Abstract
The ability of the Model for End-Stage Liver Disease (MELD) score to capture the urgency of transplantation may not be generalizable to patients with primary sclerosing cholangitis (PSC) because such patients face unique risks of death or removal from the liver transplant waitlist due to disease-specific complications (e.g. repeated bouts of bacterial cholangitis and cholangiocarcinoma). We constructed Cox regression models to determine whether disease-based differences exist in waitlist mortality prior to liver transplantation. We compared time to death on or withdrawal from the waitlist due to clinical deterioration among patients with or without PSC in the United States following implementation of the MELD allocation score. Over an eight-year period, 14,073 (20.5%) non-PSC patients died or were removed, compared with 432 (13.6%) patients with PSC (P<0.0001). The adjusted hazard ratio for PSC was 0.72 (95% CI: 0.66-0.79), indicating that these patients had a lower time-dependent risk of death on or removal from the waitlist than patients without PSC. This difference was explained, in part, by differences in the groups’ probabilities of having complications of portal hypertension at listing, as adjustment for these intermediate endpoints moved the hazard ratio closer to the null (0.84; 95% CI 0.74-0.97). Because patients with PSC are less like to die or be removed from the waitlist due to clinical deterioration than are patients with other forms of end-stage liver disease, reconsideration should be given to prevailing practices in some centers and regions of pre-emptive referral of PSC patients for living donor transplantation or provision of exception points.
Keywords: waitlist mortality, living donor transplantation, MELD score, exception points, cholangiocarcinoma
Primary sclerosing cholangitis (PSC) is a chronic, cholestatic liver disease of unclear etiology with an estimated incidence of approximately 1 per 100,000 person-years.(1, 2) Liver transplantation is the only known beneficial therapy for this progressive and potentially fatal disease, and survival rates post-transplantation are favorable for patients with PSC, exceeding 80% and 70% at 5 and 8 years, respectively.(3)
However, there are reasons to suspect that the Model for End Stage Liver Disease (MELD) score, used to allocate deceased donor livers in the United States since February 27, 2002, may poorly predict waitlist mortality among patients with PSC. First, although patients with PSC suffer general complications of portal hypertension, they also are at increased risk for specific adverse outcomes including cholangiocarcinoma (CCA), ascending cholangitis due to biliary strictures, and, among the approximately 70-80% of patients with PSC with concomitant inflammatory bowel disease (IBD), complications such as colon cancer.(1) The risk for CCA is of particular concern because it develops in 6%-36% of patients with PSC and commonly precludes liver transplantation.(4-16) Second, few patients with PSC were included in the cohort used to derive the MELD score, and recent evidence suggests that providing greater weight to bilirubin (the component of MELD most affected in PSC) would provide superior prediction of waitlist mortality.(17)
To circumvent these perceived disadvantages for patients with PSC, some have speculated that early or “preemptive” transplantation be considered for such patients.(18) Indeed, we have recently shown that PSC patients preferentially receive living donor transplants, perhaps due to preemptive referral patterns.(19) Additionally, some patients with PSC (e.g. those with two documented episodes of cholangitis requiring intravenous antibiotics and hospitalization within one year) are awarded exception points to increase their access to transplantation. (20)
Although these efforts to increase access for PSC patients appear meritorious, there is little evidence available to assess whether they are sufficient or, by contrast, necessary to promote equal access to transplantation. To date, only two studies have addressed the waitlist mortality of patients with PSC. The first was a study looking at the waitlist mortality of all patients in the United States one year after the implementation of the MELD allocation score, which showed that patients with PSC had a lower risk of death or removal, however this was a limited one-year study that presented an unadjusted analysis.(21) The only long-term study to focus of the outcomes of patients with PSC on a transplant waitlist was performed in Scandinavia where the MELD score is not used for liver allocation. The authors observed waitlist mortality of 3% among patients with PSC, compared with 7% of patients with other liver diseases, but also found that 9% (n=24) of patients with PSC, as compared to 4% of others, were withdrawn from the waiting list, mainly due to CCA. These findings suggest that patients with PSC listed for transplantation may have reduced access to organs. Such disadvantages may manifest even more strongly in the U.S., where waitlist times are typically longer, thereby exposing patients with PSC to greater time at risk for complications.(22)
We designed the present study to determine whether, and why, patients with PSC may have different risks for death or removal from the waitlist than do patients with other forms of end-stage liver disease (ESLD). Our goal was to provide evidence regarding the appropriateness, necessity, or sufficiency of pre-emptive transplantation or provision of exception points to patients with PSC.
