Abstract
Successful kidney transplantation corrects many of the metabolic abnormalities associated with chronic kidney disease (CKD); however, skeletal and cardiovascular morbidity remain prevalent in pediatric kidney transplant recipients and current recommendations from the Kidney Disease Improving Global Outcomes (KDIGO) working group suggest that bone disease—including turnover, mineralization, volume, linear growth, and strength—as well as cardiovascular disease be evaluated in all patients with CKD. Although few studies have examined bone histology after renal transplantation, current data suggest that bone turnover and mineralization are altered in the majority of patients and that biochemical parameters are poor predictors of bone histology in this population. Dual energy X-ray absorptiometry (DXA) scanning, although widely performed, has significant limitations in the pediatric transplant population and values have not been shown to correlate with fracture risk; thus, DXA is not recommended as a tool for the assessment of bone density. Newer imaging techniques, including computed tomography (quantitative CT (QCT), peripheral QCT (pQCT), high resolution pQCT (HR-pQCT) and magnetic resonance imaging (MRI)), which provide volumetric assessments of bone density and are able to discriminate bone microarchitecture, show promise in the assessment of bone strength; however, future studies are needed to define the value of these techniques in the diagnosis and treatment of renal osteodystrophy in pediatric renal transplant recipients.
Keywords: Pediatrics, CKD-MBD, Renal osteodystrophy, DXA, Cardiovascular calcification
Introduction
Childhood and adolescence are critical periods for bone mass gain since about 90% of peak bone mass is acquired before the age of 18 years and decreased peak bone mass may increase fractures risk in adulthood [1]. In both adults and children with chronic kidney disease (CKD), cardiovascular disease accompanies all subtypes of bone disease, necessitating that discussions of renal osteodystrophy also consider cardiovascular pathology [2]. Although successful kidney transplantation corrects many of the metabolic abnormalities associated with CKD, morbidity—including osteopenia, growth failure, spontaneous fractures, avascular necrosis, and increased cardiovascular mortality—remains prevalent in pediatric kidney transplant recipients [3–6]. Over the past decade, studies from the United States and Europe have highlighted the skeletal and vascular morbidities experienced by the pediatric renal transplant population; Bartosh et al. demonstrated a 44% prevalence of short stature (height Z score less than −1.88 for age), a 41% prevalence of bone-joint abnormalities and a 23% prevalence of fractures [3]; Groothoff et al. reported short stature (height below –2 SD for age) in 61%, bone disease in 35% [4], and nearly 50% with cardiovascular disease [5]; while Helenius et al. reported a markedly increased vertebral fracture rate, along with scoliosis, back pain, and degenerated discs, in children receiving solid organ transplantation, the majority of whom had received kidney transplants [7, 8]. Although current recommendations from the Kidney Disease Improving Global Outcomes (KDIGO) working group suggest that bone disease—including turnover, mineralization, volume, linear growth, and strength—as well as cardiovascular disease be evaluated in all patients with CKD, including those post renal transplantation [2], large gaps exist in our current knowledge as to the pathophysiology of mineral, bone, and vascular disease post-transplantation and few evidence-based treatment paradigms are available. This review discusses the known features of CKD mineral and bone disorder (CKD-MBD) in the pediatric renal transplantation, highlighting gaps in current knowledge and areas of ongoing research.
Mineral metabolism
Dramatic changes in serum mineral metabolism occur immediately after renal transplantation as circulating levels of phosphorus, magnesium, and PTH decline and 25(OH)vitamin D and 1,25(OH)2vitamin D values increase [9–11]. Calcineurin inhibition, which induces renal magnesium wasting, often induces hypomagnesemia [11], while hyperparathyroidism and elevated circulating fibroblast growth factor 23 (FGF-23) concentrations promote renal phosphate excretion and often cause serum phosphate concentrations to decline to below the normal range [9, 12, 13].
Chronically increased circulating FGF-23 levels may subsequently contribute to hypophosphatemia and low calcitriol levels, which persist for months after transplantation [12–15]. Elevated PTH values may also persist in some patients and hypercalcemia, in combination with hyperparathyroidism, has been traditionally considered a sign of autonomous PTH secretion and an indication for parathyroidectomy in transplant recipients [16, 17]. Cinacalcet, a calcimimetic agent, has been used in recent years as an alternative to parathyroidectomy in this population as it suppresses PTH and normalizes serum calcium concentrations in almost all patients within a few weeks [18–21]. Unfortunately, the systemic benefit of this form of therapy is unclear; indeed, recent data have demonstrated a 50% prevalence of adynamic bone disease in hypercalcemic, hyperparathyroid renal transplant recipients [22] with long-term calcimimetic therapy exacerbating this form of renal osteodystrophy [23].
25(OH)Vitamin D deficiency is also a common problem due to dietary restrictions [24], reduced sun exposure as a result of chronic illness, decreased skin synthesis of vitamin D in response to sunlight compared with individuals with normal kidney function [25, 26], and increased catabolism of 25(OH)vitamin D through 24-hydroxylase [27]. Although 25(OH)vitamin D stores improve after renal transplantation, values remain low in a significant percentage of patients, particularly in African Americans, contributing to increased PTH levels in these individuals [10].
Bone disease
In 2003, Bartosh et al. reported the historical prevalence of skeletal morbidities in 217 children undergoing transplantation from 1967 to 1969 at the University of Wisconsin [3]. The following year, Groothoff et al. published a similar study on the experience of 397 Dutch children undergoing renal transplantation from 1972 to 1992 [4]. In these two large cohorts, clinical bone disease was found to persist into adulthood in many patients who had undergone renal transplantation as children, with long-term bone and joint disorders reported in 35–41% and with as many as 18% complaining of disability from their bone disease [4]. Fractures were reported to have occurred in 23%, with avascular necrosis developing in a similar number of patients [3, 4]. In 2009, Valta et al. described an 8% prevalence of vertebral fractures [6] in a cohort of 106 pediatric renal transplant recipients who had been followed for 0 to 16 (median 5) years, while Helenius et al. reported that rates of non-vertebral fractures in 196 children receiving organ transplants (135 with kidney transplants) between 1983 and 2002 were similar to healthy controls, although the age and sex adjusted hazard rate for vertebral fractures in transplant recipients was a startling 61.3 as compared to controls [8]. These clinical findings highlight the high prevalence of skeletal (and particularly vertebral) fragility in the pediatric transplant population; however, accurate prediction of which patients are likely to experience fractures remains problematic.
