Abstract
Background. Proinflammatory cytokines play a critical role in antiviral immune responses. Large-scale genome studies have found correlations between single-nucleotide polymorphisms (SNPs) in the interleukin (IL) 18 promoter and spontaneous control of hepatitis C virus (HCV), suggesting a role in clearance.
Methods. Plasma IL-18, IL-1β, IL-6, IL-8, IL-12, interferon-γ, tumor necrosis factor–α, alanine aminotransferase (ALT), and HCV RNA levels were assessed longitudinally in subjects with known dates of HCV acquisition and analyzed according to IL-18 SNPs and outcome, either spontaneous clearance (SC) (n = 13) or persistent infection (PI) (n = 25).
Results. No significant change in plasma proinflammatory cytokine expression was observed with the exception of IL-18, which increased in every subject with initial detection of HCV RNA. In every SC subject, IL-18 returned to the preinfection baseline concomitant with HCV control. In PI subjects, IL-18 declined following the acute phase of infection but remained above the preinfection baseline throughout chronic infection and did not correlate with HCV RNA or ALT levels.
Conclusions. Plasma IL-18 was an early and the most reliably detected host response to HCV infection measured in blood. Reduced IL-18 production with transition to chronic infection without correlation with HCV RNA or ALT levels suggests modulation of the innate response with persistent infection.
Hepatitis C virus (HCV) is among the major etiologic agents of chronic liver disease. An estimated 170 million chronic infections with this virus occur worldwide, with a 1%–2% prevalence rate in most countries [1]. HCV evades clearance mechanisms and establishes persistent infection (PI) in the majority of infections, leading to cirrhosis in 5%–25% and hepatocellular carcinoma in 2% of infected persons [2, 3]. The outcome of infection is determined by early events in the virus–host interaction because failure to control HCV within the first 120 days of infection is strongly associated with PI [3]. Unfortunately, investigating early HCV pathogenesis and the mechanism underlying spontaneous control of HCV has been severely hindered by the difficulty in identifying persons acutely infected with HCV because most are asymptomatic during this period [3].
Proinflammatory cytokines, including interleukin (IL) 18, have been shown to play a role in control of viral infections [4]. In murine models of herpes simplex virus and vaccinia virus infections, IL-18 deficiency generated either by gene knockout or cytokine neutralization renders animals more susceptible to infection [5–7]. In humans, along with other inflammatory cytokines, IL-18 is elevated in persons with uncontrolled HIV and falls as the virus is suppressed by antiretroviral therapy [8, 9]. Associations between IL-18 gene polymorphisms and hepatitis B virus clearance have been observed in humans [10], and IL-18 has been shown to inhibit HBV replication in vivo in the livers of transgenic mice [11].
IL-18 is a member of the IL-1 cytokine family and is structurally related to IL-1β, and both are associated with the inflammasome [12]. IL-18 favors Th1 polarization and induces the expression of other proinflammatory cytokines, including nitric oxide, Fas ligand, and several chemokines. In addition to its role in infectious diseases, elevated serum IL-18 levels have been found in patients with autoimmune diseases, acute graft-versus-host disease, hematological malignancies, sepsis, and inflammatory liver diseases, suggesting a fundamental role in immunity [4].
The role of IL-18 in the pathogenesis of HCV remains unclear. Genetic associations between single-nucleotide polymorphisms (SNPs) in the IL-18 promoter (A/A or C/A genotype at [–607] rs1946518 and G/C or C/C genotype at [–137] rs187238) and spontaneous HCV clearance have been reported in African-American injection drug users (IDUs) [13]. In small cross-sectional studies, serum IL-18 levels 3–4 times higher than those in healthy controls have been observed in persons with chronic HCV and cirrhosis due to HCV [14–17]. The levels appear to decrease after successful treatment with interferon (IFN)-α–containing regimens [17]. In one study, subjects with HCV also had higher IL-18 levels than did those with nonalcoholic fatty liver disease, a nonviral cause of liver disease [15]. Additional cytokine-associated SNPs associated with increased likelihood of spontaneous HCV clearance include rs3750912 (IL-18 binding protein) [18] and the C/C haplotype at rs12979860 SNP (IL-28B) [19]. However, the mechanism underlying these associations remains unclear.
The present study was undertaken to determine the point during HCV infection at which IL-18 becomes elevated, whether plasma levels of other proinflammatory cytokines are altered in HCV-infected individuals, whether serum IL-18 levels differ before and during HCV infection based on SNP polymorphisms in the IL-18 promoter, and whether serum IL-18 level correlates with HCV RNA levels, liver inflammation, or outcome of infection.