Patients and Methods
Patients
We used the United Network for Organ Sharing Database (UNOS) Organ Procurement and Transplant Network (OPTN) database to identify all patients listed for liver transplantation on or after February 27, 2002 (the first date on which all such patients were assigned a MELD score). We also included patients remaining on the waitlist from prior to February 27th, 2002 because such patients were assigned a MELD score on that date. By including the person-time they accrued from February 27, 2002 forward, we were able to fully compare time to death or waitlist removal among patients with different etiologies of liver disease in the post-MELD era. Follow-up was through May 31, 2009, at which time we censored all patients remaining on the waitlist.
We excluded patients under age 18 for two reasons: first, for patients under age 12 livers are allocated by a distinct model, the Pediatric End-Stage Liver Disease (PELD) score; second, patients between the ages of 12-18 exhibit a substantially different spectrum of primary diagnoses than do those older than 18. We also excluded patients listed for retransplantation so as to ensure that all observations represent unique individuals. Lastly, we excluded patients who were listed as status 1 (defined as fulminant hepatic failure with a life expectancy of less than seven days without liver transplantation) because such patients rarely have chronic liver diseases and they are allocated organs without using the MELD score. Of the 72,158 eligible patients, we excluded 362 (0.5%) due to missing primary diagnoses.
Outcome
The primary outcome variable was death on or removal from the waitlist due to becoming too sick for transplantation or otherwise medically unsuitable (which we collectively refer to as clinical deterioration). We considered patients removed from the transplant list due to clinical deterioration as equivalent to those who died because these chronic liver diseases are almost uniformly fatal in the short term without transplantation. Such grouping is consistent with prior research in the field.(23, 24) All other outcomes were censored, with the most common censoring events being transplantation or removal due to an improved condition that no longer required transplantation. In secondary analyses, we examined the perhaps more clinically salient outcome of whether or not listed patients died or deteriorated clinically while on the waitlist, regardless of the waitlist time accrued beforehand. For this purpose, we used the binary outcome of death/removal due to clinical deterioration vs. all other removals (including transplantation).
Statistical Analysis
To compare patients with or without PSC, we used Fisher’s exact tests and chi-square tests for dichotomous variables and student t tests or Wilcoxon rank-sum tests for continuous variables, depending on their distributions.
We developed a Cox regression model to determine whether the relative hazard of death or withdrawal from the waitlist differed among patients with or without PSC after accounting for the proportion of such hazards captured by the MELD score. MELD score was treated as a time-varying covariate to enable adjustment for the full proportion of risk over time captured by MELD. We selected other independent variables for inclusion in the final model if they were independently associated with the outcome (P<0.05) or if their removal from the model changed the coefficient for PSC by ≥10%. Variables tested using these criteria included age, gender, race, blood type, UNOS region, and insurance type (private vs. public).
For any patient given MELD exception points (including but not limited to complications such as hepatocellular carcinoma), we used the higher MELD value (incorporating exception points) because the higher value reflects the patient’s true prioritization on the waitlist. However, to adjust for the fact that patients with PSC may have improved survival compared to other patients given the non-standardized practice of granting exception points for these patients, we created another time-varying binary covariate to indicate whether exception points were granted. That is, among patients with PSC, for each individual MELD score that was greater than what would be calculated using INR, bilirubin, and creatinine alone, the covariate was assigned a value of 1; for all other scores it was assigned a value of 0. By incorporating this adjustment, we could ascertain the extent to which outcomes among patients with PSC were influenced by the non-systematic granting of exception points.
We introduced a final time-varying covariate to account for the time that waitlist patients were temporally inactive (status 7) because such time enabled patient to accrue risk for outcomes such as death but not for transplantation. Inserting a time-varying covariate to adjust for this time prevented spurious results that could arise if patients with and without PSC differed in the time they spent inactive.
We stratified the baseline hazard function by transplant center to accommodate heterogeneity in the hazard of death on or removal from the transplant list across centers. We used the sandwich variance estimator to properly account for the correlation due to clustering of patients with centers. Doing so accounted for patients who were transplanted at centers where living donation was not an option.
Secondary Analyses
We conducted a set of secondary analyses with the goals of determining whether a) differential rates of portal hypertensive complications accounted for outcome differences among the groups; b) differential rates of living donor transplantation explained the results; c) the results were explained, in part, by the granting of exception points; and d) whether the exclusion of patients with hepatocellular carcinoma changed the results.
First, to determine whether the complications of portal hypertension account for observed differences in waitlist outcomes among patients with and without PSC, we performed a secondary Cox regression, adjusting for the occurrence of spontaneous bacterial peritonitis, development of ascites, hepatic encephalopathy, and recent variceal bleeding (defined as within 2 weeks prior to listing) at the time of listing. Complications after listing could not be adjusted for because they are not captured in the UNOS database.