Bone histology remains the gold standard for the diagnosis of lesions of bone turnover and mineralization; unfortunately, bone biopsy is an invasive technique that is not widely performed and a paucity of data exist as to the spectrum of bone histologic lesions in pediatric renal transplant recipients treated with current-day immunosuppressive regimens. In adult kidney recipients, the fibrosis and markedly increased rates of bone turnover present in dialysis patients with secondary hyperparathyroidism are much improved 6 months after kidney transplantation [28]. However, cross-sectional studies demonstrate that even years after transplantation the majority of transplant recipients continue to display abnormal bone histology. Some patients display persistently elevated rates of bone turnover while others develop adynamic lesions and, unfortunately, biochemical parameters, including serum PTH levels, are unable to discriminate between these various bone lesions [29–32]. Although only one study has been performed in pediatrics, a similar disconnect exists between bone formation rate and PTH levels in younger patients. A cross-sectional study published in 1998 reported the bone biopsy data from 47 pediatric kidney recipients who received steroids, cyclosporin, and antimetabolites as immune-suppression. In this study, 10% of patients had evidence of adynamic bone lesion while another 23% had high turnover renal osteodystrophy [33]. Similar to the adult studies, PTH measurements did not correlate with bone turnover and many patients with adynamic bone displayed PTH levels greater than 100 pg/ml [33]. Although bone turnover was variably normal, increased, or decreased, defective skeletal mineralization was highly prevalent with increased osteoid volumes and reduced mineral apposition rates [33]. The etiology of these observed abnormalities in bone turnover and mineralization was likely multifactorial, including persistent and de novo abnormalities in mineral metabolism [14] and medication-mediated changes in mineral metabolism and bone histology. Glucocorticoids have been shown to decrease intestinal calcium absorption [34] and increase FGF-23 levels [15] while inhibiting osteoblastic activity, decreasing bone formation, inhibiting genes for type I collagen, the IGFs, BMPs, TGF-beta, and RANK-Ligand [35–37] and increasing osteoclastic activity and bone resorption [38–41]. As a result, glucocorticoid use is linked to a decrease in trabecular bone by BMD and a decrease in bone formation rate [42]. Cyclosporine increases both bone formation and bone resorption while reducing cancellous bone volume [43, 44] and sirolimus has been shown to impair longitudinal growth by disrupting VEGF and IGF-1 signaling [45]. By contrast, azathioprine appears to have minimal impact on skeletal remodeling [46]. The specific roles of other immunosuppressive agents, such as mycophenolate mofetil, on bone formation and mineralization have yet to be evaluated. Due to the myriad of effects that glucocorticoids exert on bone histology, the use of steroid-free immune-suppressant regimens has the potential to greatly improve bone histology in the post-transplant period. Unfortunately, the bone histology associated with currently used immunosuppressant treatment regimens, including steroid-free protocols, is unknown.
Imaging studies assess skeletal mineral content and structure and are thus often used to assess bone health in both adults and children post kidney transplantation. The most widely used technique is DXA scanning, and in adult patients, this technique has demonstrated significant vertebral bone loss soon after renal transplantation; indeed, an 8.8 ± 7.0% loss in spinal bone mineral density has been noted by 18 months post-transplantation, the majority occurring in the first 6 months. Although over 50% of the subjects evaluated in one study had spinal bone mineral densities that were in a range associated with an increased fracture risk [28], a reduction of BMD in the spine after transplantation may represent a normalization of trabecular bone structure with stable or increasing spinal bone density reflecting the actions of persistently elevated PTH levels on trabecular bone. Interestingly, and consistent with the concept that decreasing bone density may actually reflect a normalization of renal osteodystrophy after transplantation, radial shaft (i.e., cortical bone) density has not been consistently shown to decrease but rather increases in some patients [28]. Although bone loss also occurs in children, its characterization is incomplete due to problems with the use of DXA scanning. The major limitations of DXA in pediatric CKD include: a reliance on areal rather than volumetric density, a parameter which changes with growth; an inability to distinguish between trabecular and cortical bone which may be independently altered in CKD; and an inability to evaluate trabecular microarchitecture, a key determinant of bone quality. The presence of vascular calcification also confounds the DXA technique since calcium deposits in vascular tissue may be interpreted by the scan as bone density; indeed, the use of lateral DXA has been advocated as a method for determining the amount of vascular calcification in adult CKD patients [47]. The difficulty in determining appropriate “normal” ranges for growth-retarded and chronically ill children pose additional problems [48]. Likely as a result of these limitations, bone density determined by DXA does not correlate with fracture risk in pediatric transplant recipients and current ICSD guidelines state that DXA scanning is inappropriate for use in children with CKD [49, 50]. In 2001, the evaluation of a cohort of 33 pediatric renal transplant recipients using three different evaluation criteria, with norms based on chronological age, height-age, and gender-matched controls, respectively, illustrated some of these problems, demonstrating that the apparent prevalence of low bone density varies considerably depending on the technique used to evaluate the data [51]. In a more recent study, Valta et al. [6] attempted to address these issues by correcting for bone age in a cohort of 106 prevalent pediatric renal transplant recipients. Using this technique, average Z score for bone density at the lumbar spine, hip and whole body were −0.5, –0.2, and −0.3, respectively. Higher PTH levels, female gender, and an age greater than 15 years were all independently associated with lower bone density while the use of recombinant growth hormone was associated with improved bone density at the lumbar spine. Although BMD decreased in the first year post-transplantation, values increased subsequently to an average Z score of −0.2 at 5 years post-transplantation. Interestingly, despite the fairly normal BMD values, the authors reported a concerning high (8%) rate of vertebral fractures [6], suggesting that normal values on DXA did not adequately reflect underlying bone pathology. Despite its limitations, serial DXA scans are recommended for therapeutic monitoring in children with symptomatic metabolic bone disease [50] and, although further studies are warranted, serial DXA measurements may prove useful in the therapeutic monitoring of some selected patients post-renal transplantation [52].
Due to the technical limitations for DXA, new bone-imaging techniques derived from QCT, pQCT, HR-pQCT, and MRI, ultrasound and bone texture analysis, have been developed. These non-invasive techniques are associated with little or no radiation exposure and both CT and MRI provide three-dimensional assessments of bone area and density, avoiding many of the growth-related issues of DXA in children [52]. Peripheral QCT is a promising method for assessing bone mass and mineral density determined by this method predicts future fractures in adults [48, 53]. Newer, high-resolution pQCT scanners have improved precision and the feasibility of this technique has been demonstrated in healthy children [54] and in those with primary hyperoxaluria [55]. Although the ability of pQCT to predict future fracture risk has not been evaluated in renal transplant recipients, Rüth et al. identified a decrease in cortical thickness and muscular force at the forearm in 55 adolescent renal transplant recipients, a change that may contribute to the increased fracture risk in these patients [56]. Bone ultrasound has also been evaluated in a cohort of 40 children and young adults with renal transplants; despite normal overall mineral density, a decrease in apparent cortical thickness was identified in this study [57]. MRI is another promising technique; however, data are lacking on its use in the pediatric renal transplantation population. Outcomes-based studies are lacking with all of these novel techniques, and future studies are warranted; however, their ability to assess volumetric bone density, to discriminate cortical from trabecular bone, and to resolve trabecular microarchitecture suggest the potential to yield clearer understanding of bone mass and structure.
