(432.5)a P = .8377 | 266.2 (58.4)a P < .0001 | −102.2(262.6)a P = .6978 |
| Schizont (RNA) | 38.7 (420.4)a P = .9266 | −51.8 (478.7)a P = .9138 | 355.2 (63.2)a P < .0001 | 113.7 (295.6)a P = .7010 | 2.9 (500.5)a P = .9952 | −192.8(470.3)a P = .6824 | 333.1 (63.6)a P < .0001 | −119.6(285.6)a P = .6760 |
| Gametocyte (RNA) | −72.8 (385.7)a P = .8504 | 123.6 (439.2)a P = .7787 | 312.4 (58.0)a P < .0001 | 170.5 (271.2)a P = .5304 | −82.5 (456.7)a P = .8567 | 18.3 (429.1)a P = .9659 | 284.1 (58.0)a P < .0001 | −9.9(260.6)a P = .9694 |
| Sporozoite (protein) | 31.5 (388.4)a P = .9353 | 82.7 (442.2)a P = .8518 | 300.4 (58.4)a P < .0001 | 175.7 (273.1)a P = .5209 | 19.0 (450.0)a P = .9662 | −77.7(422.8)a P = .8545 | 332.1 (57.1)a P < .0001 | −24.7(256.8)a P = .9234 |
| Merozoite (protein) | −123.0 (443.2)a P = .7818 | −22.4 (504.6)a P = .9645 | 445.4 (66.6)a P < .0001 | 146.7 (311.6)a P = .6384 | −6.6 (524.4)a P = .9899 | −13.6(492.8)a P = .9778 | 405.4 (66.6)a P < .0001 | −60.6(299.2)a P = .8396 |
| Trophozoite (protein) | 65.4 (416.7)a P = .8754 | 130.4 (474.5)a P = .7838 | 321.3 (62.6)a P < .0001 | 145.4 (293.0)a P = .6204 | 211.8 (498.9)a P = .6718 | 104.3 (468.8)a P = .8242 | 303.3 (63.3)a P < .0001 | −55.0(284.7)a P = .8468 |
| Gametocyte (protein) | 21.0 (383.0)a P = .9562 | 134.8 (436.1)a P = .7576 | 253.0 (57.6)a P < .0001 | 152.4 (269.3)a P = .5722 | 100.9 (447.8)a P = .8220 | −71.2(420.8)a P = .8658 | 238.0 (56.9)a P < .0001 | −10.6(255.5)a P = .9669 |
| PEXEL or HT | −279.2 (640.6)a P = .6635 | −207.0 (729.3)a P = .7769 | 586.0 (96.3)a P < .0001 | 113.1 (450.4)a P = .8020 | −225.4 (837.9)a P = .7883 | 27.4 (787.4)a P = .9722 | 480.0 (106.4)a P < .0001 | −457.1(478.1)a P = .3410 |
| RBC surface expression | −54.5 (540.7)a P = .9197 | −126.1 (615.7)a P = .8379 | 373.8 (81.3)a P < .0001 | 164.5 (380.2)a P = .6658 | 88.2 (695.5)a P = .8992 | 95.9 (653.6)a P = .8835 | 253.9 (88.3)a P = .0047 | −112.3(396.9)a P = .7776 |
| PfEMP1 | −24.0 (567.2)a P = .9663 | −83.7 (645.8)a P = .8970 | 410.7 (85.3)a P < .0001 | 494.0 (398.8)a P = .2175 | −561.3 (816.1)a P = .4929 | −447.2(766.9)a P = .5608 | 509.0 (103.7)a P < .0001 | 165.1 (465.7)a P = .7235 |
| RIFIN | −22.9 (655.5)a P = .9721 | −642.7 (746.4)a P = .3907 | 355.9 (98.5)a P = .0004 | 275.7 (460.9)a P = .5507 | −222.0 (973.5)a P = .8199 | −953.4 (914.8)a P = .2993 | 211.0 (123.6)a P = .0906 | 45.5 (555.5)a P = .9348 |
| STEVOR | −58.1 (866.8)a P = .9466 | 75.5 (986.9)a P = .9390 | 843.6 (130.3)a P < .0001 | 1240.9 (609.4)a P = .0436 | −282.8 (1364.9)a P = .8361 | −1898.7 (1282.6)a P = .1413 | 1247.8 (173.4)a P < .0001 | 1173.9 (778.9)a P = .1343 |
| SURFIN | −37.7 (295.6)a P = .8986 | −431.5 (336.6)a P = .2020 | 383.8 (44.4)a P < .0001 | 151.0 (207.8)a P = .4686 | −375.9 (413.0)a P = .3645 | −354.5 (388.1)a P = .3628 | 399.7 (52.4)a P < .0001 | −145.2(235.7)a P = .5390 |
Abbreviations: Hb, hemoglobin; PEXEL, Plasmodium export element; HT, host-targeting signal.