Second, because patients with PSC are preferentially referred for living donor transplantation, we wanted to determine if this practice of pre-emptive living donor transplantation in patients with PSC impacted the results of our primary analysis.(26) We conducted a secondary analysis in which we assumed that living donor transplantation was not possible to determine if differential rates of living donor transplantation mitigated the potential increased hazard of death in patients with PSC due to early living donor transplantation. In this analysis, we recoded varying proportions of actual living donor recipients as having either died or received a deceased donor liver. Specifically, we tested the most plausible scenario, in which all patients who actually received a living donor transplant were instead coded probabilistically based on the actual distributions of outcomes of other patients in their diagnostic group. We also report results if all patients who actually received a living donor transplant were instead coded as having died, because although implausible, this would portray the “worst-case scenario” for outcomes among PSC patients. Lastly, we constructed a Cox regression model that excluded centers that performed living donor transplants as another means to assess the impact of differential rates of living donor transplantation on our results.
Third, we re-ran our primary analyses after first excluding all patients transplanted with an exception-point-adjusted MELD, and second after excluding all patients other than those with hepatocellular carcinoma who were transplanted with an exception-point-adjusted MELD.
Finally, we re-ran our primary analyses after excluding all patients with hepatocellular carcinoma, as these patients systematically receive exception points which impacts their chances of receiving a transplant.
All analyses were conducted using Stata 11.(29)
Results
There were 71,976 patients meeting the inclusion criteria, of whom 3,165 (4.4%) had PSC. Table 1 displays the demographic characteristics of patients with and without PSC. As expected given the epidemiology of the different disease processes, patients with PSC were younger and more commonly male and white. Patients with PSC were also more likely to have private insurance.
Table 1.
Demographics of all listed patients
| PSC | Non-PSC | P-Value | |
|---|---|---|---|
| Age at listing | 47.0 ± 13.0 | 52.7 ± 9.3 | <0.0001 |
| Gender (N, % male) | 2,104 (67.9) | 43,447 (64.4) | <0.0001 |
| MELD at listing* | 15.2 ± 7.3 | 16.5 ± 8.2 | <0.0001 |
| Bilirubin at listing, mg/dL* | 6.5 ± 7.9 | 4.5 ± 7.1 | <0.0001 |
| INR at listing* | 1.3 ± 0.60 | 1.6 ± 0.86 | <0.0001 |
| Creatinine at listing, mg/dL* | 1.0 ± 0.80 | 1.3 ± 1.2 | <0.0001 |
| Ethnicity, N (%) | White: 2,565 (82.7) | White: 48,712 (72.2) | |
| Black: 379 (12.2) | Black: 5,039 (7.5) | ||
| Hispanic: 105 (3.4) | Hispanic: 9,911 (14.7) | ||
| Asian: 41 (1.3) | Asian: 3,103 (4.6) | ||
| Private Insurance, N (%)* | 2,507 (80.9) | 43,066 (63.8) | <0.0001 |
Data only available for patients listed after February 26th, 2002
Insurance is coded as private if the UNOS code for insurance equals private, and public if the insurance is coded as public (defined by UNOS as Medicare, Medicaid, or other governmental insurance).
Deaths/Removals
Table 2 displays the reasons for which patients were removed from the waitlist during the study. Among patients listed for transplantation, a significantly greater proportion of patients without PSC died or were removed from the waitlist due to clinical deterioration (20.5% vs. 13.6%, P<0.0001). Deaths alone were also more common among patients without PSC (15.1% vs. 9.2%, P<0.0001).
Table 2.
Reasons for removal
| Removal Reason | Non-PSC, N (%)* | PSC, N (%)* |
|---|---|---|
| Deceased donor transplant | 30,297 (44.0) | 1,351 (42.7) |
| Medically Unsuitable | 20 (0.03) | 0 |
| Refused Transplant | 456 (0.7) | 15 (0.5) |
| Transferred to another center | 84 (0.1) | 8 (0.3) |
| Died | 10,358 (15.1) | 291 (9.2) |
| Other | 5,461 (7.9) | 154 (4.9) |
| Condition improved | 1,879 (2.7) | 87 (2.7) |
| Too sick to transplant | 3,695 (5.4) | 141 (4.4) |
| Transplanted at another center | 1,215 (1.8) | 142 (4.5) |
| Living donor transplant | 1,344 (2.0) | 249 (7.9) |
| Died during transplant procedure | 116 (0.2) | 5 (0.2) |
| Total numbers | 55,041 (80.0) | 2,443 (77.2) |
% is defined as the percentage of patients with that outcome among all listed patients
Diagnostic group differences among those who died
Tables 3 and 4 report the characteristics of patients with PSC and with other diagnoses who died or were removed due to clinical deterioration. Patients with PSC who died or were removed experienced significantly greater wait times prior to removal than did patients without PSC. Among all patients listed for transplantation and among those who died or removed due to clinical deterioration, patients without PSC more commonly experienced complications of portal hypertension. Among patients who died or were removed from the waitlist, MELD scores at the time of listing (difference in means = 0.89, 95% CI: −0.21, 2.00) and at death/removal (difference in means = 0.04, 95% CI: −1.18, 1.26) were similar between patients with and without PSC. Patients with PSC had significantly higher values of serum bilirubin, with lower values for INR and creatinine.