Growth
In both Europe and the United States, a 44–61% prevalence of decreased final adult height has been reported [3, 4], thus representing the most prevalent long-term skeletal morbidity in the pediatric population. At the time of renal transplantation, the mean height deficit for all pediatric patients is 1.78 SD below normal height for age and gender, representing a height percentile of less than the 4th percentile [58]. The deficit is greater for males (SD: –1.82 for males versus −1.72 for females), younger patients, and those with prior transplants. Post-transplantation, children less than 6 years of age experience an improvement in mean growth deficit, while older children do not [58]. Immunosuppressant medications, persistent secondary hyperparathyroidism, low levels of circulating vitamin D [59], skeletal resistance to growth hormone, and the persistence of defective skeletal mineralization may all contribute [33]. Early case reports and clinical series suggested that children receiving steroid-free immunosuppressive regimens, those treated with alternate day steroids and those with better height SDS at the time of transplant attain the greatest final adult height [58, 60–62]. Recently, the TWIST trial, the first large (98 patients per arm) randomized, controlled trial evaluating the effect of early steroid withdrawal versus a standard steroid-based regimen has demonstrated greater catch-up growth at 6-month post-transplantation in children in the early steroid withdrawal arm of the study. In this study, the greatest gains in height were observed in pre-pubertal children [63]. Longer follow-up is needed in order to determine whether these height gains are sustained in the long term.
Cardiovascular calcification
Although renal transplantation improves survival, cardiovascular disease remains prevalent in the post-transplant period. The mortality rate in patients with CKD is markedly higher than that of the general population and cardiovascular disease is the leading cause of death in both children and adults post renal transplantation [58, 64, 65]. In contrast to the calcifications of atherosclerotic plaques that develop with age in the vascular intima of individuals with normal kidney function, vascular calcification in the uremic milieu develops primarily in the tunica media. Electron beam computed tomography (EBCT) measurements in young adults who were treated with maintenance dialysis as children have demonstrated the presence of vascular calcification in a significant percentage [64]. Arteries obtained from patients undergoing renal transplantation have been shown to express core binding factor-1 (Cbfa1), a protein believed to trigger mesenchymal cell to osteoblast transformation [66]. Upregulation of the sodium-dependent phosphate transporter PIT-1 [67] and other pro-mineralization factors such as osteopontin, bone sialoprotein, osteonectin, alkaline phosphatase, type I collagen, and bone morphogenic protein-2 (BMP-2) are also potentiated by the uremia [68–71]. By contrast, expression of calcification inhibitors, such as fetuin A, matrix gla protein, and Klotho is suppressed [72–75]. Increased levels of circulating FGF-23 may also contribute to progressive vascular disease, as elevated values are correlated with vascular calcification in adult dialysis patients [76] and mortality in the general population [77] as well as in dialysis [78] and CKD patients [79].
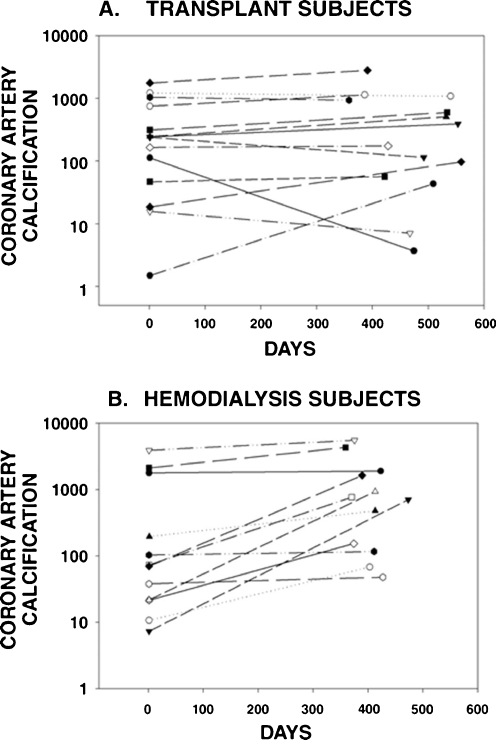
In the majority of adult patients, the rate of vascular calcification slows considerably post-transplantation; however, despite normalization of serum calcium and phosphorus levels, these lesions often do not regress [80, 81] (Fig. 1), and, in some patients, particularly those treated with warfarin therapy, an accelerated progression of vascular calcifications may even be observed [82]. EBCT is not used to evaluate vascular calcification in children; however, EBCT data from young adults who developed renal failure as children, as well as autopsy and biopsy data indicate that vascular calcification is present in children with late stages of CKD [83] and those treated with maintenance dialysis [64, 80, 84, 85], particularly those treated with high doses of calcium-containing phosphate binders [64]. Moreover, studies using carotid ultrasound have repeatedly demonstrated abnormal intimal-medial thickness (IMT) and vascular distensibility in pediatric renal transplant recipients in comparison with healthy controls [86–90]. Mitsnefes et al. have reported that the presence of hypertension is strongly linked to increased IMT and poor vessel distensibility in children after renal transplantation [86], while Bilginer et al. have related the length of time on dialysis to increased IMT [87], and Van Summeren et al. described lower fetuin A levels in transplanted patients than in controls, although these levels were not directly correlated with degree of vascular thickness [88].
Fig. 1.
Change in coronary artery calcification in renal transplant recipients (a) and hemodialysis patients (b) in subjects with baseline calcification. Reprinted with permission from Moe et al. NDT 2004; 19:2387–2393
Treatment options
Because mineral, bone, and vascular metabolism are integrally linked in patients with CKD [91], treatment must take each of these factors into consideration. As detailed above, the data on the spectrum of CKD-MBD is limited and reliable imaging techniques for the assessment of bone and vascular disease are not widely available. In both adults and children, minimizing or avoiding steroids have yielded the most promising results; indeed, total dose of glucocorticoid exposure has also been linked to bone loss [35, 92] and children treated on steroid-free immunosuppressant protocols experience greater catch-up growth post-transplantation than do those treated with steroids [60]. Alternate forms of corticosteroids have also been shown promise in lessening the deleterious effects on bone; the use of deflazacort has been reported to cause less bone loss than prednisone [93]. Recombinant human growth hormone (rhGH) has been used in children with significant height deficit after kidney transplantation. A substantial increase in linear growth occurs within the first year of rhGH therapy, but the magnitude of growth response may decline thereafter [94].
Vitamin D (in both 25(OH)vitamin D and 1,25(OH)2vitamin D forms) and calcium have also been advocated by some for the prevention of bone loss; however, initiation of therapy after the first year of transplantation in this population has not been shown to have any sustained or significant benefit [95]. By contrast, the use of bisphosphonates (alendronate) in adults an average of 5 years post-transplantation increases BMD by as much as 4.5%, compared with a 5.8% decrease in BMD in calcitriol treated patients [96]. However, bisphosphonates are controversial in patients with renal impairment, may induce adynamic bone disease [97], are not recommended by KDIGO in patients with advanced stages of CKD [2], and are controversial in children with immature skeletons, particularly as the risk to future pregnancies is unknown.
