Abstract
Paraquat (1,1'-dimethyl-4,4'-bipyridinium dichloride; PQ), an effective and widely used herbicide, was commercially introduced in 1962. It is reduced by the electron donor NADPH, and then reduced PQ transfers the electrons to molecular oxygen, resulting in the production of reactive oxygen species (ROS), which are related to cellular toxicity. However, the influence of continuous hypoxia on PQ-induced ROS production has not fully been investigated. We evaluated in vitro the protective effect of continuous hypoxia on PQ-induced cytotoxicity in the human carcinogenic alveolar basal epithelial cell line (A549 cells) by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay and live and dead assay, and by measuring lactate dehydrogenase (LDH) release. To elucidate the mechanism underlying this effect, we monitored the immunofluorescence of intracellular ROS and measured malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GPx) activities. Continuous hypoxia protected the A549 cells from PQ-induced cytotoxicity. Continuous hypoxia for a period of 24 h significantly reduced intracellular ROS, decreased MDA concentration in the supernatant, and normalized SOD and GPx activities. Continuous hypoxia attenuated PQ-induced cell toxicity in A549 cells. This protective effect might be attributable to the suppression of PQ-induced ROS generation.
Keywords: cell death, hypoxia, oxidative stress, paraquat, reactive oxygen species
Introduction
Paraquat (PQ, 1,1'-dimethy-4,4'-bipyridinium dichloride) is a common vegetative herbicide widely used globally since its introduction in the 1960s. To date, many cases of accidental or suicidal PQ poisoning have been reported. PQ and its poisoning have resulted in unfavorable outcomes for humans and is now becoming an important concern in clinical toxicology. A number of researches have demonstrated that the mechanism of PQ cytotoxicity entails interfering with the intracellular electron transfer systems, which generate the formation of superoxide anion, singlet oxygen, and other reactive oxygen species (ROS), leading to the depletion of cellular Nicotinamide adenine dinucleotide phosphate (NADPH) and lipid peroxidation of cell membranes (Smith et al., 1978; Bus and Gibson, 1984; Suntres, 2002; Yeh et al., 2006). Therefore, various antioxidants with scavenging activity, such as vitamin E, vitamin C, β-carotene, glutathione, N-acetylcysteine, melatonin, and the enzyme superoxide dismutase (SOD), have been studied and used for PQ poisoning therapy (Melchiorri et al., 1996; Suntres, 2002; Yeh et al., 2006). However, these antioxidant therapies yielded unsatisfactory results with respect to mortality.
The lung is considered the primary target organ for PQ toxicity as a consequence of the accumulation of PQ against a concentration gradient via the highly developed polyamine-uptake system (Dinis-Oliveira et al., 2006a, 2006b; Chang et al., 2009). The primary effect of PQ toxicity is seen in the lungs, where PQ gets accumulated via a process of active transport in the Clara cells and alveolar type I and type II epithelial cells, leading initially to pulmonary edema, infiltration of inflammatory cells, and damage to the alveolar epithelium, and later progressing to lung fibrosis and respiratory failure (Smith and Heath, 1975; Thurlbeck and Thurlbeck, 1976; Tomita et al., 2007). In addition, PQ comes in direct contact with oxygen in the lungs and generates toxic activated oxygen. Therefore, anaerobic conditions or hypoxia could be necessary to prevent or attenuate the generation of harmful ROS by PQ in the lungs. Although the underlying molecular mechanisms have not been fully demonstrated, it has been reported that nonlethal levels of hypoxia can sometimes lead to the upregulation of cell survival genes and signaling pathways (Seta et al., 2004).
Cheng and colleagues (Cheng et al., 2007) reported that hypoxia reduced PQ-induced cellular damage in human corneal endothelial cells. However, the protective effect of hypoxia on human lung cell line, which is the primary target for PQ toxicity, has not been investigated in vitro, and the detailed mechanisms of cellular responses to hypoxia on PQ-induced ROS production are still unclear.
Therefore, we investigated whether nonlethal levels of continuous hypoxia could protect lung epithelial cells against PQ-induced oxidative cell death, and demonstrated whether hypoxic protection is associated with reduction in PQ-induced ROS generation.