Table 3.
Demographic, clinical and laboratory data on all patients who died or were removed
| PSC | Non-PSC | P-Value | |
|---|---|---|---|
| Died/Removed, N (%)* | 432 (13.9) | 14,073 (20.9) | N/A |
| Wait time prior to removal, median (25th-75th percentile) |
451 (98-1154) | 320 (69-963) | N/A |
| Male gender, N(%) | 281 (65.1) | 8,627 (61.3) | N/A |
| Race/ethnicity | White-362 (83.8) | White: 10,019 | N/A |
| Black: 49 (11.3) | (71.2) | ||
| Hispanic: 16 (3.7) | Black: 1,022 | ||
| Asian: 5 (1.2) | (7.3) | ||
| Hispanic: 2,361 (16.8) |
|||
| Asian: 505 (3.6) | |||
| Variceal bleeding within 2 weeks of listing, N (%)† |
17 (4.4) | 810 (6.6) | 0.093 |
| History of ascites at listing, N(%)†‡ | 314 (78.5) | 11,376 (87.8) | <0.0001 |
| History of SBP at listing, N(%)† | 14 (3.5) | 864 (6.7) | 0.012 |
| History of hepatic encephalopathy at listing, N(%)†‡ |
172 (52.4) | 8,090 (71.5) | <0.0001 |
| Initial MELD | 17.8 ± 8.8 | 18.7 ± 9.5 | 0.2502 |
| Initial bilirubin, mg/dL | 8.6 + 10.0 | 5.8 ± 8.9 | <0.0001 |
| Initial INR | 1.4 ± 0.6 | 1.7 ± 1.1 | <0.0001 |
| Initial creatinine, mg/dL | 1.2 + 1.3 | 1.4 + 1.3 | <0.0001 |
| Final MELD | 24.6 ± 12.5 | 24.6 ± 12.6 | 0.97 |
| Final bilirubin, mg/dL | 14.0 ± 14.0 | 9.8 ± 12.4 | <0.0001 |
| Final INR | 2.2 ± 2.9 | 2.2 ± 2.2 | <0.0001 |
| Final creatinine, mg/dL | 1.6 ± 1.2 | 1.9 ± 1.5 | <0.0001 |
% is defined as percentage among those patients with available data
Data available only on patients listed after February 26th, 2002. Presence of complication defined as dichotomous yes/no.
Table 4.
Portal Hypertensive complications among all patients
| PSC | Non-PSC | P-Value | |
|---|---|---|---|
| Variceal bleeding within 2 weeks of listing, N (%) | 99 (3.6) | 3,217 (5.5) | <0.0001 |
| History of ascites at listing* | 1,749 (62.2) | 48,272 (78.1) | <0.0001 |
| History of SBP at listing | 59 (2.0) | 3,410 (5.4) | <0.0001 |
| History of hepatic encephalopathy at listing* | 945 (37.2) | 36,843 (63.4) | <0.0001 |
Data available only on patients listed after February 26th, 2002. Presence of complication defined as dichotomous yes/no.
Waitlist mortality of patients with vs. without PSC
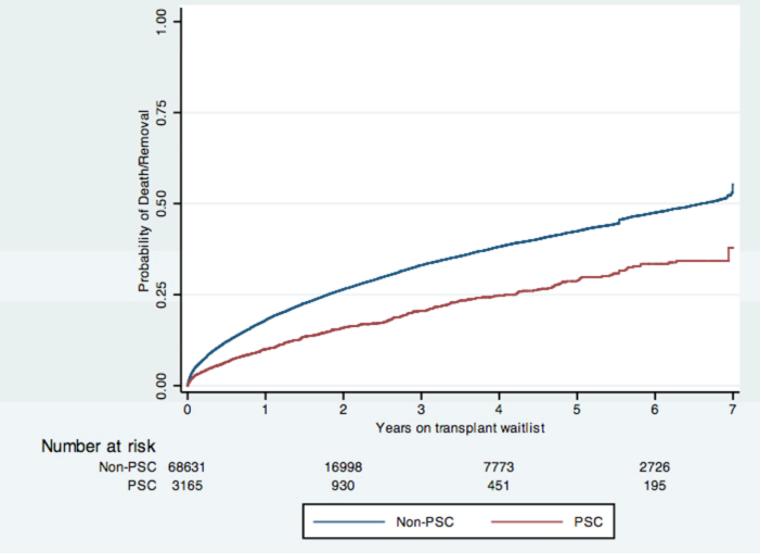
Figure 1 displays the Kaplan-Meier survival estimates for the two groups of patients. The log rank test comparing the two unadjusted failure functions had a p-value <0.0001, indicating that patients with PSC had greater unadjusted survival than patients without PSC.