METHODS
Study Participants
The incidence, immunology, and virology of HCV infection are being examined prospectively among young IDUs enrolled in the Baltimore Before and After Acute Study of Hepatitis (BBAASH). HCV-seronegative, RNA-negative persons at risk for HCV infection because of active injection drug use are invited to enroll. Those who consent are provided with counseling to reduce the risks of drug use, and monthly blood samples are collected as previously described [3]. The study protocol was approved by the institutional review board of the Johns Hopkins School of Medicine. Between 1997 and 2010, 147 subjects acquired HCV infection. HCV RNA measurements are used to identify the time of infection and to determine the outcome of infection. All participants with acute HCV infection are referred for evaluation and treatment if indicated. From this group, we selected a subset of 13 subjects with spontaneous clearance (SC), 25 with persistent infection (PI), and 13 who were reinfected after spontaneously clearing their initial HCV infection for the study of proinflammatory cytokine expression. The subjects included had plasma available for all time points to allow 2:1 matching of PI to SC and were treatment naive. Each subject in the cohort with known outcome of infection and plasma available from time points prior to infection, within the first 6 months and at 12 months of infection, was assessed to avoid selection bias.
HCV RNA Assays
Total RNA was extracted from serum using a Qiagen MinElute Virus column (Qiagen) per the manufacturer’s instructions. HCV RNA concentration in blood samples was determined using a quantitative reverse-transcription polymerase chain reaction assay with TaqMan HCV analyte-specific reagent (Roche Applied Science). The generation of DNA amplification products was monitored using a Cobas TaqMan analyzer (Roche Applied Science). This assay has a lower limit of detection (LLOD) of 50 IU/mL; all samples between 0 and 50 IU/mL were assigned a value of 25 IU/mL.
Alanine Aminotransferase Assay
Plasma alanine aminotransferase (ALT) level was determined by the Johns Hopkins Hospital clinical laboratory.
IL-18 Measurement
Plasma IL-18 was measured using the human IL-18 Platinum enzyme-linked immunosorbent assay (eBiosciences). The assay was performed per the manufacturer’s recommendations using 50 μL of plasma from each study time point. Each sample was tested in duplicate and the average calculated. Data were acquired using a SpectaMax M5 (Molecular Devices). The LLOD of IL-18 in plasma samples is 40 pg/mL.
Cytokine Measurement
The Meso Scale Discovery multispot assay was used to measure IL-1β, IL-6, IL-8, IL-12 p70, IFN-γ, and tumor necrosis factor (TNF)–α levels. The assay was performed per the manufacturer’s recommendations using 25 μL of plasma from each time point. Data were acquired on a SECTOR Imager SI2400. Results were analyzed using Meso Scale Discovery Workbench software. The LLOD is 0.5 pg/mL for each cytokine.
IL-18 Allelic Discrimination