Results
The toxicity of PQ & continuous hypoxia
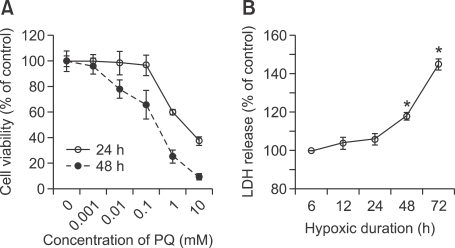
Exposure to PQ for 8 h resulted in a concentration-dependent decrease in cell survival, as shown by the MTT assay (Figure 1A). A total of 60% and 80% loss were measured in cells exposed to 1 mM PQ after 24 and 48 h, respectively. To determine the effect of continuous hypoxia on A549 cells in our culture system, we subjected the A549 cells to hypoxia (1% oxygen) for 6, 12, 24, 48, and 72 h, and assessed continuous hypoxia-induced damage using the LDH assay. During the first 24 h of exposure, no significant increase in LDH levels was observed (Figure 1B). But, after that the LDH levels increased in proportion to the duration of hypoxia.
Figure 1.
Cytotoxic activity of PQ & continuous hypoxia in A549 cells. (A) A549 cells were treated with various concentrations of PQ for 8 h. Cytotoxicity was determined by the MTT assay after 24 and 48 h, and relative absorbance is expressed as % of control (not subjected to PQ treatment). (B) Medium lactate dehydrogenase (LDH) activities were measured immediately after hypoxic exposure. Values are expressed as % of control levels from sister cultures maintained in normoxia for the same durations. Data are represented as mean ± standard deviation of the mean (n = 6). *P < 0.05.
Protective effects of continuous hypoxia on the viability of cells subjected to PQ
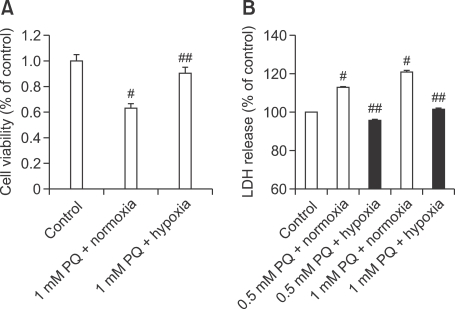
Because continuous hypoxia showed no significant injury to A549 cells within the first 24 h of exposure in our culture system, as shown in Figure 1, we tested whether a 24-h period of hypoxia protects these cells from PQ toxicity using the MTT and LDH assays (Figure 2). Treatment with PQ for 8 h yielded a significant increase and decrease in LDH level and MTT reduction, respectively, compared with the control. As shown in Figure 2, this PQ-induced MTT degradation and LDH release were abolished by a 24-h exposure to nonlethal hypoxia.
Figure 2.
Protective effect of continuous nonlethal hypoxia on PQ-induced cytotoxicity in A549 cells. (A) MTT Assay: Degree of viability was quantified at 24 h after 1 mM PQ treatment using the MTT reduction assay. PQ + Normoxia, 24-h normoxia (21% O2) after 1 mM PQ treatment for 8 h; PQ + Hypoxia, 24-h hypoxia (1% O2) after 1 mM PQ treatment for 8 h (B) LDH Assay: Inhibition of PQ (0.5 and 1 mM)-induced LDH release by hypoxia. Cell death was assessed by measuring the release of the cytosolic marker LDH and is expressed as percentage of net LDH release induced by PQ (% of total LDH, n = 6) Results are expressed as a percentage of the value obtained in normoxic cultures (control, not subjected to PQ treatment). #Significantly different from control group, P < 0.05. ##Significantly different from PQ + Normoxia group, P < 0.05.
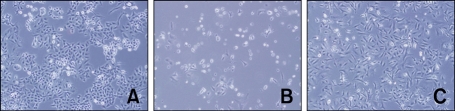
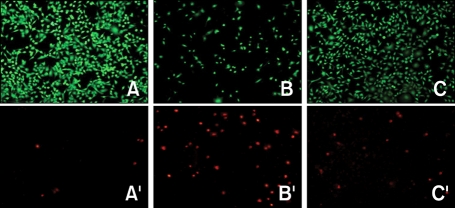
In addition, we identified the protective effect of 24-h period of continuous hypoxia on the viability of PQ-treated cells with microscopic imaging (Figure 3) and a live and dead assay (Figure 4). Collectively, all these data suggest that continuous hypoxia reduces PQ-induced death in A549 cells.
Figure 3.
Representative microscopic images of cell viability in hypoxic vs normoxic conditions after PQ-induced cell death. At 48 h after 500 µM PQ treatment for 8 h, a significant reduction in cell number was observed in PQ-treatment group in normoxia (B). On the other hand, 24 h of hypoxia after PQ treatment suppressed PQ-induced cell decease, as per the microscopic observations (C). The morphology of the cells was observed under phase contrast microscopy (×100); A, B, and C show Control, PQ + Normoxia, and PQ + Hypoxia cultures, respectively.