Figure 1.
In developing the multivariable Cox model assessing the survival of patients with PSC, each of the independent variables tested were significantly associated with the hazard of death/removal due to clinical deterioration, and were included in the final model (Table 5). The adjusted hazard ratio for PSC in this final model was 0.72 (95% CI: 0.66-0.79), revealing that waitlist survival is greater among patients with PSC than among those with other forms of liver disease.
Table 5.
Univariable and Multivariable Cox Models Assessing Factors Associated with Death or Removal from the Waitlist
| Variable | Univariable | Multivariable*, PSC vs. others |
P-Value† |
|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) |
|||
| PSC | 0.58 (0.52-0.63) | 0.72 (0.66-0.79 | <0.0001 |
| Age‡ | 1.35 (1.32-1.37) | 1.34 (1.31-1.38) | <0.0001 |
| Blood type | |||
| O | 1.0 | 1.0 | |
| A | 0.95 (0.92-0.99) | 0.97 (0.93-1.00) | 0.094 |
| B | 0.87 (0.82-0.92) | 0.88 (0.82-0.93) | <0.0001 |
| AB | 0.75 (0.67-0.84) | 0.75 (0.64-0.87) | <0.0001 |
| Male gender | 1.02 (0.99-1.06) | 1.04 (0.99-1.08) | 0.102 |
| Race/Ethnicity | |||
| White | 1.0 | 1.0 | |
| Black | 1.13 (1.06-1.21) | 1.04 (0.95-1.13) | 0.421 |
| Hispanic | 1.11 (1.06-1.16) | 1.02 (0.95-1.09) | 0.622 |
| Asian | 0.81 (0.74-0.88) | 0.80 (0.73-0.88) | <0.0001 |
| Insurance† | |||
| Public | 1.0 | 1.0 | |
| Private | 0.70 (0.68-0.72) | 0.79 (0.75-0.82) | <0.0001 |
Multivariable model included three time-varying covariates: MELD score, exception score (yes/no), and temporally inactive status (status 7)
Reported P-values are from the multivariable model
Age reported as increased hazard ratio for every increase in 10 years of age
Insurance is coded as private if the UNOS code for insurance equals private, and public if the insurance is coded as public (defined by UNOS as Medicare, Medicaid, or other governmental insurance).
Secondary analyses
Complications of portal hypertension at the time of listing were more prevalent among patients without PSC. Full information on the presence or absence of complications of portal hypertension was available on 52,625 patients (73.1% of the total cohort). The individual hazard ratios for the complications of portal hypertension at listing were: a) esophageal variceal bleed within 2 weeks, HR=1.62 (95% CI 1.31-1.99); b) hepatic encephalopathy, HR=1.40 (95% CI 1.32, 1.48); c) spontaneous bacterial peritonitis, HR=1.43 (95% CI 1.30-1.58); d) ascites, HR=2.18 (95% CI 1.94, 2.44). Running the primary model on this restricted cohort (i.e., adjusting for the same covariates) yielded a hazard ratio for PSC of 0.67 (95% CI 0.59-0.76). Further adjustment for the four markers of portal hypertension produced a hazard ratio for PSC that was closer to the null but still significant (HR = 0.84; 95% CI 0.74-0.97).
When we recoded living donor organ recipients according to the observed outcomes among other patients in their diagnostic groups (i.e., 21% of non-PSC living donor recipients were reclassified as having died or removed, vs. 14% among living-donor recipients with PSC), the observed hazard ratio for PSC increased slightly to 0.75 (95% CI: 0.69-0.82). In the “worst-case scenario,” in which all living donor recipients were reclassified as having died or been removed from the waitlist due to clinical deterioration, the hazard ratio for PSC was 0.96 (95% CI 0.87-1.07). The hazard ratio for PSC was unchanged, HR=0.70 (95% CI: 0.55-0.90), when we excluded centers that performed living donor transplants.
When we excluded all patients transplanted with an exception-point-adjusted MELD (28.8% of transplanted non-PSC patients compared with 14.8% of transplanted PSC patients) and all patients other than those with hepatocellular carcinoma who were transplanted with an exception-point-adjusted MELD (12.0% of transplanted PSC patients as opposed to 6.5% of transplanted non-PSC patients), the results did not significantly change, with respective hazard ratios of 0.70 (95% CI 0.64-0.77) and 0.75 (0.68-0.82).
Finally, excluding patients with hepatocellular carcinoma yielded a hazard ratio of PSC that was unchanged (HR=0.68, 95% CI: 0.62-0.75).