Treatment options aimed specifically at curbing cardiovascular calcifications are also limited. In an open-label trial evaluating the effect of fluvastatin on cardiovascular mortality and graft loss, cardiac mortality was decreased by 29% after 6.7 years of follow-up in adults after renal transplantation, although all-cause mortality and graft loss did not differ between groups [98]. Pre-clinical data has also suggested that statins may also reverse the effects of corticosteroids on bone, preventing osteonecrosis by decreasing adipogenic and increasing osteogenic bone marrow stromal cell differentiation [99, 100] and, in a single-arm observational study, Pritchet reported a 1% incidence of osteonecrosis in 284 transplant recipients followed for an average of 7.5 years, which appeared to be an improvement from historical controls [101]. However, in a subsequent prospective study of 2,881 patients, 383 of whom received statins, no difference in the rates of osteonecrosis was noted between groups [102]. Studies in children have not been performed.
Conclusions
Although successful transplantation corrects many of the metabolic abnormalities associated with the development of CKD-MBD, abnormalities in mineral metabolism and bone and vascular biology persist after successful renal transplantation. Limited data exist on bone histology after transplantation while DXA, the most widely used imaging technique, does not predict fracture risk and is thus not recommended in children with CKD. New imaging techniques based on CT and MRI have the potential to provide more detailed information as to bone quality and microarchitecture, however, the correlation between these techniques and clinical outcomes are unknown. Current recommendations from the Kidney Disease Improving Global Outcomes working group suggest that all patients with CKD be evaluated for mineral, bone, and vascular consequences of CKD-MBD, and further research is much needed in order to enhance skeletal and cardiovascular health in this population.
Questions (answers appear following the reference list)
- In post-transplantation renal osteodystrophy
-
A.PTH levels correlate closely with bone turnover
-
B.bone turnover returns to the normal range in all patients
-
C.defects in skeletal mineralization are common
-
D.adynamic bone disease is rare
-
A.
- In pediatric renal transplant recipients, bone density
-
A.is best assessed using DXA with age-matched controls
-
B.is best assessed using DXA with height-matched controls
-
C.is best assessed using DXA with gender-matched controls
-
D.cannot reliably be assessed by DXA
-
A.
- Post renal transplantation, cardiovascular disease
-
A.resolves within the first 2 years
-
B.improves (in adults) with the use of lipid-lowering agents
-
C.is unrelated to traditional risk factors, such as hypertension
-
D.is minimal in children
-
A.
- The leading cause of death in children post renal transplantation is
-
A.cardiovascular disease
-
B.cancer
-
C.infection
-
D.trauma
-
A.
- After renal transplantation growth
-
A.improves equally in all age groups, from infancy to adolescence
-
B.improves with steroid-avoidant and steroid-minimizing immunosuppressant protocols
-
C.does not respond to growth hormone
-
D.is no longer a clinical problem
-
A.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
ANSWERS:
1. C
2. D
3. B
4. A
5. B
References
- 1.Soyka LA, Fairfield WP, Klibanski A. Clinical review 117: hormonal determinants and disorders of peak bone mass in children. J Clin Endocrinol Metab. 2000;85:3951–3963. doi: 10.1210/jcem.85.11.6994. [DOI] [PubMed] [Google Scholar]
- 2.KDIGO Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int. 2009;76:s1–s130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 3.Bartosh SM, Leverson G, Robillard D, Sollinger HW. Long-term outcomes in pediatric renal transplant recipients who survive into adulthood. Transplantation. 2003;76:1195–1200. doi: 10.1097/01.TP.0000092524.75807.84. [DOI] [PubMed] [Google Scholar]
- 4.Groothoff JW, Cransberg K, Offringa M. Long-term follow-up of renal transplantation in children: a Dutch cohort study. Transplantation. 2004;78:453–460. doi: 10.1097/01.tp.0000128616.02821.8b. [DOI] [PubMed] [Google Scholar]
- 5.Groothoff JW, Lilien MR, van de Kar NC, Wolff ED, Davin JC. Cardiovascular disease as a late complication of end-stage renal disease in children. Pediatr Nephrol. 2005;20:374–379. doi: 10.1007/s00467-004-1624-8. [DOI] [PubMed] [Google Scholar]
- 6.Valta H, Makitie O, Ronnholm K, Jalanko H. Bone health in children and adolescents after renal transplantation. J Bone Miner Res. 2009;24:1699–1708. doi: 10.1359/jbmr.090407. [DOI] [PubMed] [Google Scholar]
- 7.Helenius I, Remes V, Tervahartiala P, Salminen S, Sairanen H, Holmberg C, Palmu P, Helenius M, Peltonen J, Jalanko H. Spine after solid organ transplantation in childhood: a clinical, radiographic, and magnetic resonance imaging analysis of 40 patients. Spine. 2006;31:2130–2136. doi: 10.1097/01.brs.0000231717.63974.f3. [DOI] [PubMed] [Google Scholar]
- 8.Helenius I, Remes V, Salminen S, Valta H, Mäkitie O, Holmberg C, Palmu P, Tervahartiala P, Sarna S, Helenius M, Peltonen J, Jalanko H. Incidence and predictors of fractures in children after solid organ transplantation: a 5-year prospective, population-based study. J Bone Miner Res. 2006;21:380–387. doi: 10.1359/JBMR.051107. [DOI] [PubMed] [Google Scholar]
- 9.Garabedian M, Silve C, Levy-Bentolila D, Bourdeau A, Ulmann A, Nguyen TM, Lieberherr M, Broyer M, Balsan S. Changes in plasma 1,25 and 24,25-dihydroxyvitamin D after renal transplantation in children. Kidney Int. 1981;20:403–410. doi: 10.1038/ki.1981.153. [DOI] [PubMed] [Google Scholar]