Figure 4.
Live and dead fluorescent assay for effect of continuous nonlethal hypoxia on PQ-induced cytotoxicity. Cells were exposed to normoxia (control), 8 h of treatment with 500 µM PQ + 24 h of normoxia (21% oxygen), and 8 h of 500 µM PQ + 24 h of hypoxia (1% oxygen). Culture dishes were subsequently stained with the live and dead assay (Molecular Probes) and viewed under a fluorescence microscope. A, B, and C show the Control, 8-h 500 µM PQ + 24-h normoxia, and 8-h 500 µM PQ + 24-h hypoxia cultures, respectively, with intracellular calcein fluorescence signifying viable cells. A', B', and C' show ethidium homodimer fluorescence of the same fields as A, B, and C, respectively, and demonstrate injured and nonviable cells. Note that PQ increased cell injury/death after 24 h, and this was diminished by nonlethal continuous hypoxia.
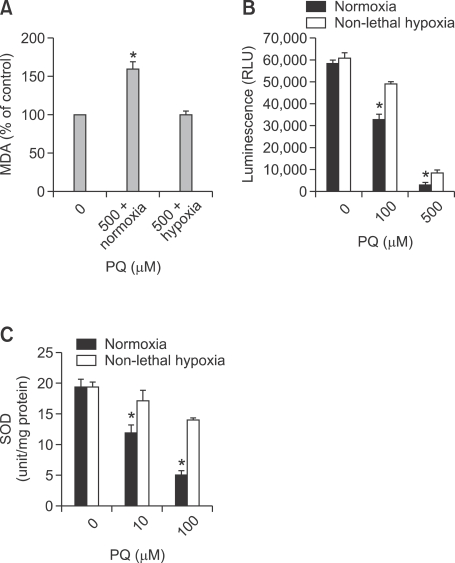
Effects of continuous hypoxia on PQ-induced oxidative stress
In A549 cells, we measured the lipid peroxide level (Figure 5A), GSH level (Figure 5B), and SOD activity (Figure 5C) as the markers of oxidative stress. We compared the levels of these markers between the group that underwent 24-h hypoxia exposure after an 8-h PQ treatment and the control group (normoxia-exposed group) with only 8-h PQ exposure. A significantly decreased MDA level was observed in the hypoxia-exposed group in comparison to the normoxia-exposed group, suggesting attenuated PQ-induced oxidative damage in the hypoxia-exposed group. The GPx activity in the hypoxia-exposed group was increased by half as compared with the normoxia-exposed group (P < 0.05), consistent with the fact that it is being utilized to combat the effects of PQ (Figure 5B). After PQ treatment, the total activity of SOD in the hypoxia-exposed group increased significantly as compared with that in the normoxia-exposed group.
Figure 5.
(A) Measurement of lipid peroxide levels. A549 cells were exposed to normoxia (control) or to hypoxia for 24 h after an 8-h 0.5 mM PQ exposure. Then, A549 cells and media were harvested for MDA level assessment. Data (mean ± SD) were normalized to the control (normoxia-exposed group), and was taken as 100%. (B) Measurement of GPx activity. A549 cells were exposed to normoxia or to hypoxia for 24 h after an 8-h 0.1 & 0.5 mM PQ exposure. Then, after another 24-h incubation in normoxia, A549 cells and media were harvested for assessing GSH levels. (C) Measurement of total SOD activity A549 cells were exposed to normoxia or to hypoxia for 24 h after an 8-h 10 & 100 µM PQ exposure. Then, after another 24-h incubation in normoxia, A549 cells and media were harvested for assessing total SOD levels. Values are mean ± SD; statistically significant differences (P < 0.05): *between hypoxia group and normoxia group.
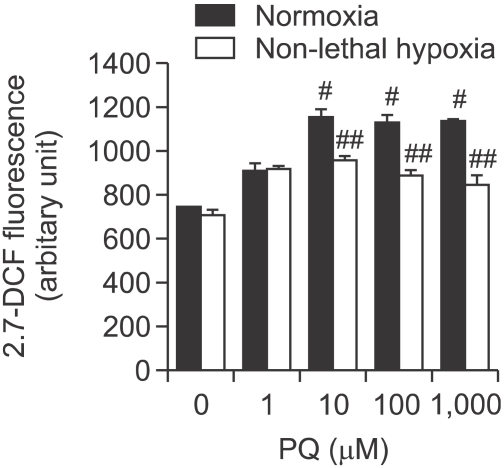
Effects of continuous hypoxia on PQ-induced ROS generation
The levels of ROS in the PQ treatment group increased significantly compared with the normoxia-exposed group (P < 0.05; Figure 6). On the other hand, the levels of ROS in the hypoxia-exposed group decreased significantly compared with the normoxia-exposed group (P < 0.05; Figure 6).