Discussion
This analysis of the UNOS database of patients on the liver transplant waitlist since the introduction of the MELD allocation system demonstrates that the risk of death or removal from the waitlist due to clinical deterioration for patients with PSC is lower than that for patients with other end-stage liver diseases. This reduced risk was identified in both time-dependent and time-independent analyses, and remained after adjusting for: a) the MELD score throughout a patient’s entire time on the waitlist; b) complications of portal hypertension at the time of listing; c) differential rates of living donor transplantation; and d) the granting of exception points for patients with PSC and other diseases. This central finding calls into question the need for the current practices of preemptively referring PSC patients for living donor transplantation and/or providing them with exception points(20) in efforts to enhance their access.
In the initial study validating the MELD score in predicting survival of patients post-TIPS placement, patients with alcohol-related and cholestatic liver diseases had better post-TIPS 3-month survival.(27) Consequently, the initial MELD formula gave additional points to patients with hepatocellular etiologies of liver disease. However, because the MELD score did not incorporate complications of cholestasis or PSC-specific complications, it was deemed “inequitable” to give points only to patients with hepatocellular etiologies of liver disease, and the formula was modified.(28) Over time, many have speculated that the weighting of the MELD score still disadvantaged patients with PSC and other forms of cholestatic liver disease by not capturing their unique clinical features that better reflect their severity of illness. Thus, some physicians may recommend living donor transplantation preferentially for PSC patients, and in certain regions, such as Region 9, patients with PSC are granted exception points if they suffer from repeated bouts of cholangitis. Initially, these patients are given a MELD score of 25, and certain Regional Review Boards will grant additional points if a patient suffers from subsequent bouts of cholangitis.(20) In contrast to the beneficent intentions of such practices, if the overall survival of patients with PSC listed for liver transplantation is superior to that for patients with other diseases, as this study suggests, then such practices intended to level the playing field for patients with PSC may inadvertently disadvantage other patients.
Our results also provide insights into the mechanisms that do and do not contribute to patients with PSC experiencing lower risks of death or removal compared to other patients with end-stage liver disease. Our results suggest that differential rates in living donor transplantation among patients with PSC may explain a proportion of the observed effect, but cannot be the sole explanation because the effect favoring patients with PSC remained even when we reclassified large proportions of patients receiving living donor transplants as having died instead and when we excluded centers that perform living donor liver transplants. Second, by adjusting in our primary analysis for whether patients with PSC were transplanted with exception points, and by excluding such patients in secondary analyses, we show that the survival benefit among patients with PSC exists independently from these practices.
Finally, our results suggest that differential rates of complications of portal hypertension at the time of listing among groups explains a sizeable proportion of the survival benefit experienced by PSC patients. Adjusting for the occurrence of these intermediate outcomes moved the hazard ratio for PSC towards the null, from 0.67 to 0.85, suggesting that the presence of complications of portal hypertension mediates, in part, the observed survival benefit. Because patients with PSC are less likely to develop complications of portal hypertension, they may be less likely to die or be removed from the waitlist due to clinical deterioration. However, because the UNOS dataset does not provide the specific reasons for removal from the waitlist, we cannot quantify precisely the proportion of the effect explained by portal hypertensive complications.
The UNOS dataset limits our ability to explore other potential mechanisms by which patients with PSC have better waitlist survival than patients with other forms of end-stage liver disease. We intend to explore this question in future studies, relying on patient-level data from individual centers.
The results of our study are different from those of the study by Brandsaeter et al.(22) One possible explanation is the substantially shorter wait times in the Nordic study (median of 1 month) compared those in our study (median of 1 year), leading to fewer patients who developed complications of portal hypertension on the waitlist, and thus the increased waitlist mortality of patients without PSC observed in our study. Additionally, the case mixes are substantially different in the two studies: PSC is the most common indication for liver transplantation in the Nordic countries, whereas in the United States, hepatitis C and alcoholic liver disease are the most common indications. As we have shown, patients with these diseases more commonly manifest complications of portal hypertension compared to patients with PSC. However, we were limited in that we could not identify specific reasons why patients were removed due to clinical deterioration. As reported by Brandsaeter (22), in Scandanavian nations many such patients develop cholangiocarcinoma. Future studies using other data sources are needed to better delineate the reasons for removal among patients with PSC in the United States.
Our study has several limitations. First, use of the UNOS database limited the data elements available for covariate adjustment in the secondary analyses. Data regarding the presence of ascites, spontaneous bacterial peritonitis, and hepatic encephalopathy were only available for patients listed after the MELD was introduced, thus we could only perform our secondary analysis including complications of portal hypertension on 73.1% of our cohort. Also, the severity of these complications could not be validated. We dichotomized the outcomes of ascites and hepatic encephalopathy to limit the risk of exposure misclassification based on grade/stage of disease. Data on MELD scores and the laboratory elements that make up the MELD are limited on patients in the pre-MELD era, thus we chose only to focus on patients listed in the post-MELD era so that we could adjust for severity of illness. Furthermore, the question we hoped to answer was whether there are differences in waitlist mortality in the post-MELD era, thus a pre-MELD comparison was not needed. It is impossible to predict the outcomes of patients who received exception points on a case-by-case basis (i.e. patients with PSC with repeated bouts of bacterial cholangitis) were they not granted exception points, as patients may have sought high donor-risk organs or been listed in regions with a lower average MELD score at the time of transplantation, and thus received a transplant anyway. However, exclusion of these patients this did not significantly impact our results.