- 10.Tuchman S, Kalkwarf HJ, Zemel BS, Shults J, Wetzsteon RJ, Foerster D, Strife CF, Leonard MB. Vitamin D deficiency and parathyroid hormone levels following renal transplantation in children. Pediatr Nephrol. 2010;25(12):2509–2516. doi: 10.1007/s00467-010-1612-0. [DOI] [PubMed] [Google Scholar]
- 11.June CH, Thompson CB, Kennedy MS, Nims J, Thomas ED. Profound hypomagnesemia and renal magnesium wasting associated with the use of cyclosporine for marrow transplantation. Transplantation. 1985;39:620–624. doi: 10.1097/00007890-198506000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Bhan I, Shah A, Holmes J, Isakova T, Gutierrez O, Burnett SM, Jüppner H, Wolf M. Post-transplant hypophosphatemia: tertiary 'hyper-phosphatoninism'? Kidney Int. 2006;70:1486–1494. doi: 10.1038/sj.ki.5001788. [DOI] [PubMed] [Google Scholar]
- 13.Evenepoel P, Meijers BK, de Jonge H, Naesens M, Bammens B, Claes K, Kuypers D, Vanrenterghem Y. Recovery of hyperphosphatoninism and renal phosphorus wasting one year after successful renal transplantation. Clin J Am Soc Nephrol. 2008;3:1829–1836. doi: 10.2215/CJN.01310308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evenepoel P, Naesens M, Claes K, Kuypers D, Vanrenterghem Y. Tertiary 'hyperphosphatoninism' accentuates hypophosphatemia and suppresses calcitriol levels in renal transplant recipients. Am J Transplant. 2007;7:1193–1200. doi: 10.1111/j.1600-6143.2007.01753.x. [DOI] [PubMed] [Google Scholar]
- 15.Bacchetta J, Dubourg L, Harambat J, Ranchin B, Abou-Jaoude P, Arnaud S, Carlier MC, Richard M, Cochat P. The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J Clin Endocrinol Metab. 2010;95:1741–1748. doi: 10.1210/jc.2009-1576. [DOI] [PubMed] [Google Scholar]
- 16.D'Alessandro AM, Melzer JS, Pirsch JD, Sollinger HW, Kalayoglu M, Vernon WB, Belzer FO, Starling JR. Tertiary hyperparathyroidism after renal transplantation: operative indications. Surgery. 1989;106:1049–1055. [PubMed] [Google Scholar]
- 17.Gwinner W, Suppa S, Mengel M, Hoy L, Kreipe HH, Haller H, Schwarz A. Early calcification of renal allografts detected by protocol biopsies: causes and clinical implications. Am J Transplant. 2005;5:1934–1941. doi: 10.1111/j.1600-6143.2005.00938.x. [DOI] [PubMed] [Google Scholar]
- 18.Kruse AE, Eisenberger U, Frey FJ, Mohaupt MG. The calcimimetic cinacalcet normalizes serum calcium in renal transplant patients with persistent hyperparathyroidism. Nephrol Dial Transplant. 2005;20:1311–1314. doi: 10.1093/ndt/gfh924. [DOI] [PubMed] [Google Scholar]
- 19.Leca N, Laftavi M, Gundroo A, Kohli R, Min I, Karam J, Sridhar N, Blessios G, Venuto R, Pankewycz O. Early and severe hyperparathyroidism associated with hypercalcemia after renal transplant treated with cinacalcet. Am J Transplant. 2006;6:2391–2395. doi: 10.1111/j.1600-6143.2006.01475.x. [DOI] [PubMed] [Google Scholar]
- 20.Szwarc I, Argiles A, Garrigue V, Delmas S, Chong G, Deleuze S, Mourad G. Cinacalcet chloride is efficient and safe in renal transplant recipients with posttransplant hyperparathyroidism. Transplantation. 2006;82(5):675–680. doi: 10.1097/01.tp.0000232452.80018.ad. [DOI] [PubMed] [Google Scholar]
- 21.Srinivas TR, Schold JD, Womer KL, Kaplan B, Howard RJ, Bucci CM, Meier-Kriesche HU. Improvement in hypercalcemia with cinacalcet after kidney transplantation. Clin J Am Soc Nephrol. 2006;1(2):323–326. doi: 10.2215/CJN.00500705. [DOI] [PubMed] [Google Scholar]
- 22.Borchhardt K, Sulzbacher I, Benesch T, Fodinger M, Sunder-Plassmann G, Haas M. Low-turnover bone disease in hypercalcemic hyperparathyroidism after kidney transplantation. Am J Transplant. 2007;7(11):2515–2521. doi: 10.1111/j.1600-6143.2007.01950.x. [DOI] [PubMed] [Google Scholar]
- 23.Borchhardt KA, Diarra D, Sulzbacher I, Benesch T, Haas M, Sunder-Plassmann G. Cinacalcet decreases bone formation rate in hypercalcemic hyperparathyroidism after kidney transplantation. Am J Nephrol. 2010;31(6):482–489. doi: 10.1159/000304180. [DOI] [PubMed] [Google Scholar]
- 24.Coburn JW, Koppel MH, Brickman AS, Massry SG. Study of intestinal absorption of calcium in patients with renal failure. Kidney Int. 1973;3:264–272. doi: 10.1038/ki.1973.40. [DOI] [PubMed] [Google Scholar]
- 25.Holick MF. Vitamin D and the kidney. Kidney Int. 1987;32:912–929. doi: 10.1038/ki.1987.295. [DOI] [PubMed] [Google Scholar]
- 26.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 27.Helvig CF, Cuerrier D, Hosfield CM, Ireland B, Kharebov AZ, Kim JW, Ramjit NJ, Ryder K, Tabash SP, Herzenberg AM, Epps TM, Petkovich M. Dysregulation of renal vitamin D metabolism in the uremic rat. Kidney Int. 2010;78(5):463–472. doi: 10.1038/ki.2010.168. [DOI] [PubMed] [Google Scholar]
- 28.Julian BA, Laskow DA, Dubovsky J, Dubovsky EV, Curtis JJ, Quarles LD. Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med. 1991;325:544–550. doi: 10.1056/NEJM199108223250804. [DOI] [PubMed] [Google Scholar]
- 29.Velasquez-Forero F, Mondragon A, Herrero B, Pena JC. Adynamic bone lesion in renal transplant recipients with normal renal function. Nephrol Dial Transplant. 1996;11(Suppl 3):58–64. doi: 10.1093/ndt/11.supp3.58. [DOI] [PubMed] [Google Scholar]
- 30.Carlini RG, Rojas E, Arminio A, Weisinger JR, Bellorin-Font E. What are the bone lesions in patients with more than four years of a functioning renal transplant? Nephrol Dial Transplant. 1998;13(Suppl 3):103–104. doi: 10.1093/ndt/13.suppl_3.103. [DOI] [PubMed] [Google Scholar]
- 31.Lehmann G, Ott U, Stein G, Steiner T, Wolf G. Renal osteodystrophy after successful renal transplantation: a histomorphometric analysis in 57 patients. Transplant Proc. 2007;39:3153–3158. doi: 10.1016/j.transproceed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Cruz EA, Lugon JR, Jorgetti V, Draibe SA, Carvalho AB. Histologic evolution of bone disease 6 months after successful kidney transplantation. Am J Kidney Dis. 2004;44:747–756. [PubMed] [Google Scholar]