Regression coefficient.
Table 3.
Poisson Regression Results for the Breadth of the Immunoglobulin G Response Specific for Plasmodium falciparum Antigen Categories Before and After the Malaria Season by Hemoglobin Type, Age, and Gender
| Before malaria season (May) |
After malaria season (Dec) |
|||||||
| P. falciparum protein categories | Hb type (AC vs AA) | Hb type (AS vs AA) | Age | Gender | Hb type (AC vs AA) | Hb type (AS vs AA) | Age | Gender |
| Merozoite (RNA) | 0.0281 (0.1389)a P = .8394 | −0.0495 (0.2051)a P = .8090 | 0.1323 (0.0221)a P < .0001 | 0.0582 (0.1096)a P = .5952 | 0.0623 (0.1032)a P = .5461 | 0.0518 (0.0919)a P = .5727 | 0.0554 (0.0146)a P = .0002 | 0.0309 (0.0677)a P = .6478 |
| Ring (RNA) | −0.0055(0.1607)a P = .9726 | −0.0282(0.2358)a P = .9047 | 0.1347 (0.0263)a P < .0001 | 0.0263 (0.1278)a P = .8369 | 0.0617 (0.1076)a P = .5662 | 0.0309 (0.1105)a P = .7794 | 0.0709 (0.0169)a P < .0001 | 0.0119 (0.0781)a P = .8782 |
| Trophozoite (RNA) | 0.0669 (0.1625)a P = .6803 | −0.0252(0.2198)a P = .9086 | 0.1311 (0.0258)a P < .0001 | 0.0603 (0.1267)a P = .6342 | 0.0917 (0.1305)a P = .4821 | 0.0171 (0.1128)a P = .8791 | 0.0732 (0.0180)a P < .0001 | 0.0375 (0.0829)a P = .6509 |
| Schizont (RNA) | 0.0159 (0.1404)a P = .9095 | 0.0196 (0.1974)a P = .9208 | 0.1151 (0.0246)a P < .0001 | 0.0283 (0.1175)a P = .8091 | 0.0391 (0.1196)a P = .7433 | 0.0201 (0.1137)a P = .8596 | 0.0565 (0.0172)a P = .0010 | 0.0360 (0.0797)a P = .6515 |
| Gametocyte (RNA) | 0.0339 (0.1731)a P = .8445 | −0.0235(0.2197)a P = .9146 | 0.1283 (0.0279)a P < .0001 | 0.0637 (0.1364)a P = .6403 | 0.0281 (0.1378)a P = .8383 | 0.0209 (0.1195)a P = .8606 | 0.0690 (0.0194)a P = .0004 | 0.0402 (0.0914)a P = .6601 |
| Sporozoite (protein) | 0.0021 (0.1638)a P = .9894 | 0.0401 (0.2228)a P = .8568 | 0.1307 (0.0259)a P < .0001 | 0.0381 (0.1267)a P = .7634 | 0.0345 (0.1337)a P = .7958 | 0.0031 (0.1219)a P = .9795 | 0.0777 (0.0180)a P < .0001 | 0.0231 (0.0838)a P = .7822 |
| Merozoite (protein) | −0.0001(0.1722)a P = .9993 | −0.0431(0.2481)a P = .8618 | 0.1341 (0.0277)a P < .0001 | 0.0641 (0.1352)a P = .6352 | 0.1121 (0.1251)a P = .3701 | 0.0596 (0.1167)a P = .6090 | 0.0866 (0.0184)a P < .0001 | 0.0630 (0.0846)a P = .4559 |
| Trophozoite (protein) | 0.0464 (0.1579)a P = .7685 | −0.0359(0.2353)a P = .8785 | 0.1417 (0.0272)a P < .0001 | 0.0634 (0.1320)a P = .6307 | 0.0919 (0.1292)a P = .4764 | 0.0911 (0.1202)a P = .4485 | 0.0750 (0.0188)a P < .0001 | 0.0376 (0.0857)a P = .6605 |
| Gametocyte (protein) | 0.0441 (0.1599)a P = .7828 | −0.0002(0.2016)a P = .9988 | 0.1208 (0.0264)a P < .0001 | 0.0336 (0.1291)a P = .7943 | 0.0539 (0.1297)a P = .6775 | 0.0179 (0.1171)a P = .8780 | 0.0593 (0.0187)a P = .0015 | 0.0401 (0.0859)a P = .6399 |