A secondary goal of our study was to determine if the waitlist survival is different for patients with PSC who have developed cholangiocarcinoma and/or bacterial cholangitis, as compared to those without these complications. However, careful exploration of the UNOS dataset revealed significant underreporting of these complications, thereby precluding us from performing such an analysis. Because waitlist survival for the total group of patients with PSC is significantly better than for patients without PSC, the group of patients with PSC and complications (cholangiocarcinoma and/or bacterial cholangitis) would have to be rather large, and have substantially worse outcomes than patients without PSC, for the waitlist survival of patients with PSC and complications to be significantly worse than that of patients without PSC. We are beginning to plan future studies to address this question using patient-level data acquired directly from individual centers.
Finally, these pooled data cannot be applied perfectly to a given patient with PSC. It may be that some patients with PSC are in fact “sicker” than their MELD scores indicate and may have reductions in their quality of life that are not captured by their scores. However, even a conservative interpretation of these data would yield the conclusion that the vast majority of patients with PSC have at least comparable survival to those with other forms of ESLD following listing for liver transplantation.
For these reasons, we see no basis for changing the MELD score explicitly to promote access among patients with PSC. By contrast, our results suggest caution in providing exception points for these patients, and that if physicians are referring patients with PSC for living donor transplantation to prevent them from developing cholangiocarcinoma, such choices may erode access for other patients and/or lead to more living donations than is truly necessary to promote acceptable outcomes for patients with PSC, thereby causing unnecessary risks to donors.(30)
Acknowledgments
Financial Support
1. NIH/NIDDK F32 1-F32-DK-089694-01 Grant
2. K08 HS018406 (SDH)
3. This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
4. Leonard Davis Institute of Health Economics, University of Pennsylvania
List of Abbreviations
- PSC
Primary sclerosing cholangitis
- CCA
Model for End-Stage Liver Disease
- MELD
Cholangiocarcinoma
- UNOS
United Network for Organ Sharing
- OPTN
Organ Procurement and Transplantation Network
- CTP
Child-Turcotte-Pugh
- ESLD
End-Stage Liver Disease
References
- 1.Bambha K, Kim WR, Talwalkar J, Torgerson H, Benson JT, Therneau TM, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003 Nov;125(5):1364–9. doi: 10.1016/j.gastro.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Lindkvist B, Benito de Valle M, Gullberg B, Bjornsson E. Incidence and prevalence of primary sclerosing cholangitis in a defined adult population in Sweden. Hepatology. 2010 Aug;52(2):571–7. doi: 10.1002/hep.23678. [DOI] [PubMed] [Google Scholar]
- 3.Roberts MS, Angus DC, Bryce CL, Valenta Z, Weissfeld L. Survival after liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transpl. 2004 Jul;10(7):886–97. doi: 10.1002/lt.20137. [DOI] [PubMed] [Google Scholar]
- 4.Schrumpf E, Abdelnoor M, Fausa O, Elgjo K, Jenssen E, Kolmannskog F. Risk factors in priry sclerosing cholangitis. J Hepatol. 1994 Dec;21(6):1061–6. doi: 10.1016/s0168-8278(05)80618-x. [DOI] [PubMed] [Google Scholar]
- 5.Rosen CB, Nagorney DM, Wiesner RH, Coffey RJ, Jr., LaRusso NF. Cholangiocarcinoma complicating primary sclerosing cholangitis. Ann Surg. 1991 Jan;213(1):21–5. doi: 10.1097/00000658-199101000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miros M, Kerlin P, Walker N, Harper J, Lynch S, Strong R. Predicting cholangiocarcinoma in patients with primary sclerosing cholangitis before transplantation. Gut. 1991 Nov;32(11):1369–73. doi: 10.1136/gut.32.11.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005 Sep;50(9):1734–40. doi: 10.1007/s10620-005-2927-8. [DOI] [PubMed] [Google Scholar]
- 8.Kornfeld D, Ekbom A, Ihre T. Survival and risk of cholangiocarcinoma in patients with primary sclerosing cholangitis. A population-based study. Scand J Gastroenterol. 1997 Oct;32(10):1042–5. doi: 10.3109/00365529709011222. [DOI] [PubMed] [Google Scholar]
- 9.Knechtle SJ, D’Alessandro AM, Harms BA, Pirsch JD, Belzer FO, Kalayoglu M. Relationships between sclerosing cholangitis, inflammatory bowel disease, and cancer in patients undergoing liver transplantation. Surgery. 1995 Oct;118(4):615–9. doi: 10.1016/s0039-6060(05)80026-1. discussion 9-20. [DOI] [PubMed] [Google Scholar]