- 33.Sanchez CP, Salusky IB, Kuizon BD, Ramirez JA, Gales B, Ettenger RB, Goodman WG. Bone disease in children and adolescents undergoing successful renal transplantation. Kidney Int. 1998;53:1358–1364. doi: 10.1046/j.1523-1755.1998.00866.x. [DOI] [PubMed] [Google Scholar]
- 34.Hahn TJ, Halstead LR, Baran DT. Effects off short-term glucocorticoid administration on intestinal calcium absorption and circulating vitamin D metabolite concentrations in man. J Clin Endocrinol Metab. 1981;52:111–115. doi: 10.1210/jcem-52-1-111. [DOI] [PubMed] [Google Scholar]
- 35.McIntyre HD, Menzies B, Rigby R, Perry-Keene DA, Hawley CM, Hardie IR. Long-term bone loss after renal transplantation: comparison of immunosuppressive regimens. Clin Transplant. 1995;9:20–24. [PubMed] [Google Scholar]
- 36.Ponticelli C, Aroldi A. Osteoporosis after organ transplantation. Lancet. 2001;357:1623. doi: 10.1016/S0140-6736(00)04765-6. [DOI] [PubMed] [Google Scholar]
- 37.Grotz W, Mundinger A, Gugel B, Exner V, Reichelt A, Schollmeyer P. Missing impact of cyclosporine on osteoporosis in renal transplant recipients. Transplant Proc. 1994;26:2652–2653. [PubMed] [Google Scholar]
- 38.Allen DB, Goldberg BD. Stimulation of collagen synthesis and linear growth by growth hormone in glucocorticoid-treated children. Pediatrics. 1992;89:416–421. [PubMed] [Google Scholar]
- 39.Root AW, Bongiovanni AM, Eberlein WR. Studies of the secretion and metabolic effects of human growth hormone in children with glucocorticoid-induced growth retardation. J Pediatr. 1969;75:826–832. doi: 10.1016/s0022-3476(69)80306-9. [DOI] [PubMed] [Google Scholar]
- 40.Ortoft G, Oxlund H. Qualitative alterations of cortical bone in female rats after long-term administration of growth hormone and glucocorticoid. Bone. 1996;18:581–590. doi: 10.1016/8756-3282(96)00077-4. [DOI] [PubMed] [Google Scholar]
- 41.Wehrenberg WB, Bergman PJ, Stagg L, Ndon J, Giustina A. Glucocorticoid inhibition of growth in rats: partial reversal with somatostatin antibodies. Endocrinology. 1990;127:2705–2708. doi: 10.1210/endo-127-6-2705. [DOI] [PubMed] [Google Scholar]
- 42.Rubin MR, Bilezikian JP. Clinical review 151: the role of parathyroid hormone in the pathogenesis of glucocorticoid-induced osteoporosis: a re-examination of the evidence. J Clin Endocrinol Metab. 2002;87:4033–4041. doi: 10.1210/jc.2002-012101. [DOI] [PubMed] [Google Scholar]
- 43.Aubia J, Serrano S, Marinoso L, Hojman L, Diez A, Lloveras J, Masramon J. Osteodystrophy of diabetics in chronic dialysis: a histomorphometric study. Calcif Tissue Int. 1988;42:297–301. doi: 10.1007/BF02556363. [DOI] [PubMed] [Google Scholar]
- 44.Movsowitz C, Epstein S, Fallon M, Ismail F, Thomas S. Cyclosporin-A in vivo produces severe osteopenia in the rat: effect of dose and duration of administration. Endocrinology. 1988;123:2571–2577. doi: 10.1210/endo-123-5-2571. [DOI] [PubMed] [Google Scholar]
- 45.Alvarez-Garcia O, Garcia-Lopez E, Loredo V, Gil-Peña H, Rodríguez-Suárez J, Ordóñez FA, Carbajo-Pérez E, Santos F. Rapamycin induces growth retardation by disrupting angiogenesis in the growth plate. Kidney Int. 2010;78:561–568. doi: 10.1038/ki.2010.173. [DOI] [PubMed] [Google Scholar]
- 46.Bryer HP, Isserow JA, Armstrong EC, Mann GN, Rucinski B, Buchinsky FJ, Romero DF, Epstein S. Azathioprine alone is bone sparing and does not alter cyclosporin A-induced osteopenia in the rat. J Bone Miner Res. 1995;10:132–138. doi: 10.1002/jbmr.5650100119. [DOI] [PubMed] [Google Scholar]
- 47.Toussaint ND, Pedagogos E, Lau KK, Heinze S, Becker GJ, Beavis J, Polkinghorne KR, Damasiewicz MJ, Kerr PG. Lateral lumbar X-ray assessment of abdominal aortic calcification in Australian haemodialysis patients. Nephrology (Carlton) 2010 doi: 10.1111/j.1440-1797.2010.01420.x. [DOI] [PubMed] [Google Scholar]
- 48.Bacchetta J, Boutroy S, Juillard L, Vilayphiou N, Guebre-Egziabher F, Pelletier S, Delmas PD, Fouque D. Bone imaging and chronic kidney disease: will high-resolution peripheral tomography improve bone evaluation and therapeutic management? J Ren Nutr. 2009;19:44–49. doi: 10.1053/j.jrn.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 49.Weber LT, Mehls O. Limitations of dual x-ray absorptiometry in children with chronic kidney disease. Pediatr Nephrol. 2010;25:3–5. doi: 10.1007/s00467-009-1248-0. [DOI] [PubMed] [Google Scholar]
- 50.Bianchi ML, Baim S, Bishop NJ, Gordon CM, Hans DB, Langman CB, Leonard MB, Kalkwarf HJ, International Society for Clinical Densitometry (ISCD) Official positions of the International Society for Clinical Densitometry (ISCD) on DXA evaluation in children and adolescents. Pediatr Nephrol. 2010;25:37–47. doi: 10.1007/s00467-009-1249-z. [DOI] [PubMed] [Google Scholar]
- 51.Saland JM, Goode ML, Haas DL, Romano TA, Seikaly MG. The prevalence of osteopenia in pediatric renal allograft recipients varies with the method of analysis. Am J Transplant. 2001;1:243–250. doi: 10.1034/j.1600-6143.2001.001003243.x. [DOI] [PubMed] [Google Scholar]
- 52.Chesney RW. Bone mineral density in chronic renal insufficiency and end-stage renal disease: how to interpret the scans. J Pediatr Endocrinol Metab. 2004;17(Suppl 4):1327–1332. [PubMed] [Google Scholar]
- 53.Bachrach LK. Measuring bone mass in children: can we really do it? Horm Res. 2006;65(Suppl 2):11–16. doi: 10.1159/000091749. [DOI] [PubMed] [Google Scholar]
- 54.Burrows M, Liu D, McKay H. High-resolution peripheral QCT imaging of bone micro-structure in adolescents. Osteoporos Int. 2010;21:515–520. doi: 10.1007/s00198-009-0913-2. [DOI] [PubMed] [Google Scholar]
- 55.Bacchetta J, Fargue S, Boutroy S, Basmaison O, Vilayphiou N, Plotton I, Guebre-Egziabher F, Dohin B, Kohler R, Cochat P. Bone metabolism in oxalosis: a single-center study using new imaging techniques and biomarkers. Pediatr Nephrol. 2010;25:1081–1089. doi: 10.1007/s00467-010-1453-x. [DOI] [PubMed] [Google Scholar]