Peripheral blood mononuclear cells were isolated by Ficoll-Hypaque centrifugation of blood samples from BBAASH subjects. DNA was extracted using QIAamp DNA Blood Mini Kit (Qiagen). IL-18 SNPs rs187238 and rs1946518 were genotyped using a commercial TaqMan SNP genotyping assay from Applied Biosystems). IL-28B SNP rs12979860 was genotyped using a custom TaqMan SNP genotyping assay (Applied Biosystems). See Supplementary Table for primers and probes or context sequences, where applicable, and PCR profile. Assays contained unlabeled PCR primers and hydrolysis probes labeled with either FAM® or VIC® fluorescent dyes. The Roche LightCycler 480 System (Roche Applied Science) was used to measure the fluorescence from each reaction. Genotype was determined using Roche’s software for allelic discrimination.
Statistical Analysis
The Wilcoxon rank sum test and paired t test were used to evaluate statistically significant differences between groups. Differences were considered statistically significant when P < .05.
RESULTS
Proinflammatory Cytokine IL-18 Is Strongly Induced During Acute HCV Infection
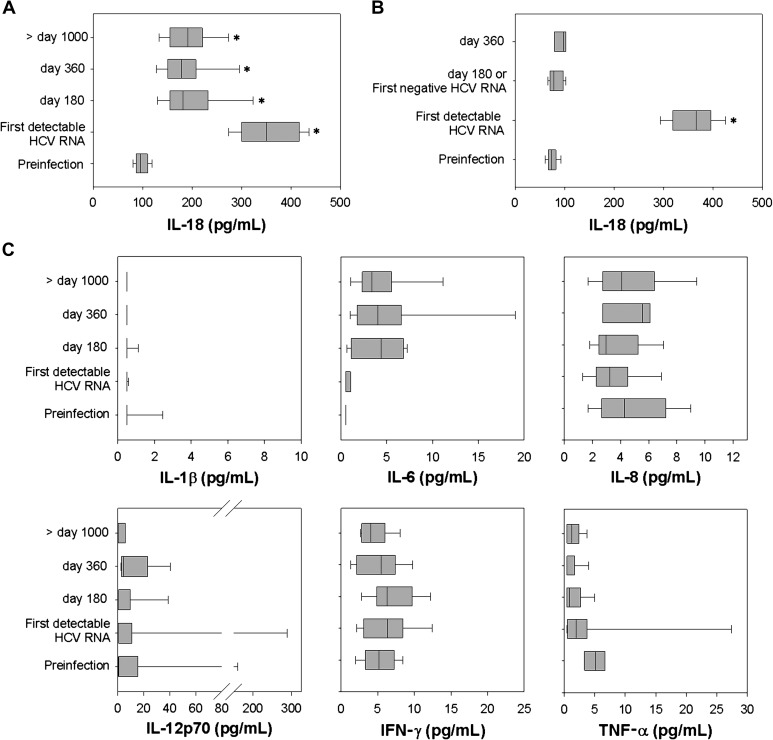
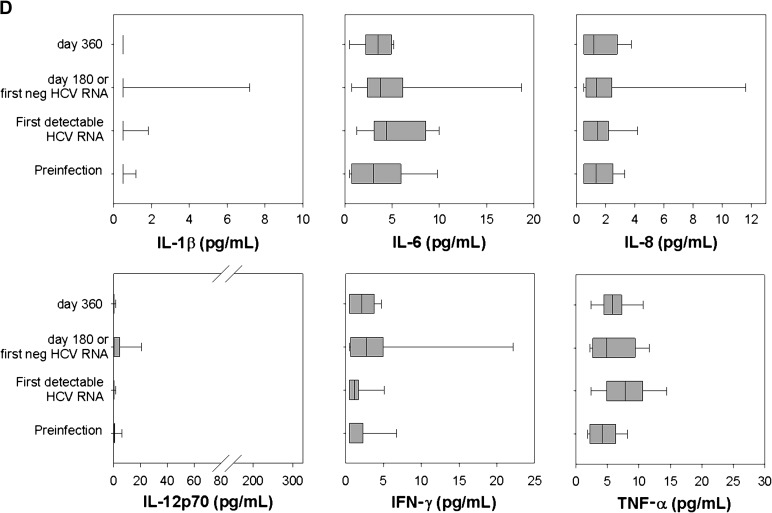
Compared to preinfection levels, plasma IL-18 rose sharply with the first detection of HCV in every subject (Figure 1A and 1B). IL-18 concentration rose from 98 ± 14 pg/mL preinfection to 353 ± 65 pg/mL at its peak in the PI group and from 72 ± 12 mg/mL to 361 ± 41 mg/mL in the SC group. IL-18 levels at initial viremia did not differ significantly between the PI group and SC group (P = .74). Thus, although HCV infection induces IL-18 production, the level produced in acute infection is not associated with the outcome of infection. The inflammasome-associated cytokine IL-1β did not increase with HCV infection, and mean values were not statistically different preinfection, at first detectable RNA, or on day 180 or day 360 after infection in PI and SC (Figure 1C and 1D). Only 4% of all plasma IL-1β measurements were above the LLOD. Similarly, no other proinflammatory cytokine showed statistically significant increases when preinfection time points were compared with the initial infection time point, or 180, 360, and >1000 days after infection (Figure 1C and 1D).
Figure 1.
Plasma interleukin (IL) 18 remains elevated in subjects with persistent hepatitis C virus (HCV) infection but returns to baseline with clearance. Plasma IL-18 concentration (pg/mL) was measured by enzyme-linked immunosorbent assay in 25 subjects who developed persistent HCV infection (A) and 13 subjects who spontaneously cleared HCV infection (B). A, Plasma IL-18 concentration at a preinfection time point, on the day of first detectable HCV RNA, at approximately day 180 (range, 150–202), day 360 (range, 348–392), and a time point >1000 days of positive HCV RNA (range, 1078–1741). Median, upper, and lower quartile ranges are shown; (*) denotes P < .05 compared with preinfection level. B, Plasma IL-18 concentration at a preinfection time point, on the day of first detectable HCV RNA, and at the first day at which HCV RNA becomes undetectable (174 ± 86 days). Plasma IL-1β, IL-6, IL-8, IL-12 p70, interferon (IFN)–γ, and tumor necrosis factor (TNF)–α concentrations (pg/mL) were measured at identical time points for subjects with persistent HCV infection (C) and subjects who spontaneously cleared HCV (D).