| PEXEL or HT | −0.1720 (0.0969)a P = .0761 | −0.0828 (0.1756)a P = .6371 | 0.1261 (0.0180)a P < .0001 | 0.0566 (0.0798)a P = .4778 | 0.0166 (0.0517)a P = .7478 | 0.0154 (0.0493)a P = .7543 | 0.0393 (0.0082)a P < .0001 | 0.0102 (0.0359)a P = .7756 |
| RBC surface expression | −0.0013 (0.1119)a P = .9902 | −0.0656 (0.1719)a P = .7027 | 0.1055 (0.0184)a P < .0001 | 0.0334 (0.0873)a P = .7014 | 0.0809 (0.0794)a P = .3081 | 0.0098 (0.0636)a P = .8769 | 0.0423 (0.0106)a P < .0001 | 0.0013 (0.0479)a P = .9778 |
| PfEMP1 | −0.1133 (0.0612)a P = .0662 | 0.0120 (0.0696)a P = .8629 | 0.0273 (0.0092)a P = .0035 | 0.0375 (0.0430)a P = .3838 | 1.2583 (0.8244)a P = .1269 | −0.2486 (1.1789)a P = .8329 | −0.5315 (0.2134)a P = .0127 | −0.6306 (0.7596)a P = .4064 |
| RIFIN | 0.4726 (0.6521)a P = .4686 | 0.0903 (0.7342)a P = .9021 | −0.2604 (0.1119)a P = .0199 | −0.5155 (0.4955)a P = .2981 | −1.2313 (4.5039)a P = .9723 | −1.5210 (5.9891)a P = .9689 | −0.2858 (0.1514)a P = .0255 | −1.4801 (1.1431)a P = .1953 |
| STEVOR | 0.4189 (0.7201)a P = .5607 | 1.3505 (0.7537)a P = .0731 | −0.8246 (0.1509)a P < .0001 | −0.0527 (0.4779)a P = .9121 | −0.3866 (0.9531)a P = .6850 | 0.1751 (0.9150)a P = .8482 | −0.9603 (0.2290)a P < .0001 | 0.0006 (0.5753)a P = .9990 |
| SURFIN | −0.3894 (0.6310)a P = .5371 | −0.0056 (0.6496)a P = .9930 | −0.5354 (0.1023)a P < .0001 | −0.2299 (0.4080)a P = .5729 | −1.8858 (1.1321)a P = .0957 | −1.1453 (0.9089)a P = .2076 | −0.6083 (0.1422)a P < .0001 | −0.0140 (0.4812)a P = .9766 |
Abbreviations: Hb, hemoglobin; PEXEL, Plasmodium export element; HT, host-targeting signal.
Regression coefficient.
HbAS and HbAC Are Not Associated With Enhanced IgG Responses to P. falciparum Proteins Expressed on the iRBC Surface
Next we tested the hypothesis that HbAS and HbAC selectively enhance IgG responses to P. falciparum proteins expressed on the iRBC surface. In age-adjusted regression analysis, we determined the magnitude and breadth of IgG responses specific for the 491 proteins for which there is evidence of expression on the iRBC surface by proteomic analysis [35] or by the presence of a Plasmodium export element (PEXEL) motif [36] or host-targeting (HT) signal (Supplementary Table 1) [37]. Whether classified by proteomic data or the presence of a PEXEL motif or HT signal, Hb type did not predict IgG levels specific for proteins putatively or known to be expressed on the iRBC surface before or after the malaria season (Table 2). Likewise, the number of P. falciparum–encoded iRBC surface proteins recognized before or after the malaria season did not differ significantly by Hb type (Table 3). Again, the magnitude and breadth of IgG responses specific for P. falciparum proteins expressed on the iRBC surface increased with age (Tables 2 and 3).