- 56.Ruth EM, Weber LT, Schoenau E, Wunsch R, Seibel MJ, Feneberg R, Mehls O, Tönshoff B. Analysis of the functional muscle-bone unit of the forearm in pediatric renal transplant recipients. Kidney Int. 2004;66:1694–1706. doi: 10.1111/j.1523-1755.2004.00937.x. [DOI] [PubMed] [Google Scholar]
- 57.Mussa A, Porta F, Gianoglio B, Gaido M, Nicolosi MG, De Terlizzi F, de Sanctis C, Coppo R. Bone alterations in children and young adults with renal transplant assessed by phalangeal quantitative ultrasound. Am J Kidney Dis. 2007;50:441–449. doi: 10.1053/j.ajkd.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 58.(2008) North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) Annual Report
- 59.Quesada JM, Serrano I, Borrego F, Martin A, Pena J, Solana R. Calcitriol effect on natural killer cells from hemodialyzed and normal subjects. Calcif Tissue Int. 1995;56:113–117. doi: 10.1007/BF00296341. [DOI] [PubMed] [Google Scholar]
- 60.Sarwal MM, Yorgin PD, Alexander S, Millan MT, Belson A, Belanger N, Granucci L, Major C, Costaglio C, Sanchez J, Orlandi P, Salvatierra O., Jr Promising early outcomes with a novel, complete steroid avoidance immunosuppression protocol in pediatric renal transplantation. Transplantation. 2001;72:13–21. doi: 10.1097/00007890-200107150-00006. [DOI] [PubMed] [Google Scholar]
- 61.Sarwal MM, Vidhun JR, Alexander SR, Satterwhite T, Millan M, Salvatierra O., Jr Continued superior outcomes with modification and lengthened follow-up of a steroid-avoidance pilot with extended daclizumab induction in pediatric renal transplantation. Transplantation. 2003;76:1331–1339. doi: 10.1097/01.TP.0000092950.54184.67. [DOI] [PubMed] [Google Scholar]
- 62.Hokken-Koelega AC, van Zaal MA, van Bergen W, de Ridder MA, Stijnen T, Wolff ED, de Jong RC, Donckerwolcke RA, de Muinck Keizer-Schrama SM, Drop SL. Final height and its predictive factors after renal transplantation in childhood. Pediatr Res. 1994;36:323–328. doi: 10.1203/00006450-199409000-00009. [DOI] [PubMed] [Google Scholar]
- 63.Grenda R, Watson A, Trompeter R, Tönshoff B, Jaray J, Fitzpatrick M, Murer L, Vondrak K, Maxwell H, van Damme-Lombaerts R, Loirat C, Mor E, Cochat P, Milford DV, Brown M, Webb NJ. A randomized trial to assess the impact of early steroid withdrawal on growth in pediatric renal transplantation: the TWIST study. Am J Transplant. 2010;10:828–836. doi: 10.1111/j.1600-6143.2010.03047.x. [DOI] [PubMed] [Google Scholar]
- 64.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 65.Chavers BM, Li S, Collins AJ, Herzog CA. Cardiovascular disease in pediatric chronic dialysis patients. Kidney Int. 2002;62:648–653. doi: 10.1046/j.1523-1755.2002.00472.x. [DOI] [PubMed] [Google Scholar]
- 66.Moe SM, Duan D, Doehle BP, O'Neill KD, Chen NX. Uremia induces the osteoblast differentiation factor Cbfa1 in human blood vessels. Kidney Int. 2003;63:1003–1011. doi: 10.1046/j.1523-1755.2003.00820.x. [DOI] [PubMed] [Google Scholar]
- 67.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 68.Ahmed S, O'Neill KD, Hood AF, Evan AP, Moe SM. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:1267–1276. doi: 10.1053/ajkd.2001.24533. [DOI] [PubMed] [Google Scholar]
- 69.Bostrom K. Insights into the mechanism of vascular calcification. Am J Cardiol. 2001;88:20E–22E. doi: 10.1016/s0002-9149(01)01718-0. [DOI] [PubMed] [Google Scholar]
- 70.Moe SM, O'Neill KD, Duan D, Ahmed S, Chen NX, Leapman SB, Fineberg N, Kopecky K. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int. 2002;61:638–647. doi: 10.1046/j.1523-1755.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- 71.Chen NX, O'Neill KD, Duan D, Moe SM. Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells. Kidney Int. 2002;62:1724–1731. doi: 10.1046/j.1523-1755.2002.00625.x. [DOI] [PubMed] [Google Scholar]
- 72.Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schinke T, Amendt C, Trindl A, Poschke O, Muller-Esterl W, Jahnen-Dechent W. The serum protein alpha2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. A possible role in mineralization and calcium homeostasis. J Biol Chem. 1996;271:20789–20796. doi: 10.1074/jbc.271.34.20789. [DOI] [PubMed] [Google Scholar]
- 74.Sweatt A, Sane DC, Hutson SM, Wallin R. Matrix Gla protein (MGP) and bone morphogenetic protein-2 in aortic calcified lesions of aging rats. J Thromb Haemost. 2003;1:178–185. doi: 10.1046/j.1538-7836.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 75.Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280:1015–1020. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 76.Imanishi Y, Inaba M, Nakatsuka K, Nagasue K, Okuno S, Yoshihara A, Miura M, Miyauchi A, Kobayashi K, Miki T, Shoji T, Ishimura E, Nishizawa Y. FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int. 2004;65:1943–1946. doi: 10.1111/j.1523-1755.2004.00604.x. [DOI] [PubMed] [Google Scholar]
- 77.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the heart and soul study. Ann Intern Med. 2010;152:640–648. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seiler S, Reichart B, Roth D, Seibert E, Fliser D, Heine GH. FGF-23 and future cardiovascular events in patients with chronic kidney disease before initiation of dialysis treatment. Nephrol Dial Transplant. 2010;25:3983–3989. doi: 10.1093/ndt/gfq309. [DOI] [PubMed] [Google Scholar]
- 80.Ishitani MB, Milliner DS, Kim DY, Bohorquez HE, Heimbach JK, Sheedy PF, 2nd, Morgenstern BZ, Gloor JM, Murphy JG, McBane RD, Bielak LF, Peyser PA, Stegall MD. Early subclinical coronary artery calcification in young adults who were pediatric kidney transplant recipients. Am J Transplant. 2005;5:1689–1693. doi: 10.1111/j.1600-6143.2005.00914.x. [DOI] [PubMed] [Google Scholar]
- 81.Moe SM, O'Neill KD, Reslerova M, Fineberg N, Persohn S, Meyer CA. Natural history of vascular calcification in dialysis and transplant patients. Nephrol Dial Transplant. 2004;19:2387–2393. doi: 10.1093/ndt/gfh303. [DOI] [PubMed] [Google Scholar]