Figure 6.
Effects of continuous nonlethal hypoxia on the generation of PQ-induced reactive oxygen species (ROS). A549 cells were treated with increasing concentrations of PQ for 8 h and were incubated in normoxic vs hypoxic conditions for 24 h. Then, the amount of ROS was measured by DCFH-DA intensity. The error bars represent the standard deviation. #Significantly different from control group, P < 0.05. ##Significantly different from PQ + Normoxia group, P < 0.05.
These results indicate that although PQ could increase free radical generation in A549 cells, exposure of A549 cells to hypoxia after exposure to PQ could decrease free radical generation.
Discussion
The major findings of the present study are (1) continuous nonlethal hypoxia may protect and rescue lung epithelial cells against PQ-induced toxicity; (2) continuous nonlethal hypoxia attenuated PQ-induced oxidative stress; (3) the protective effect of continuous nonlethal hypoxia on PQ toxicity was involved in the suppression of ROS generation.
PQ is a highly toxic herbicide that is harmful to humans and animals as well. Over the past few decades, several cases of accidental or suicidal PQ poisoning and death have been reported (Gram, 1997; Chang et al., 2009). PQ-mediated cellular damage is essentially due to the continuous generation of the superoxide anion (O2·-) from molecular oxygen (O2) by the PQ radicals which are converted by interaction with the mixed function system of microsomes in the presence of NADPH (Fairshter, 1981). The di-cation of PQ can be reduced again, via a redox cycle, which yields a new and spontaneous reaction with O2 resulting in the generation of ROS such as O2·-. The dismutation of O2·- leads to the formation of the hydroxyl radical (HO·), which is a highly toxic free radical and reacts with other molecules such as proteins or membrane fatty acids that have been implicated in the initiation of membrane injury by lipid peroxidation during exposure to PQ (Bus et al., 1974; Youngman and Elstner, 1981; Chen and Lua, 2000; Cho et al., 2010).
The major cause of death in PQ poisoning is respiratory failure, which occurs as a result of oxidative injury to the alveolar epithelium. This redox cycle originating from PQ is sustained by the extensive supply of electrons and oxygen in the lungs, making it the most vulnerable organ in PQ toxicity (Smith, 1987).
Hypoxia has been shown to produce protective effects in some in vitro and in vivo systems, but its effects in lung cell line have not been studied in vitro (Rhodes et al., 1976; Demeere, 1984; Cheng et al., 2007). In addition, it remains unclear at present whether the protective mechanisms of hypoxia on PQ-induced oxidative damage are related to ROS generation.
It is also unclear whether hypoxic protection is related with ROS suppression. Therefore, in our study, we tried to corroborate the hypothesis that hypoxia could reduce PQ-induced ROS production via the decrease in oxygen supply, and that oxygen might be an important substrate for PQ-induced ROS formation, particularly in lung cell lines that are highly exposed to oxygen.
In this study, we assessed hypoxia-induced reduction in cellular damage of A549 cells by 3 independent methods-LDH assay, MTT assay, and live and dead assay. Among these approaches, the LDH assay is a reliable indicator of cellular injury as it assesses the release of intracellular LDH into the extracellular fluids after PQ exposure. We also observed that hypoxic injury in A549 cells could be determined by the MTT assay. Namely, we demonstrated that nonlethal hypoxia reduces PQ-induced injury to lung cells.
PQ brings about significant changes in the endogenous antioxidant system and oxidative damage, such as lipid peroxidation, where the SOD and GSH-Px activities were decreased and the level of MDA increased, indicating excessive lipid peroxidation. Although the assessment of PQ-induced oxidative stress is complicated by the presence of universal oxidative stress markers, we were able to assess the hypoxia-induced change in the PQ-induced antioxidant status in vitro using 3 oxidative markers-MDA, SOD, and GSH-Px. Here, we used these 3 oxidative markers to demonstrate that hypoxia-induced protection is linked with a decrease in PQ-induced oxidative stress. This study may be the first to assess the effects of hypoxia on PQ toxicity using oxidative stress markers in lung cell line in vitro.