HbAS and HbAC Are Not Associated With Enhanced IgG Responses to P. falciparum VSAs
Lastly, we tested the hypothesis that HbAS and HbAC selectively enhance IgG responses to P. falciparum VSAs, namely, members of the PfEMP1, RIFIN, STEVOR, and SURFIN families expressed on the iRBC surface. Of the 491 immunoreactive proteins, 7 PfEMP1, 3 RIFIN, 1 STEVOR, and 2 SURFIN proteins were represented (Supplementary Table 1). Hb type did not predict IgG levels specific for VSAs before or after the malaria season in age-adjusted analysis (Table 2). Furthermore, the proportion of children with IgG specific for at least 1 protein in each VSA family did not differ significantly by Hb type in age-adjusted analysis (Table 3). Consistent with the IgG responses to other P. falciparum protein categories, the magnitude and breadth of IgG responses specific for proteins in each VSA family increased with age (Tables 2 and 3).
DISCUSSION
We tested the hypothesis that HbAS and HbAC accelerate the acquisition of humoral immunity to malaria by profiling IgG responses of Malian children to a diverse array of P. falciparum proteins expressed at all stages of parasite development in humans. Because these children were young and lived continuously in their village, they were actively acquiring malaria immunity during this study. Accordingly, we found that the magnitude and breadth of IgG responses specific for 491 P. falciparum proteins increased with age. We also found that P. falciparum–specific IgG responses increased from before to after the malaria season, during which nearly all children experienced ≥1 P. falciparum infection. Together these data indicate that P. falciparum proteins on the microarray are folded adequately enough to bind at least some proportion of the P. falciparum–specific IgG repertoire of Malian children, and that the microarray can detect expected differences in IgG responses between different groups of Malian children sampled simultaneously (younger versus older), or the same group of children sampled longitudinally (before and after the malaria season). Importantly, we previously found that IgG responses to P. falciparum proteins expressed in the high-throughput translation system correlated with IgG responses to correctly folded recombinant proteins spotted onto the same microarray [31]. Moreover, in children of all ages, we detected IgG specific for purified EBNA-1 printed on the same microarray, but we did not observe consistent changes in EBNA-1–specific IgG from before to after the malaria season [31].
Using this protein microarray, we compared P. falciparum–specific IgG profiles of children who differed by Hb type. In age-adjusted analyses, we found that the magnitude and breadth of IgG responses did not differ among HbAS, HbAC, and HbAA children either before or after the malaria season. These data suggest that Hb type does not modulate the acquisition or maintenance of P. falciparum–specific long-lived plasma cells or memory B cells—assayed indirectly by measuring IgG levels before and after the malaria season, respectively. These findings were made regardless of the life-cycle stage at which parasite proteins are expressed, or of whether the proteins are expressed in the interior or on the surface of iRBCs. Our findings differ from those of Verra et al [25], who found that P. falciparum–specific IgG responses are enhanced in HbAS and HbAC children in Burkina Faso. Whereas this conclusion was based on differences in IgG responses to well-characterized P. falciparum proteins expressed at different life-cycle stages, relatively few proteins (CSP, AMA1, EBA-175, MSP119, MSP-2) were studied. Furthermore, this conclusion was supported only by data obtained from children in urban Ouagadougou and not by data from children residing in nearby rural villages where immunity is more continuously acquired with age. Most importantly, age-adjusted analyses were not performed on data obtained from urban children due to lack of age data on a significant proportion of them [25].
This study has several strengths that permit a more comprehensive assessment of the effect of HbAS and HbAC on the acquisition of P. falciparum–specific humoral immunity. Importantly, we studied IgG responses to a sufficiently large and diverse number of P. falciparum proteins to permit analyses of subsets of P. falciparum–specific IgG responses by life-cycle stage and subcellular location within iRBCs. In addition, we designed the study to minimize differences in P. falciparum exposure between children who differed by Hb type. Specifically, we enrolled an age-stratified, randomly sampled population representing 15% of individuals living in a small community with no dominant body of water that experiences intense, annual P. falciparum transmission. Moreover, households of HbAS and HbAC children were distributed evenly over the study site [29]. Thus children in a given age group differed by Hb type but not cumulative P. falciparum exposure. Furthermore, the study was restricted to children without P. falciparum infection before the malaria season at the time of plasma sampling, thus limiting the potential confounding effects of concurrent P. falciparum infection and consequent boosting of P. falciparum–specific IgG.