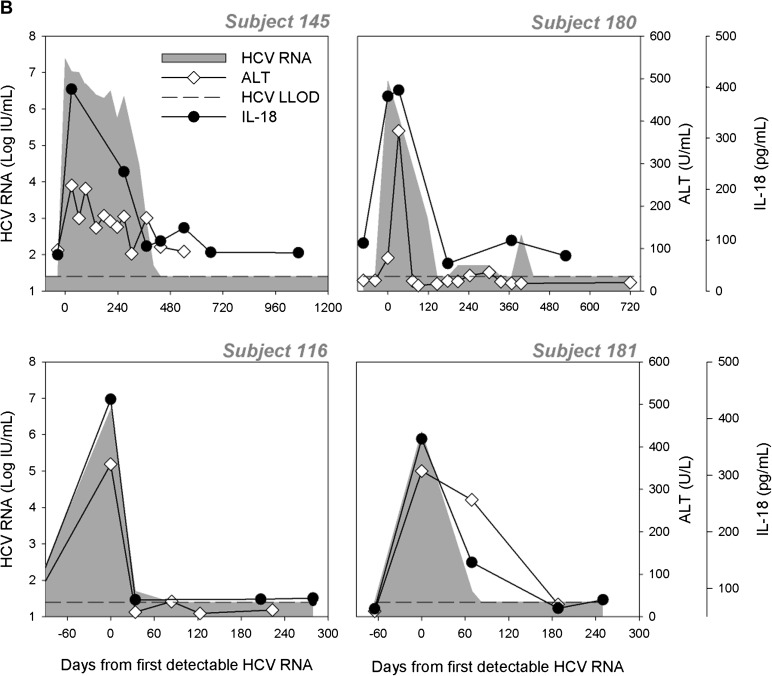
Plasma IL-18 Levels Decline With Viral Recovery But Not Persistence
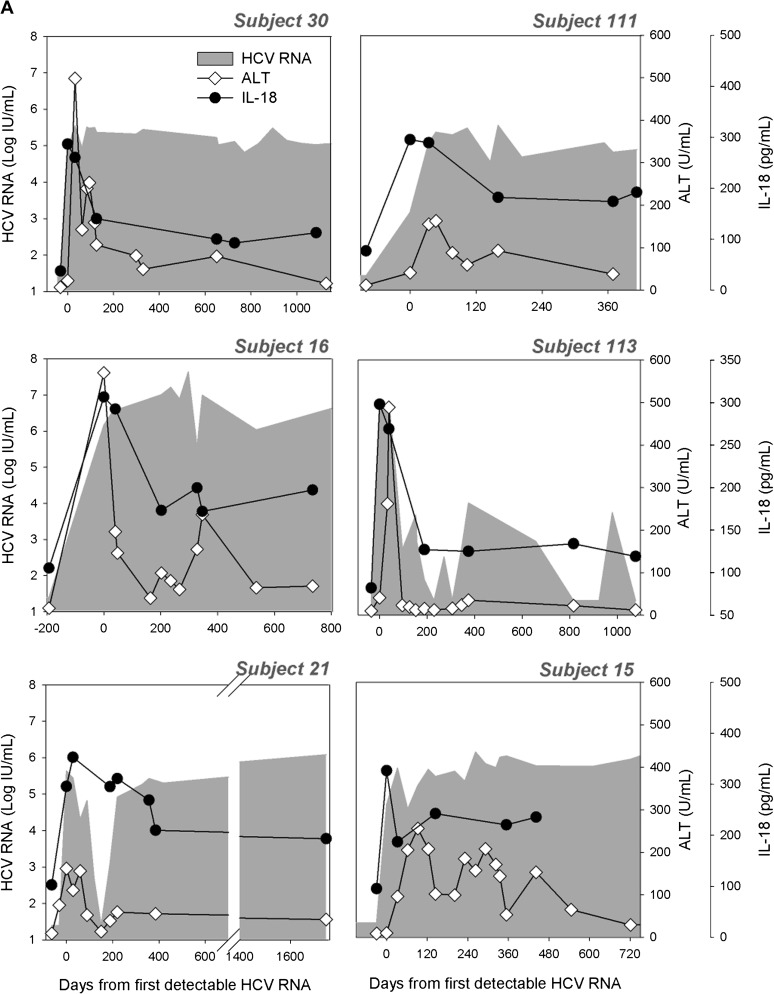
In SC subjects, plasma IL-18 rapidly returned to baseline at the first time point by which HCV RNA becomes undetectable (Figure 1B); mean IL-18 level following control of HCV infection was 79 ± 14 pg/mL and not differ statistically from preinfection in the same subjects. In contrast, IL-18 remained significantly elevated in PI subjects compared with preinfection levels after 180 days (192 ± 55 pg/mL, P < .001), 360 days (191 ± 58 pg/mL, P < .001), or 1000 days of infection (194 ± 45 pg/mL, P < .001; Figure 1A). The 180-day time point was chosen because it is close to the average number of days to first negative HCV RNA in the SC group. Levels at 180, 360, and 1000 days of infection are also statistically lower than at first detectable RNA. The kinetics of IL-18 relative to HCV RNA and ALT during the course of infection is shown for PI (Figure 2A and Supplementary Figure 1A) and SC subjects (Figure 2B and Supplementary Figure 1B). IL-18 and ALT increases are temporally correlated with the appearance of HCV in the plasma, but there is no correlation between absolute HCV RNA and IL-18 levels (Supplementary Figure 2) during early infection or PI. Instead, IL-18 was maintained within a narrow range despite fluctuations in HCV RNA levels and widely different HCV RNA levels in persistent infection. For example, subject 113 had wide variation in HCV RNA level from day 400 to day 1200 of infection, but IL-18 was maintained in a very narrow range (within 20 pg/mL) for that period (Figure 2A). For subjects with high HCV RNA levels, IL-18 is maintained at high levels for some and at low levels for others. There was also no correlation between IL-18 levels and ALT levels.