MDA is a marker of LPO, and is used to determine the degree of oxidative stress. MDA is produced by the peroxidation of polyunsaturated fatty acids that have more than 2 double bonds, such as linolenic, arachidonic, and docosahexaenoic acids (Del Rio et al., 2005). MDA level was elevated in the serum and lungs of patients with PQ ingestion, with a peak at 6-11 days after PQ poisoning (Yasaka et al., 1981; Kurisaki, 1985). However, some animal experiments did not show an elevation in MDA levels (Kurisaki, 1985; Hara et al., 1991).
The increase in MDA levels reflects the unbalance between endogenous antioxidant and oxidative stress, and the initiation of oxidative injury in our body. Intracellular antioxidant defenses increase as adaptive responses to oxidative stress.
We measured the antioxidants-GPx and SOD enzymes-that may reflect intracellular defenses. Glutathione, a major nonprotein thiol involved in many aspects of cellular metabolism and regulation, plays a crucial role in the cellular antioxidant defense system by scavenging free radicals and other ROS, removing hydrogen and lipid peroxides, and preventing oxidation of biomolecules (Wu et al., 2004). PQ-induced oxidative stress can lead to significant depletion of glutathione (Nakagawa et al., 1995; Yeh et al., 2006). Based on our observations, we can say that increased glutathione level during hypoxia is suggestive of the fact that hypoxia helps ameliorate PQ-caused oxidative stress.
SOD is a crucial enzyme in cellular antioxidant systems, as it plays critical roles in the elimination of excess ROS in living organisms. SOD converts superoxide radical to hydrogen peroxide, which is subsequently converted to water by CAT and GPX. Inadequate elimination of ROS results in oxidative stress that may cause severe metabolic malfunctions and can damage biological macromolecules (Matés, 2000). In the present study, PQ-induced A549 cells increased the levels of antioxidant enzymes. The results indicate that PQ-induced production of higher levels of ROS may have triggered the increase in the activities of antioxidant enzymes in the cells. This may be attributed to an instant active role of SOD or GPX in modulating the harmful effects of PQ. Based on our observations, we believe that increased SOD level during hypoxia is suggestive of hypoxia-induced suppression of PQ-induced superoxide generation.
There is only one clinical case report of hypoxic therapy in treatment of a patient with PQ poisoning in the English literature so far (Demeere, 1984). They applied the treatment consisted of hypoxic ventilation with 14% oxygen and 86% nitrogen, hemodialysis, and forced dieresis to a patient with PQ intoxication. The patient never exhibited either clinical or radiological signs of pulmonary complications. Therefore, our in vitro data is expected to be utilized in in vivo study and potential clinical application to treat patients with PQ poisoning.
In this study, intracellular ROS was measured by DCF fluorescence intensity. Treatment with PQ increased DCFH-DA intensity, indicating elevated ROS production. However, nonlethal continuous hypoxia attenuated PQ-induced ROS production.
In summary, the results of the present study suggest that nonlethal continuous hypoxia is able to protect and rescue lung epithelial cells against PQ-induced toxicity. These effects are attributable mainly to the hypoxic effect of reducing the oxygen supply to lessen the generation of the free radicals involved in cell death. The protective properties of hypoxia support its potential beneficial actions against PQ-induced oxidative damage and related mechanisms of toxicity that are likely to be associated with the deleterious effects of increased ROS production.
Methods
Materials
MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole], fetal bovine serum, and dimethyl sulfoxide were purchased from Sigma Chemical Co. (St. Louis, MO). The live and dead viability/cytotoxicity kit (L-3224) was obtained from Molecular Probes (Eugene, OR). Lactate dehydrogenase (LDH) cytotoxicity assay kit was acquired from Biovision Inc. (Mountain view, CA), and SOD activity assay kit was taken from Dojindo Molecular Technologies, Inc. (Rockville, MD).
Cell culture
Human lung adenocarcinoma A549 cells (KCLB, Seoul, Korea) were grown in DMEM supplemented with 10% fetal bovine serum, 1% glutamine, and 100 units/ml penicillin and streptomycin, and were maintained in a conventional humidified incubator set at 37℃ in an atmosphere of 5% CO2 (Sanyo MCO-18M; Moriguchi, Osaka, Japan).