This study also has limitations. First, the expected finding of a strong association between IgG responses and age might have confounded the relationship between IgG responses and Hb type. However, the ages of HbAA, HbAS and HbAC children did not differ significantly, and in age-adjusted analyses there was no relationship between P. falciparum–specific IgG responses and Hb type. Second, we assayed P. falciparum–specific IgG responses in a relatively small number of HbAS (n = 20) and HbAC (n = 15) children. Although HbAS and HbAC children combined represented 25% of the children in the study, a larger study may have detected more subtle differences in IgG responses. Third, it is possible that HbAA children who generated low-titer, nonprotective IgG responses early in life had already died prior to this study. If true, this may have artificially enriched the group of HbAA children for those having higher-titer and more-broadly reactive IgG responses. Finally, we measured IgG responses to a limited set of PfEMP1 proteins and other VSAs, and it is possible that all relevant epitopes of these proteins in particular are not displayed on the microarray. The role of these proteins in antigenic variation and cytoadherence strongly implicates them as important targets of naturally acquired immunity to malaria [38–40]. Given the possibility that PfEMP1 levels are reduced on the surface of HbAS and HbAC iRBCs in vivo, one might reasonably expect that these Hb variants modulate the acquisition of antibodies to PfEMP1 and other VSAs, and it will be essential that future studies quantify IgG responses to substantial numbers of PfEMP1, RIFIN, STEVOR, and SURFIN protein sequence variants.
It remains possible that HbAS and HbAC protect against malaria by enhancing antibody responses to antigens not studied here, or by immune mechanisms not measured in this study. For example, HbAS and HbAC may modulate the quality of antibody responses (fine specificity, IgG subclass ratios [41, 42]) or innate and cellular immune responses. Indeed, Ferreira et al recently demonstrated in a mouse model that sickle Hb may suppress the immune-mediated pathogenesis of cerebral malaria [43].
In conclusion, our data suggest that neither HbAS nor HbAC significantly enhances the magnitude or breadth of IgG responses specific for a diverse array of P. falciparum proteins. Further studies are needed to better understand the relationships among HbAS, HbAC, and acquired immunity to malaria.
Supplementary Data
Supplementary data are available at The Journal of infections Diseases online (http://www.oxfordjournals.org/our_journals/jid/).
Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
We sincerely thank the residents of Kambila, Mali, for their participation in this study. We also thank Julie Kim, Dr. Richard Sakai, and the staff of the Mali Service Center in Bamako for logistical support.
Financial support.
This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health; and by National Institute of Allergy and Infectious Diseases, National Institutes of Health [1R43AI066791 to P. L. F].
Potential conflicts of interest.
P. L. F. has patent applications related to protein microarray fabrication and has stock positions with Antigen Discovery. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hill AV, Allsopp CE, Kwiatkowski D, et al. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 2.Aidoo M, Terlouw DJ, Kolczak MS, et al. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet. 2002;359:1311–2. doi: 10.1016/S0140-6736(02)08273-9. [DOI] [PubMed] [Google Scholar]
- 3.Jallow M, Teo YY, Small KS, et al. Genome-wide and fine-resolution association analysis of malaria in West Africa. Nat Genet. 2009;41:657–65. doi: 10.1038/ng.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal A, Guindo A, Cissoko Y, et al. Hemoglobin C associated with protection from severe malaria in the Dogon of Mali, a West African population with a low prevalence of hemoglobin S. Blood. 2000;96:2358–63. [PubMed] [Google Scholar]
- 5.Modiano D, Luoni G, Sirima BS, et al. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature. 2001;414:305–8. doi: 10.1038/35104556. [DOI] [PubMed] [Google Scholar]