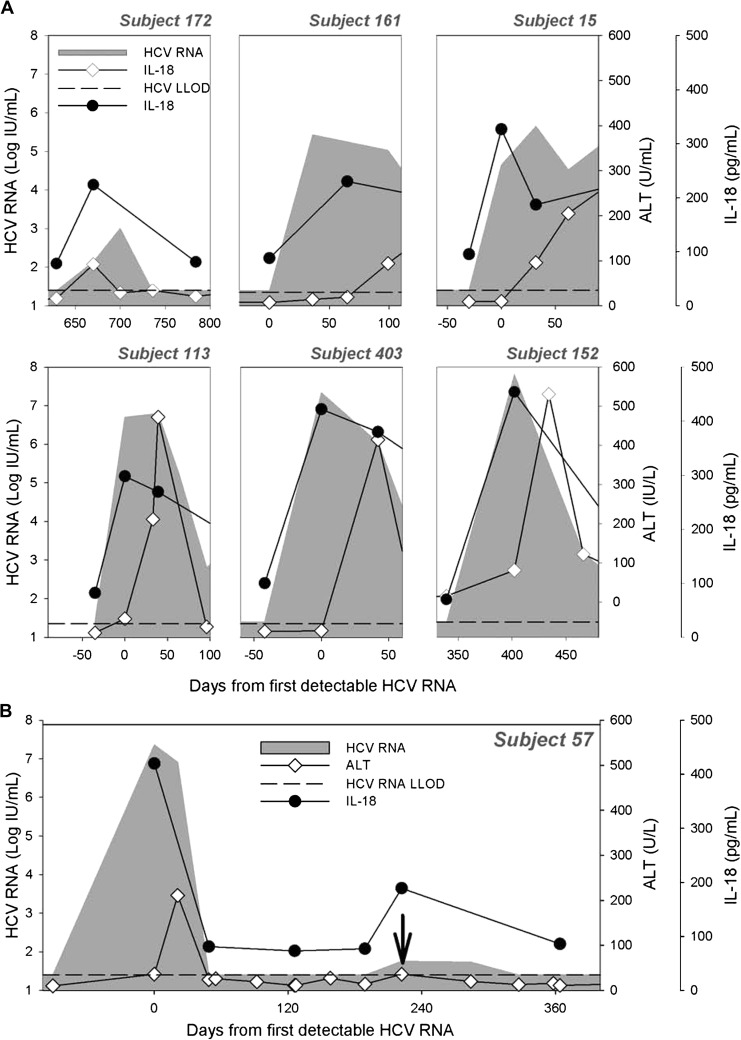
Figure 2.
Longitudinal measurement of plasma interleukin (IL) 18 in subjects with persistent hepatitis C virus (HCV) infection, spontaneous clearance, and reinfection. Plasma IL-18 concentration (pg/mL) was measured by enzyme-linked immunosorbent assay in 25 subjects who developed persistent HCV infection (A) and 13 subjects who spontaneously cleared HCV infection (B). Representative longitudinal data for 6 subjects with persistent infection (PI) (A) and 4 subjects with spontaneous clearance (B) comparing alanine aminotransferase (ALT [◊]) and plasma IL-18 concentration (•) with HCV RNA (shaded). IL-18 was also measured longitudinally in subjects who cleared their primary HCV infection and were subsequently reinfected. Representative data for 3 subjects are shown (C). Time is plotted relative to the day of first detectable HCV RNA. The lower limit of detection for HCV RNA is 25 IU/mL (dashed horizontal line).
IL-18 Responses to HCV Infection Are Not Extinguished With Repeated Exposure to HCV
Plasma IL-18 was also measured longitudinally in 13 subjects who were subsequently infected after clearing an initial HCV infection (reinfection, as previously defined [20]). This was compared with HCV RNA and ALT levels. Data for 3 representative subjects are shown in Figure 2C; initial infection is associated with a rise in plasma IL-18 concentration. This rise occurs early in infection and returns to the baseline level as the HCV RNA becomes undetectable. With each subsequent reinfection, plasma IL-18 rises with the detection of new HCV RNA. The rise in IL-18 is apparent at the first measurable HCV RNA of the reinfection, even with HCV RNA levels that are extremely low. The level to which IL-18 rises is similar regardless of the number of infections, and, in all cases, plasma IL-18 returns to the baseline as HCV RNA is cleared from the blood.
Figure 3.
IL-18 is a sensitive indicator of hepatitis C virus (HCV) infection. Plasma IL-18 concentration (•), alanine aminotransferase (ALT [◊]), and HCV RNA (shaded) at the onset of initial HCV infection are shown for 6 representative subjects in which ALT elevation occured later than initial detection of HCV RNA (A). Sensitivity of IL-18 in detecting reinfection events is shown in (B). Subject 57 was reinfected at day 222 (vertical arrow); an increase in plasma IL-18 (•) is shown, whereas no significant change in ALT (◊) at this reinfection is shown.