Continuous exposure to nonlethal levels of hypoxia
A549 cells grown in 10-cm dishes were randomly placed in a normoxic incubator (20% O2, 75% N2, and 5% CO2) or a hypoxic chamber (SANYO MCO-18M, Moriguchi, Japan; 1% O2, 94% N2, and 5% CO2) for continuous exposure to hypoxia. LDH assay was performed to determine nonlethal hypoxic time. For other experiments, culture dishes were randomly divided into 2 groups, 1 was placed in a normoxic incubator and the other in a hypoxic chamber after PQ exposure for 8 h. After continuous exposure to nonlethal levels of hypoxia and normoxia, the cells were assessed for cell viability and oxidative stress markers (Supplemental Data Figure S1).
Assessment of cell viability and cell injury
MTT assay: MTT assay was employed to quantitatively evaluate cell viability. The MTT assay was performed 24 and 48 h after PQ treatment and 24 h after a 24 h period of normoxia or continuous hypoxia following an 8 h PQ treatment. In brief, viable adherent cells were stained with MTT (2 mg/ml) for 2 h. The media were removed, and the formazan crystals produced were dissolved by adding 200 µl of dimethyl sulfoxide. Then, the absorbance at 540 nm was determined using a SpectraMax 250 microplate reader (Molecular Probes, Eugene, OR).
LDH release assay: As a marker of cellular lysis, LDH released from injured cells into the medium was determined after time-course hypoxic exposure. LDH release assay was performed 24 h after a 24 h period of normoxia or continuous hypoxia following an 8 h PQ treatment by using an LDH assay kit. LDH activity was measured using a SpectraMax 250 microplate reader (Molecular Probes).
Morphological observation of A549 cells: Morphological changes in A549 cells were assessed at the 24 h time point after a 24 h period of normoxia or continuous hypoxia following an 8 h PQ treatment. Microphotographs of cultured cells were taken using an Olympus phase-contrast microscope (Olympus Optical Co., Tokyo, Japan).
Immunofluorescence analysis for the live and dead assay: For assessing cell survival, we performed the live and dead assay according to the manufacturer's protocol. A549 cells were exposed to ethidium homodimer-1 (4 µM), which binds to nucleic acids and cell-permeant calcein AM (3 µM), which is hydrolyzed by intracellular esterases. Bound ethidium homodimer-1 produces red fluorescence when exposed to a wavelength of 528 nm, allowing for the identification of dead cells, and calcein, the cleavage product of calcein AM, produces green fluorescence when exposed to a wavelength of 494 nm and is used to identify live cells. The cells were analyzed under a fluorescence microscope (Zeiss Axiovert 24, Sony Progressive 3CCD and Camera Adapter CMA-D2; blue excitation filter, 488/515 nm; green excitation filter, 514/550 nm).
Assessment of oxidative stress markers
We measured lipid peroxidation, GSH content, and SOD activity as the markers of oxidative stress.
Thiobarbituric acid-reactive substances assay: Lipid peroxide content in A549 cells was determined by using the modified thiobarbituric acid-reactive substances (TBARS) assay method as described previously (Mihara and Uchiyama, 1978; Sunderman et al., 1985). In brief, the culture media were collected and centrifuged at 3,000 rpm for 5 min, followed by the addition of an equal volume of 0.67% (w/v) TBA. Then, 800 µl of the supernatant was mixed with 100 µl of 0.1 M 2-TBA and heated at 100℃ for 10 min. After cooling, the absorbance was read at 532 nm using a SpectraMax 250 microplate reader (Molecular Probes). An MDA standard was prepared from 1,1,3,3-tetraethoxypropane.
Glutathione assay: This assay was performed as previously described (Baker et al., 1990). In brief, 50 µl of the cytosolic fraction and 100 µl of the reaction mixture (1 mM NADPH, 100 mM Na2HPO4, 100 units GSH reductase, and 1 mM DTNB) were incubated for 15 min at 37℃ in a 96-well plate. Absorbance was measured at 412 nm using a SpectraMax 250 microplate reader (Molecular Probes). GSH levels were determined from the standard curve constructed using 1 mM GSH, and were expressed in terms of per microgram protein.
Determination of SOD activity: After A549 cells were exposed to the different concentrations of PQ for 8 h, they were incubated in hypoxic or normoxic conditions for another 24 h. Samples of the supernatant were collected from each well to measure SOD activity using the SOD activity assay kit.