- 6.Mockenhaupt FP, Ehrhardt S, Cramer JP, et al. Hemoglobin C and resistance to severe malaria in Ghanaian children. J Infect Dis. 2004;190:1006–9. doi: 10.1086/422847. [DOI] [PubMed] [Google Scholar]
- 7.Williams TN, Mwangi TW, Wambua S, et al. Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J Infect Dis. 2005;192:178–86. doi: 10.1086/430744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreuels B, Kreuzberg C, Kobbe R, et al. Differing effects of HbS and HbC traits on uncomplicated falciparum malaria, anemia, and child growth. Blood. 2010;115:4551–8. doi: 10.1182/blood-2009-09-241844. [DOI] [PubMed] [Google Scholar]
- 9.Friedman MJ. Erythrocytic mechanism of sickle cell resistance to malaria. Proc Natl Acad Sci U S A. 1978;75:1994–7. doi: 10.1073/pnas.75.4.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasvol G, Weatherall DJ, Wilson RJ. Cellular mechanism for the protective effect of haemoglobin S against P. falciparum malaria. Nature. 1978;274:701–3. doi: 10.1038/274701a0. [DOI] [PubMed] [Google Scholar]
- 11.Ayi K, Turrini F, Piga A, Arese P. Enhanced phagocytosis of ring-parasitized mutant erythrocytes: A common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood. 2004;104:3364–71. doi: 10.1182/blood-2003-11-3820. [DOI] [PubMed] [Google Scholar]
- 12.Fairhurst RM, Baruch DI, Brittain NJ, et al. Abnormal display of PfEMP-1 on erythrocytes carrying haemoglobin C may protect against malaria. Nature. 2005;435:1117–21. doi: 10.1038/nature03631. [DOI] [PubMed] [Google Scholar]
- 13.Cholera R, Brittain NJ, Gillrie MR, et al. Impaired cytoadherence of Plasmodium falciparum–infected erythrocytes containing sickle hemoglobin. Proc Natl Acad Sci U S A. 2008;105:991–6. doi: 10.1073/pnas.0711401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayoumi RA. The sickle-cell trait modifies the intensity and specificity of the immune response against P. falciparum malaria and leads to acquired protective immunity. Med Hypotheses. 1987;22:287–98. doi: 10.1016/0306-9877(87)90193-9. [DOI] [PubMed] [Google Scholar]
- 15.Ringelhann B, Hathorn MK, Jilly P, Grant F, Parniczky G. A new look at the protection of hemoglobin AS and AC genotypes against Plasmodium falciparum infection: A census tract approach. Am J Hum Genet. 1976;28:270–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Williams TN, Mwangi TW, Roberts DJ, et al. An immune basis for malaria protection by the sickle cell trait. PLoS Med. 2005;2:e128. doi: 10.1371/journal.pmed.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–7. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 18.Cornille-Brogger R, Fleming AF, Kagan I, Matsushima T, Molineaux L. Abnormal haemoglobins in the Sudan savanna of Nigeria. II. Immunological response to malaria in normals and subjects with sickle cell trait. Ann Trop Med Parasitol. 1979;73:173–83. doi: 10.1080/00034983.1979.11687244. [DOI] [PubMed] [Google Scholar]
- 19.Edozien JC, Boyo AE, Morley DC. The relationship of serum gamma-globulin concentration to malaria and sickling. J Clin Pathol. 1960;13:118–23. doi: 10.1136/jcp.13.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Hesran JY, Personne I, Personne P, et al. Longitudinal study of Plasmodium falciparum infection and immune responses in infants with or without the sickle cell trait. Int J Epidemiol. 1999;28:793–8. doi: 10.1093/ije/28.4.793. [DOI] [PubMed] [Google Scholar]
- 21.Allen SJ, Bennett S, Riley EM, et al. Morbidity from malaria and immune responses to defined Plasmodium falciparum antigens in children with sickle cell trait in The Gambia. Trans R Soc Trop Med Hyg. 1992;86:494–8. doi: 10.1016/0035-9203(92)90083-o. [DOI] [PubMed] [Google Scholar]
- 22.Aucan C, Traore Y, Tall F, et al. High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Infect Immun. 2000;68:1252–8. doi: 10.1128/iai.68.3.1252-1258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luty AJ, Ulbert S, Lell B, et al. Antibody responses to Plasmodium falciparum: Evolution according to the severity of a prior clinical episode and association with subsequent reinfection. Am J Trop Med Hyg. 2000;62:566–72. doi: 10.4269/ajtmh.2000.62.566. [DOI] [PubMed] [Google Scholar]