IL-18 Production May Be an Earlier and More Sensitive Marker of HCV Infection Than ALT Elevation
As shown in Figures 1 and 2, IL-18 rises early in the course of infection regardless of clinical outcome. Because IL-18 levels uniformly increase with the earliest detection of HCV RNA, the rise in IL-18 precedes ALT elevation in subjects where ALT elevation is delayed. This is demonstrated for subjects 15, 113, 161, and 403, where IL-18 rises as soon as HCV RNA is detectable but ALT elevation is not observed until later (Figure 3A). This suggests that IL-18 is produced in response to HCV infection rather than as a result of subsequent liver inflammation, as measured by ALT elevation, and may be an earlier marker of HCV infection than is transaminitis. This phenomenon is also seen during HCV reinfection where IL-18 elevation may precede ALT elevation (subject 152) and may occur in the absence of significant ALT elevation (Figure 3B). In some cases, IL-18 may be an indicator of a new infection even when ALT levels are not elevated, as in subject 57 who was reinfected around day 200 with a peak HCV of 57 IU/mL. Overall, IL-18 elevation preceded ALT elevation in 43% of PI subjects by 19 ± 91 days and 47% of SC subjects by 17 ± 6 days. IL-18 elevation also preceded HCV seroconversion by 23 days and 22 days, respectively, in 45% of PI subjects and 51% of SC subjects (data not shown). ALT elevation did not precede IL-18 elevation in any subjects.
Genetic Polymorphisms and IL-18 Production in HCV Infection
In an effort to begin exploring the relationship between IL-18 promoter SNPs and protein expression we genotyped all subjects for the IL-18 promoter SNPs –137G/C (rs187238) and –607C/A (rs1946518), as well as the rs12979860 SNP in IL-28B, which has previously been associated with spontaneous clearance. We found that the baseline/preinfection plasma IL-18 level was not different among subjects with various IL-18 and IL-28B SNP genotypes. Further, the peak level of IL-18 appears to be independent of the IL-18 and IL-28B SNP genotypes such that the IL-18 concentration observed at the time point of first detectable HCV RNA is not different among genotypes (Table 1).
Table 1.
Relationship Between Interleukin (IL) 18 and IL-28B Single-Nucleotide Polymorphisms and Plasma IL-18a
| Persistent infection |
Spontaneous clearance |
||||
| SNP | Preinfection IL-18 (pg/mL) | Peak IL-18 (pg/mL) | Preinfection IL-18 (pg/mL) | Peak IL-18 (pg/mL) | |
| rs187238 (−137) | GG | 102 ± 15 | 323 ± 76 | 71 ± 14 | 360 ± 52 |
| GC + CC | 92 ± 11 | 349 ± 68 | 74 ± 9 | 348 ± 54 | |
| rs1946518 (−607) | CC | 105 ± 17 | 301 ± 66 | 73 ± 16 | 368 ± 59 |
| CA + AA | 94 ± 11 | 360 ± 67 | 73 ± 9 | 347 ± 48 | |
| rs12979860 | CC | 94 ± 13 | 363 ± 77 | 75 ± 10 | 351 ± 54 |
| CT + TT | 101 ± 15 | 318 ± 64 | 92 ± 9 | 368 ± 46 | |
Abbreviations: HCV, hepatitis C virus; IL, interleukin; SNP, single-nucleotide polymorphism.
At preinfection and at the time point at which peak IL-18 was detected in subjects with chronic HCV infection and in subjects who spontaneously cleared HCV infection.
We also assessed the correlation between SNPs in the IL-18 binding protein (rs3750912) and IL-1β (rs1424860), which have previously been identified in large-scale genome studies as associated with clearance. However, we found these to be very homogenous within the study cohort (data not shown) and thus unlikely to have an impact on the observations presented above.
DISCUSSION
The virus–host interaction during the earliest stages of infection determines the outcome of HCV infection. Although studies of acute HCV infection in humans are limited, analysis of the gene expression profiles from liver tissue of acutely infected chimpanzees suggests that HCV triggers many IFN-stimulated genes and a strong type 1 IFN response [21, 22]. In multiple infection models, induction and secretion of type 1 IFNs occur after recognition of pathogen-associated molecular patterns by pattern recognition receptors. Previous studies have identified several mechanisms by which HCV may be recognized by the immune system, including sensing of double-stranded RNA by retinoic acid inducible gene I, Toll-like receptor 3 [23], and protein kinase R [24]. Recently, new mechanisms of viral recognition have emerged, including the recently characterized nucleotide-binding oligomerization domain–like receptors, a large family of cytosolic pattern recognition receptors associated with inflammasome activation. Inflammasomes have been shown to initiate signaling cascades that lead to the production of proinflammatory cytokines, including IL-18 and IL-1β, which amplify the antiviral innate immune response [25]. Previous studies have identified an association between IL-18 SNPs and likelihood of HCV clearance; however, the mechanism of this association is not understood. In this study, we explored the relationship among HCV infection, proinflammatory cytokines, and the inflammasome-associated cytokines IL-18 and IL-1β.
Longitudinal measurement of circulating IL-18 suggests that IL-18 is an early marker of HCV infection and a sensitive indicator of persistent infection (Figure 1A and 1B). The pattern of IL-18 production in HCV infection suggests 3 phases. First, in each subject followed from preinfection to infection, a characteristic, approximately 4-fold rise in IL-18 coincided with the detection of HCV RNA in the blood. This occurred before elevation in serum ALT in approximately 45% of subjects (Figure 3), suggesting that IL-18 is among the earliest immune responses to the virus. The initial rise in IL-18 was observed regardless of clinical outcome, and there was no correlation between peak IL-18 level and the outcome of infection (Figure 1).