Measurement of intracellular ROS generation
The accumulation of ROS was determined by analyzing 2', 7'-dichlorofluorescin (DCF) fluorescence (Mattson et al., 1995; Bastianetto et al., 2000). In brief, 10 µM 2',7'-dichlorofluorescin diacetate (DCFH-DA) was applied to the culture medium at the onset of hypoxia exposure, as described previously (Keston and Brandt, 1965). The freely cell-permeable DCFH-DA is readily converted into the highly fluorescent DCF, which interacts with peroxide. DCF fluorescence was quantified (excitation/emission = 485 nm/530 nm) using a fluorescence SpectraMax 250 multi-well plate reader.
Statistical analysis
Statistical significance was examined using the Student's t-test. The 2-sample t-test was used for 2-group comparisons. Values were reported as mean ±SD. Statistical significance was set at P < 0.05.
Supplemental data
Supplemental data include a figure and can be found with this article online at http://e-emm.or.kr/article/article_files/SP-43-9-02.pdf.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0021711).
Abbreviations
- DCFH-DA
2',7'-dichlorofluorescin diacetate
- LDH
lactate dehydrogenase
- PQ
paraquat
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid-reactive substances
Supplementary Material
Supplemental Data
References
- 1.Baker MA, Cerniglia GJ, Zaman A. Microtiter plate assay for the measurement of glutathioneand glutathione disulfide in large numbers of biological samples. Anal Biochem. 1990;190:360–365. doi: 10.1016/0003-2697(90)90208-q. [DOI] [PubMed] [Google Scholar]
- 2.Bastianetto S, Ramassamy C, Dore S, Christen Y, Poirier J, Quirion R. The Ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by beta-amyloid. Eur J Neurosci. 2000;12:1882–1890. doi: 10.1046/j.1460-9568.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- 3.Bus JS, Aust SD, Gibson JE. Superoxide- and singlet oxygen-catalyzed lipid peroxidation as a possible mechanism for paraquat (methyl viologen) toxicity. Biochem Biophys Res Commun. 1974;58:749–755. doi: 10.1016/s0006-291x(74)80481-x. [DOI] [PubMed] [Google Scholar]
- 4.Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environ Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang X, Shao C, Wu Q, Wu Q, Huang M, Zhou Z. Pyrrolidine dithiocarbamate attenuates paraquat-induced lung injury in rats. J Biomed Biotechnol. 2009;2009:619487. doi: 10.1155/2009/619487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CM, Lua AC. Lung toxicity of paraquat in the rat. J Toxicol Environ Health A. 2000;60:477–487. doi: 10.1080/00984100050079548. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Q, Nguyen T, Song H, Bonanno J. Hypoxia protects human corneal endothelium from tertiary butyl hydroperoxide and paraquat-induced cell death in vitro. Exp Biol Med (Maywood) 2007;232:445–453. [PMC free article] [PubMed] [Google Scholar]
- 8.Cho SC, Rhim JH, Son YH, Lee SJ, Park SC. Suppression of ROS generation by 4,4-diaminodiphenylsulfone in non-phagocytic human diploid fibroblasts. Exp Mol Med. 2010;42:223–232. doi: 10.3858/emm.2010.42.3.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Demeere JL. Paraquat toxicity. The use of hypoxic ventilation. Acta Anaesthesiol Belg. 1984;35:219–230. [PubMed] [Google Scholar]
- 11.Dinis-Oliveira RJ, De Jesus Valle MJ, Bastos ML, Carvalho F, Sanchez Navarro A. Kinetics of paraquat in the isolated rat lung: Influence of sodium depletion. Xenobiotica. 2006a;36:724–737. doi: 10.1080/00498250600790331. [DOI] [PubMed] [Google Scholar]
- 12.Dinis-Oliveira RJ, Remiao F, Carmo H, Duarte JA, Navarro AS, Bastos ML, Carvalho F. Paraquat exposure as an etiological factor of Parkinson's disease. Neurotoxicology. 2006b;27:1110–1122. doi: 10.1016/j.neuro.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Fairshter RD. Paraquat toxicity and lipid peroxidation. Arch Intern Med. 1981;141:1121–1123. [PubMed] [Google Scholar]