- 24.Dziegiel M, Rowe P, Bennett S, et al. Immunoglobulin M and G antibody responses to Plasmodium falciparum glutamate-rich protein: Correlation with clinical immunity in Gambian children. Infect Immun. 1993;61:103–8. doi: 10.1128/iai.61.1.103-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verra F, Simpore J, Warimwe GM, et al. Haemoglobin C and S role in acquired immunity against Plasmodium falciparum malaria. PLoS One. 2007;2:e978. doi: 10.1371/journal.pone.0000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardner MJ, Hall N, Fung E, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsh K, Otoo L, Hayes RJ, Carson DC, Greenwood BM. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 28.Cabrera G, Cot M, Migot-Nabias F, Kremsner PG, Deloron P, Luty AJ. The sickle cell trait is associated with enhanced immunoglobulin G antibody responses to Plasmodium falciparum variant surface antigens. J Infect Dis. 2005;191:1631–8. doi: 10.1086/429832. [DOI] [PubMed] [Google Scholar]
- 29.Crompton PD, Traore B, Kayentao K, et al. Sickle cell trait is associated with a delayed onset of malaria: implications for time-to-event analysis in clinical studies of malaria. J Infect Dis. 2008;198:1265–75. doi: 10.1086/592224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dicko A, Klion AD, Thera MA, et al. The etiology of severe anemia in a village and a periurban area in Mali. Blood. 2004;104:1198–200. doi: 10.1182/blood-2003-11-3884. [DOI] [PubMed] [Google Scholar]
- 31.Crompton PD, Kayala MA, Traore B, et al. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc Natl Acad Sci U S A. 2010;107:6958–63. doi: 10.1073/pnas.1001323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doolan DL, Mu Y, Unal B, et al. Profiling humoral immune responses to P. falciparum infection with protein microarrays. Proteomics. 2008;8:4680–94. doi: 10.1002/pmic.200800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Florens L, Washburn MP, Raine JD, et al. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419:520–6. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 34.Le Roch KG, Zhou Y, Blair PL, et al. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301:1503–8. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- 35.Florens L, Liu X, Wang Y, et al. Proteomics approach reveals novel proteins on the surface of malaria-infected erythrocytes. Mol Biochem Parasitol. 2004;135:1–11. doi: 10.1016/j.molbiopara.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Marti M, Good RT, Rug M, Knuepfer E, Cowman AF. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science. 2004;306:1930–3. doi: 10.1126/science.1102452. [DOI] [PubMed] [Google Scholar]
- 37.Hiller NL, Bhattacharjee S, van Ooij C, et al. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306:1934–7. doi: 10.1126/science.1102737. [DOI] [PubMed] [Google Scholar]
- 38.Cham GK, Turner L, Kurtis JD, et al. Hierarchical, domain type-specific acquisition of antibodies to Plasmodium falciparum erythrocyte membrane protein 1 in Tanzanian children. Infect Immun. 2010;78:4653–9. doi: 10.1128/IAI.00593-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackintosh CL, Christodoulou Z, Mwangi TW, et al. Acquisition of naturally occurring antibody responses to recombinant protein domains of Plasmodium falciparum erythrocyte membrane protein 1. Malar J. 2008;7:155. doi: 10.1186/1475-2875-7-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su XZ, Heatwole VM, Wertheimer SP, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 41.Ntoumi F, Ekala MT, Makuwa M, Lekoulou F, Mercereau-Puijalon O, Deloron P. Sickle cell trait carriage: imbalanced distribution of IgG subclass antibodies reactive to Plasmodium falciparum family–specific MSP2 peptides in serum samples from Gabonese children. Immunol Lett. 2002;84:9–16. doi: 10.1016/s0165-2478(02)00131-1. [DOI] [PubMed] [Google Scholar]
- 42.Matondo Maya DW, Mavoungou E, Deloron P, Theisen M, Ntoumi F. Distribution of IgG subclass antibodies specific for Plasmodium falciparum glutamate-rich-protein molecule in sickle cell trait children with asymptomatic infections. Exp Parasitol. 2006;112:92–8. doi: 10.1016/j.exppara.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 43.Ferreira A, Marguti I, Bechmann I, et al. Sickle hemoglobin confers tolerance to Plasmodium infection. Cell. 2011;145:398–409. doi: 10.1016/j.cell.2011.03.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.