Regardless of clinical outcome, during acute infection there is a second phase during which IL-18 is modulated and declines. This decline begins around the time of peak viremia and occurs despite stably high or increasing HCV titers. This suggests that IL-18 is not produced solely in response to HCV but is subject to additional control mechanisms. Alternatively, this phenomenon may suggest that the host response becomes refractory to continued HCV replication or that the inflammatory response to HCV is altered with persistence.
The third phase is observed in PI subjects. IL-18 remains elevated at a level 2- to 3-fold above the subject’s preinfection baseline and appears to maintain a steady level despite fluctuations in HCV RNA in the blood. The level is not correlated with HCV RNA level or ALT levels. It is unclear what mediates the continued lower-level production and why the host does not respond to fluctuations in HCV titer as in the first phase. In SC subjects, IL-18 returns to baseline by the time HCV RNA becomes undetectable. This pattern of response was unique among all proinflammatory cytokines tested, including IL-1β, another inflammasome-associated cytokine.
In reinfected subjects, IL-18 increases, as with primary infection, with each subsequent reinfection and returns to baseline as each infection is cleared (Figure 2C). As described previously, peak HCV RNA is often lower with reinfection and ALT can rise earlier in infection, suggesting an anamnestic response to the virus [20]. Interestingly, the peak IL-18 observed during reinfection in several subjects closely matched the level observed during the primary infection despite a lower peak HCV RNA level in the reinfections. The observed variation in IL-18 levels in the different stages of HCV infection required frequent longitudinal sampling and has not previously been described in any infection.
Finally, previous reports have found an association between polymorphisms in the IL-18 promoter region or binding protein and the outcome of infection [13, 19]. In one study, the IL-18 promoter SNP genotypes –137G/C or C/C (rs187238) and –607C/A or A/A (rs1946518) were associated with HCV clearance [13]. We genotyped all subjects in this cohort and found no association between IL-18 SNP genotype and peak IL-18 level (see Table 1), suggesting that the polymorphism may not exert its effect by altering IL-18 production in response to HCV infection. There was also no strong association between the presence of the protective allele and clearance, but the small number of subjects studied may limit this analysis. SNP-type associations with disease outcome usually require subject numbers in the hundreds or thousands for detection to occur because having a polymorphism does not perfectly correlate with outcome but rather increases the risk of an outcome. However, because the number of subjects was sufficient to show significantly different levels of IL-18 between cleared and chronically infected subjects, differences based on SNP types must not be as large as those based on outcome. In addition, the racial composition of the study subjects is predominantly Caucasian and therefore does not match that of the predominantly African-American IDUs in which this association was observed. Similar to our findings, a large genome-wide association study of Caucasians also failed to find the association between IL-18 SNP and outcome of HCV infection [26]. The rs12979860 SNP (IL-28B) genotype was also compared with peak IL-18 level, and no association was found between IL-28B SNP type and IL-18 production (Table 1).
Another limitation to this analysis is that we assess plasma cytokine levels, not the levels in the liver and the environment that hepatocytes experience. There may be a direct correlation between blood and tissue levels for some cytokines, but we cannot exclude the possibility that a cytokine is in high local concentration with such a strong gradient that the levels at distant sites may be very low. Thus, the SNP types may result in levels that differ in the liver, but these differences are not detectable in the plasma. It is assumed that a higher cytokine concentration suggests a higher likelihood of biologic activity. However, because we do not have a functional assay for IL-18, we cannot make inferences regarding the biologic activity of IL-18 at target sites. Correlation of local concentration and measurements at distal sites is particularly interesting for cytokines such as IL-1β, which is thought to be activated in the same manner as IL-18 and thus might be produced in response to infection of the liver. Although the lack of concordance between IL-18 and IL-1β could be due to different sensitivity of the assays or differential degradation or binding, it raises the intriguing possibility that HCV may selectively induce IL-18 or alternatively that HCV may suppress IL-1β.
In summary, IL-18 elevation occurs uniformly with initial HCV detection. In cases where ALT elevation lags initial viremia, IL-18 elevation occurs before the rise in ALT, making IL-18 the earliest and most reliable measure of host response to HCV infection in blood. IL-18 does not return to baseline but declines in those with persistent infection. We do not know what controls the dampening of IL-18 production in response to HCV infection with persistent viremia. Although fluctuations in HCV RNA in chronic infection do not result in dramatic changes in IL-18 levels, exposure to a new HCV after clearance of an initial infection results uniformly in robust increases in IL-18, even when the amount of new HCV is barely measureable. Thus, the initial exposure to HCV does not render people incapable of producing IL-18 in response to HCV; rather, it is continued exposure in chronic HCV infection that results in decreased IL-18 production. Evasion of innate activation is an active area of interest in HCV infection, and the mechanisms responsible for the induction of and subsequent reduction in IL-18 signaling with progression to chronic HCV infection should be further investigated.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support.