- 14.Gram TE. Chemically reactive intermediates and pulmonary xenobiotic toxicity. Pharmacol Rev. 1997;49:297–341. [PubMed] [Google Scholar]
- 15.Hara S, Endo T, Kuriiwa F, Kano S. Different effects of paraquat on microsomal lipid peroxidation in mouse brain, lung and liver. Pharmacol Toxicol. 1991;68:260–265. doi: 10.1111/j.1600-0773.1991.tb01235.x. [DOI] [PubMed] [Google Scholar]
- 16.Keston AS, Brandt R. The Fluorometric Analysis of Ultramicro Quantities of Hydrogen Peroxide. Anal Biochem. 1965;11:1–5. doi: 10.1016/0003-2697(65)90034-5. [DOI] [PubMed] [Google Scholar]
- 17.Kurisaki E. Lipid peroxidation in human paraquat poisoning. J Toxicol Sci. 1985;10:29–33. doi: 10.2131/jts.10.29. [DOI] [PubMed] [Google Scholar]
- 18.Matés JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- 19.Mattson MP, Barger SW, Begley JG, Mark RJ. Calcium, free radicals, and excitotoxic neuronal death in primary cell culture. Methods Cell Biol. 1995;46:187–216. doi: 10.1016/s0091-679x(08)61930-5. [DOI] [PubMed] [Google Scholar]
- 20.Melchiorri D, Reiter RJ, Sewerynek E, Hara M, Chen L, Nistico G. Paraquat toxicity and oxidative damage. Reduction by melatonin. Biochem Pharmacol. 1996;51:1095–1099. doi: 10.1016/0006-2952(96)00055-x. [DOI] [PubMed] [Google Scholar]
- 21.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa I, Suzuki M, Imura N, Naganuma A. Enhancement of paraquat toxicity by glutathione depletion in mice in vivo and in vitro. J Toxicol Sci. 1995;20:557–564. doi: 10.2131/jts.20.5_557. [DOI] [PubMed] [Google Scholar]
- 23.Rhodes ML, Zavala DC, Brown D. Hypoxic protection in paraquat poisoning. Lab Invest. 1976;35:496–500. [PubMed] [Google Scholar]
- 24.Seta KA, Yuan Y, Spicer Z, Lu G, Bedard J, Ferguson TK, Pathrose P, Cole-Strauss A, Kaufhold A, Millhorn DE. The role of calcium in hypoxia-induced signal transduction and gene expression. Cell Calcium. 2004;36:331–340. doi: 10.1016/j.ceca.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Smith LL. Mechanism of paraquat toxicity in lung and its relevance to treatment. Hum Toxicol. 1987;6:31–36. doi: 10.1177/096032718700600105. [DOI] [PubMed] [Google Scholar]
- 26.Smith LL, Rose MS, Wyatt I. The pathology and biochemistry of paraquat. Ciba Found Symp. 1978:321–341. doi: 10.1002/9780470715413.ch18. [DOI] [PubMed] [Google Scholar]
- 27.Smith P, Heath D. The pathology of the lung in paraquat poisoning. J Clin Pathol Suppl (R Coll Pathol) 1975;9:81–93. [PMC free article] [PubMed] [Google Scholar]
- 28.Sunderman FW, Jr, Marzouk A, Hopfer SM, Zaharia O, Reid MC. Increased lipid peroxidation in tissues of nickel chloride-treated rats. Ann Clin Lab Sci. 1985;15:229–236. [PubMed] [Google Scholar]
- 29.Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology. 2002;180:65–77. doi: 10.1016/s0300-483x(02)00382-7. [DOI] [PubMed] [Google Scholar]
- 30.Thurlbeck WM, Thurlbeck SM. Pulmonary effects of paraquat poisoning. Chest. 1976;69:276–280. doi: 10.1378/chest.69.2_supplement.276. [DOI] [PubMed] [Google Scholar]
- 31.Tomita M, Okuyama T, Katsuyama H, Miura Y, Nishimura Y, Hidaka K, Otsuki T, Ishikawa T. Mouse model of paraquat-poisoned lungs and its gene expression profile. Toxicology. 2007;231:200–209. doi: 10.1016/j.tox.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 33.Yasaka T, Ohya I, Matsumoto J, Shiramizu T, Sasaguri Y. Acceleration of lipid peroxidation in human paraquat poisoning. Arch Intern Med. 1981;141:1169–1171. [PubMed] [Google Scholar]
- 34.Yeh ST, Guo HR, Su YS, Lin HJ, Hou CC, Chen HM, Chang MC, Wang YJ. Protective effects of N-acetylcysteine treatment post acute paraquat intoxication in rats and in human lung epithelial cells. Toxicology. 2006;223:181–190. doi: 10.1016/j.tox.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Youngman RJ, Elstner EF. Oxygen species in paraquat toxicity: the crypto-OH radical. FEBS Lett. 1981;129:265–268. doi: 10.1016/0014-5793(81)80180-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data