- 10.Helzberg JH, Petersen JM, Boyer JL. Improved survival with primary sclerosing cholangitis. A review of clinicopathologic features and comparison of symptomatic and asymptomatic patients. Gastroenterology. 1987 Jun;92(6):1869–75. doi: 10.1016/0016-5085(87)90618-4. [DOI] [PubMed] [Google Scholar]
- 11.Farges O, Malassagne B, Sebagh M, Bismuth H. Primary sclerosing cholangitis: liver transplantation or biliary surgery. Surgery. 1995 Feb;117(2):146–55. doi: 10.1016/s0039-6060(05)80078-9. [DOI] [PubMed] [Google Scholar]
- 12.Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004 Mar;99(3):523–6. doi: 10.1111/j.1572-0241.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 13.Broome U, Lofberg R, Veress B, Eriksson LS. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995 Nov;22(5):1404–8. doi: 10.1002/hep.1840220511. [DOI] [PubMed] [Google Scholar]
- 14.Bjornsson E, Kilander A, Olsson R. CA 19-9 and CEA are unreliable markers for cholangiocarcinoma in patients with primary sclerosing cholangitis. Liver. 1999 Dec;19(6):501–8. doi: 10.1111/j.1478-3231.1999.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 15.Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Loof L, Danielsson A, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002 Mar;36(3):321–7. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 16.Aadland E, Schrumpf E, Fausa O, Elgjo K, Heilo A, Aakhus T, et al. Primary sclerosing cholangitis: a long-term follow-up study. Scand J Gastroenterol. 1987 Aug;22(6):655–64. doi: 10.3109/00365528709011139. [DOI] [PubMed] [Google Scholar]
- 17.Sharma P, Schaubel DE, Sima CS, Merion RM, Lok AS. Re-weighting the model for end-stage liver disease score components. Gastroenterology. 2008 Nov;135(5):1575–81. doi: 10.1053/j.gastro.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Patt CH, Thuluvath PJ. Liver transplantation for primary sclerosing cholangitis: Screening for biliary malignancy and the role for biliary malignancy and role of pre-emptive transplantation. Curr Opinion Organ Transplant. 2002;2002(7):129–36. [Google Scholar]
- 19.Goldberg D, French B, A T, Reddy KR, Halpern S. Current Trends in Living Donor Liver Transplantation for Primary Sclerosing Cholangitis. doi: 10.1097/TP.0b013e31821694b3. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiano T. Personal Communication. New York, NY: 2010. [Google Scholar]
- 21.Freeman RB, Wiesner RH, Edwards E, Harper A, Merion R, Wolfe R. Results of the first year of the new liver allocation plan. Liver Transpl. 2004 Jan;10(1):7–15. doi: 10.1002/lt.20024. [DOI] [PubMed] [Google Scholar]
- 22.Brandsaeter B, Broome U, Isoniemi H, Friman S, Hansen B, Schrumpf E, et al. Liver transplantation for primary sclerosing cholangitis in the Nordic countries: outcome after acceptance to the waiting list. Liver Transpl. 2003 Sep;9(9):961–9. doi: 10.1053/jlts.2003.50169. [DOI] [PubMed] [Google Scholar]
- 23.Moylan CA, Brady CW, Johnson JL, Smith AD, Tuttle-Newhall JE, Muir AJ. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008 Nov 26;300(20):2371–8. doi: 10.1001/jama.2008.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Austin MT, Poulose BK, Ray WA, Arbogast PG, Feurer ID, Pinson CW. Model for end-stage liver disease: did the new liver allocation policy affect waiting list mortality? Arch Surg. 2007 Nov;142(11):1079–85. doi: 10.1001/archsurg.142.11.1079. [DOI] [PubMed] [Google Scholar]
- 25.French B, Heagerty PJ. Analysis of longitudinal data to evaluate a policy change. Stat Med. 2008 Oct 30;27(24):5005–25. doi: 10.1002/sim.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldberg D, French B, A T, Reddy KR, Halpern S. Current Trends in Living Donor Liver Transplantation for Primary Sclerosing Cholangitis. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000 Apr;31(4):864–71. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 28.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001 Feb;33(2):464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 29.StataCorp . Stata Statistical Software: Release 11. StataCorp LP; College Statiom, TX: 2010. [Google Scholar]
- 30.Merion RM, Shearon TH, Berg CL, Everhart JE, Abecassis MM, Shaked A, et al. Hospitalization rates before and after adult-to-adult living donor or deceased donor liver transplantation. Ann Surg. 2010 Mar;251(3):542–9. doi: 10.1097/SLA.0b013e3181ccb370. [DOI] [PMC free article] [PubMed] [Google Scholar]