This work was supported by National Institutes of Health grants U19 AI09025, R0113324, and R01AI077757.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.WHO. Hepatitis C. Global prevalence. Wkly Epidemiol Rec. 1997;72:341–4. [PubMed] [Google Scholar]
- 2.Ray S, Thomas D, Hepatitis C. Principles and practices of infectious diseases. Vol 2. Philadelphia, PA: Churchill Livingstone Elsevier; 2010. pp. 2157–85. [Google Scholar]
- 3.Cox AL, Netski DM, Mosbruger T, et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Infect Dis. 2005;40:951–8. doi: 10.1086/428578. [DOI] [PubMed] [Google Scholar]
- 4.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 5.Barr DP, Belz GT, Reading PC, et al. A role for plasmacytoid dendritic cells in the rapid IL-18-dependent activation of NK cells following HSV-1 infection. Eur J Immunol. 2007;37:1334–42. doi: 10.1002/eji.200636362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reading PC, Whitney PG, Barr DP, et al. IL-18, but not IL-12, regulates NK cell activity following intranasal herpes simplex virus type 1 infection. J Immunol. 2007;179:3214–21. doi: 10.4049/jimmunol.179.5.3214. [DOI] [PubMed] [Google Scholar]
- 7.Reading PC, Smith GL. Vaccinia virus interleukin-18-binding protein promotes virulence by reducing gamma interferon production and natural killer and T-cell activity. J Virol. 2003;77:9960–8. doi: 10.1128/JVI.77.18.9960-9968.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe D, Uehira T, Yonemoto H, et al. Sustained high levels of serum interferon-gamma during HIV-1 infection: a specific trend different from other cytokines. Viral Immunol. 2010;23:619–25. doi: 10.1089/vim.2010.0065. [DOI] [PubMed] [Google Scholar]
- 9.Iannello A, Boulassel MR, Samarani S, et al. HIV-1 causes an imbalance in the production of interleukin-18 and its natural antagonist in HIV-infected individuals: implications for enhanced viral replication. J Infect Dis. 2010;201:608–17. doi: 10.1086/650314. [DOI] [PubMed] [Google Scholar]
- 10.Cheong JY, Cho SW, Oh B, et al. Association of interleukin-18 gene polymorphisms with hepatitis B virus clearance. Dig Dis Sci. 2010;55:1113–9. doi: 10.1007/s10620-009-0819-z. [DOI] [PubMed] [Google Scholar]
- 11.Kimura K, Kakimi K, Wieland S, Guidotti LG, Chisari FV. Interleukin-18 inhibits hepatitis B virus replication in the livers of transgenic mice. J Virol. 2002;76:10702–7. doi: 10.1128/JVI.76.21.10702-10707.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 13.An P, Thio CL, Kirk GD, Donfield S, Goedert JJ, Winkler CA. Regulatory polymorphisms in the interleukin-18 promoter are associated with hepatitis C virus clearance. J Infect Dis. 2008;198:1159–65. doi: 10.1086/592047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma A, Chakraborti A, Das A, Dhiman RK, Chawla Y. Elevation of interleukin-18 in chronic hepatitis C: implications for hepatitis C virus pathogenesis. Immunology. 2009;128:e514–22. doi: 10.1111/j.1365-2567.2008.03021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vecchiet J, Falasca K, Cacciatore P, et al. Association between plasma interleukin-18 levels and liver injury in chronic hepatitis C virus infection and non-alcoholic fatty liver disease. Ann Clin Lab Sci. 2005;35:415–22. [PubMed] [Google Scholar]
- 16.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoneda S, Umemura T, Katsuyama Y, et al. Association of serum cytokine levels with treatment response to pegylated interferon and ribavirin therapy in genotype 1 chronic hepatitis C patients. J Infect Dis. 2011;203:1087–95. doi: 10.1093/infdis/jiq165. [DOI] [PubMed] [Google Scholar]
- 18.Mosbruger TL, Duggal P, Goedert JJ, et al. Large-scale candidate gene analysis of spontaneous clearance of hepatitis C virus. J Infect Dis. 2010;201:1371–80. doi: 10.1086/651606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osburn WO, Fisher BE, Dowd KA, et al. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2010;138:315–24. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bigger CB, Brasky KM, Lanford RE. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J Virol. 2001;75:7059–66. doi: 10.1128/JVI.75.15.7059-7066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bigger CB, Guerra B, Brasky KM, et al. Intrahepatic gene expression during chronic hepatitis C virus infection in chimpanzees. J Virol. 2004;78:13779–92. doi: 10.1128/JVI.78.24.13779-13792.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meurs EF, Breiman A. The interferon inducing pathways and the hepatitis C virus. World J Gastroenterol. 2007;13:2446–54. doi: 10.3748/wjg.v13.i17.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Post J, Ratnarajah S, Lloyd AR. Immunological determinants of the outcomes from primary hepatitis C infection. Cell Mol Life Sci. 2009;66:733–56. doi: 10.1007/s00018-008-8270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol. 2010;10:688–98. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rauch A, Kutalik Z, Descombes P, et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010;138:1338–45. doi: 10.1053/j.gastro.2009.12.056. 1345 e1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.