- 82.Hristova M, Van BC, Schurgers LJ, Lanske B, Danziger J. Rapidly progressive severe vascular calcification sparing the kidney allograft following warfarin initiation. Am J Kidney Dis. 2010;56:1158–1162. doi: 10.1053/j.ajkd.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shroff RC, McNair R, Skepper JN, Figg N, Schurgers LJ, Deanfield J, Rees L, Shanahan CM. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol. 2010;21:103–112. doi: 10.1681/ASN.2009060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Milliner DS, Zinsmeister AR, Lieberman E, Landing B. Soft tissue calcification in pediatric patients with end-stage renal disease. Kidney Int. 1990;38(5):931–936. doi: 10.1038/ki.1990.293. [DOI] [PubMed] [Google Scholar]
- 85.Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F. Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation. 2002;106:100–105. doi: 10.1161/01.cir.0000020222.63035.c0. [DOI] [PubMed] [Google Scholar]
- 86.Mitsnefes MM, Kimball TR, Witt SA, Glascock BJ, Khoury PR, Daniels SR. Abnormal carotid artery structure and function in children and adolescents with successful renal transplantation. Circulation. 2004;110:97–101. doi: 10.1161/01.CIR.0000133412.53089.26. [DOI] [PubMed] [Google Scholar]
- 87.Bilginer Y, Ozaltin F, Basaran C, Aki TF, Karabulut E, Duzova A, Besbas N, Topaloglu R, Ozen S, Bakkaloglu M, Al B. Carotid intima-media thickness in children and young adults with renal transplant: internal carotid artery vs. common carotid artery. Pediatr Transplant. 2007;11:888–894. doi: 10.1111/j.1399-3046.2007.00760.x. [DOI] [PubMed] [Google Scholar]
- 88.van Summeren MJ, Hameleers JM, Schurgers LJ, Hoeks AP, Uiterwaal CS, Krüger T, Vermeer C, Kuis W, Lilien MR. Circulating calcification inhibitors and vascular properties in children after renal transplantation. Pediatr Nephrol. 2008;23:985–993. doi: 10.1007/s00467-007-0743-4. [DOI] [PubMed] [Google Scholar]
- 89.Delucchi A, Dinamarca H, Gainza H, Whitttle C, Torrealba I, Iniguez G. Carotid intima-media thickness as a cardiovascular risk marker in pediatric end-stage renal disease patients on dialysis and in renal transplantation. Transplant Proc. 2008;40:3244–3246. doi: 10.1016/j.transproceed.2008.03.126. [DOI] [PubMed] [Google Scholar]
- 90.Siirtola A, Kallio T, Ala-Houhala M, Lehtimäki T, Solakivi T, Antikainen M, Salo MK, Holmberg C. Carotid intima-media thickness after pediatric renal or liver transplantation at high-resolution B-mode ultrasonography. Transplant Proc. 2010;42:1695–1698. doi: 10.1016/j.transproceed.2010.02.096. [DOI] [PubMed] [Google Scholar]
- 91.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 92.Patel S, Kwan JT, McCloskey E, McGee G, Thomas G, Johnson D, Wills R, Ogunremi L, Barron J. Prevalence and causes of low bone density and fractures in kidney transplant patients. J Bone Miner Res. 2001;16:1863–1870. doi: 10.1359/jbmr.2001.16.10.1863. [DOI] [PubMed] [Google Scholar]
- 93.Lippuner K, Casez JP, Horber FF, Jaeger P. Effects of deflazacort versus prednisone on bone mass, body composition, and lipid profile: a randomized, double-blind study in kidney transplant patients. J Clin Endocrinol Metab. 1998;83:3795–3802. doi: 10.1210/jcem.83.11.5235. [DOI] [PubMed] [Google Scholar]
- 94.Fine RN, Yadin O, Nelson PA, Pyke-Grimm K, Boechat MI, Lippe BH, Sherman BM, Ettenger RB, Kamil E. Recombinant human growth hormone treatment of children following renal transplantation. Pediatr Nephrol. 1991;5:147–151. doi: 10.1007/BF00852873. [DOI] [PubMed] [Google Scholar]
- 95.Cueto-Manzano AM, Konel S, Freemont AJ, Adams JE, Mawer B, Gokal R, Hutchison AJ. Effect of 1, 25-dihydroxyvitamin D3 and calcium carbonate on bone loss associated with long-term renal transplantation. Am J Kidney Dis. 2000;35:227–236. doi: 10.1016/s0272-6386(00)70331-3. [DOI] [PubMed] [Google Scholar]
- 96.Fan SL, Almond MK, Ball E, Evans K, Cunningham J. Pamidronate therapy as prevention of bone loss following renal transplantation. Kidney Int. 2000;57:684–690. doi: 10.1046/j.1523-1755.2000.00890.x. [DOI] [PubMed] [Google Scholar]
- 97.Amerling R, Harbord NB, Pullman J, Feinfeld DA. Bisphosphonate use in chronic kidney disease: association with adynamic bone disease in a bone histology series. Blood Purif. 2010;29:293–299. doi: 10.1159/000276666. [DOI] [PubMed] [Google Scholar]
- 98.Holdaas H, Fellstrom B, Cole E, Nyberg G, Olsson AG, Pedersen TR, Madsen S, Grönhagen-Riska C, Neumayer HH, Maes B, Ambühl P, Hartmann A, Staffler B, Jardine AG, Assessment of LEscol in Renal Transplantation (ALERT) Study Investigators Long-term cardiac outcomes in renal transplant recipients receiving fluvastatin: the ALERT extension study. Am J Transplant. 2005;5:2929–2936. doi: 10.1111/j.1600-6143.2005.01105.x. [DOI] [PubMed] [Google Scholar]
- 99.Cui Q, Wang GJ, Su CC, Balian G. The Otto Aufranc Award. Lovastatin prevents steroid induced adipogenesis and osteonecrosis. Clin Orthop Relat Res. 1997;344:8–19. [PubMed] [Google Scholar]
- 100.Maritz FJ, Conradie MM, Hulley PA, Gopal R, Hough S. Effect of statins on bone mineral density and bone histomorphometry in rodents. Arterioscler Thromb Vasc Biol. 2001;21:1636–1641. doi: 10.1161/hq1001.097781. [DOI] [PubMed] [Google Scholar]
- 101.Pritchett JW. Statin therapy decreases the risk of osteonecrosis in patients receiving steroids. Clin Orthop Relat Res. 2001;386:173–178. doi: 10.1097/00003086-200105000-00022. [DOI] [PubMed] [Google Scholar]
- 102.Ajmal M, Matas AJ, Kuskowski M, Cheng EY. Does statin usage reduce the risk of corticosteroid-related osteonecrosis in renal transplant population? Orthop Clin North Am. 2009;40:235–239. doi: 10.1016/j.ocl.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]