Abstract
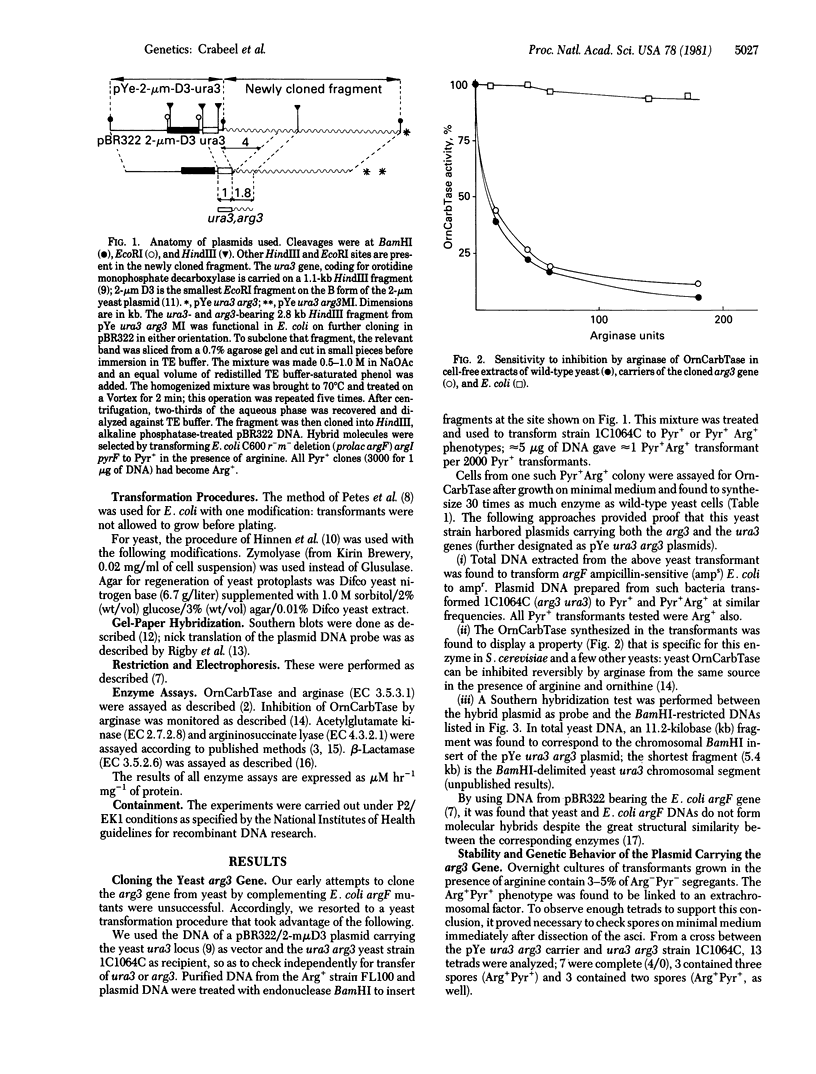
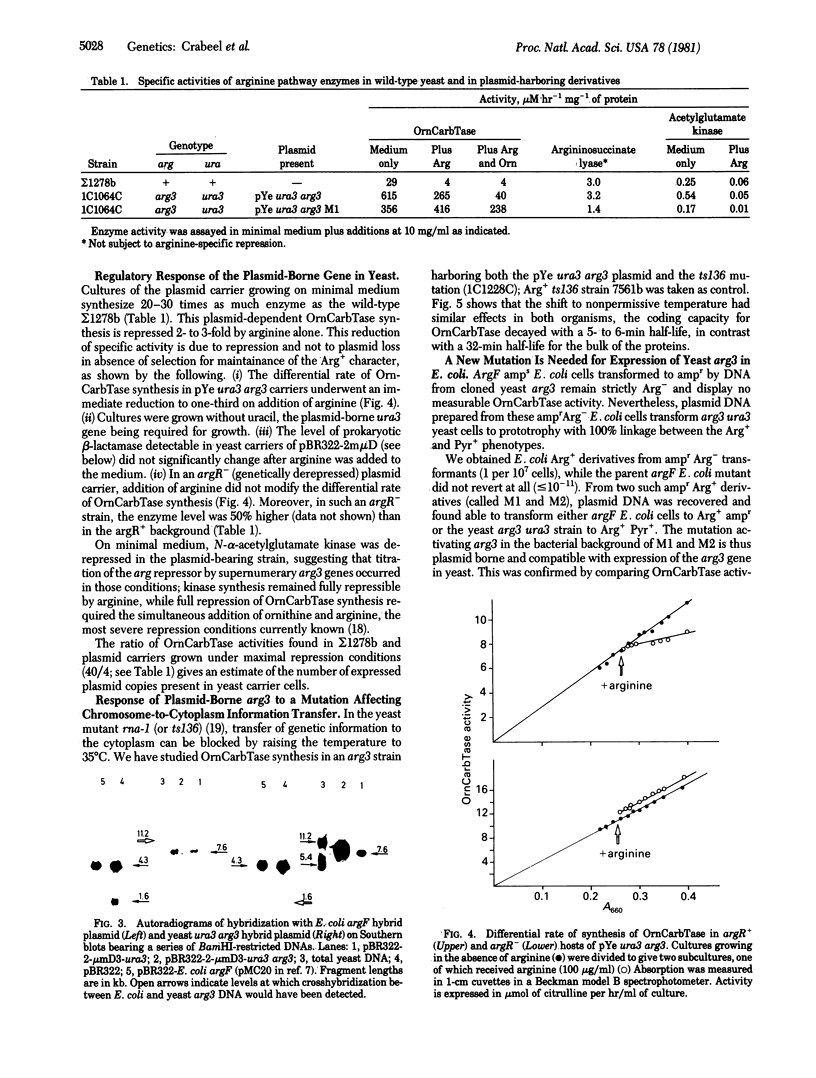
The yeast arg3 gene, coding for ornithine carbamoyltransferase (carbamoylphosphate:L-ornithine carbamoyltransferase, EC 2.1.3.3), has been cloned on a hybrid pBR322-2-micrometers plasmid. The cloned gene gives a normal regulatory response in yeast. It is not expressed at 35 degrees C when a mutation preventing mRNA export from the nucleus at this temperature is included in the genetic make-up of the carrier strain. In Escherichia coli, no functional expression can be observed from the native yeast arg3 gene. The study of a mutant plasmid (M1) producing low levels of yeast carbamoyltransferase in E. coli has permitted the localization and orientation of arg3 on the plasmid. The mutation involved is a deletion that alters the regulatory response of arg3 in yeast. The plasmid bla gene produces detectable amounts of beta-lactamase (penicillin amido-beta-lactamhydrolase, EC 3.5.2.6) in yeast: the data provide an estimate of the beta-lactamase activity associated with one exemplar of the plasmid expressing arg3 (0.6 units).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. L., Lacroute F., Botstein D. Evidence for transcriptional regulation of orotidine-5'-phosphate decarboxylase in yeast by hybridization of mRNA to the yeast structural gene cloned in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):386–390. doi: 10.1073/pnas.76.1.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechet J., Greenson M., Wiame J. M. Mutations affecting the repressibility of arginine biosynthetic enzymes in Saccharomyces cerevisiae. Eur J Biochem. 1970 Jan;12(1):31–39. doi: 10.1111/j.1432-1033.1970.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Chevallier M. R., Aigle M. Qualitative detection of penicillinase produced by yeast strains carrying chimeric yeast-coli plasmids. FEBS Lett. 1979 Dec 1;108(1):179–180. doi: 10.1016/0014-5793(79)81204-1. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli: specific complementation of argininosuccinate lyase (argH) mutations. J Mol Biol. 1978 Apr 25;120(4):517–532. doi: 10.1016/0022-2836(78)90351-0. [DOI] [PubMed] [Google Scholar]
- Crabeel M., Charlier D., Cunin R., Glansdorff N. Cloning and endonuclease restriction analysis of argF and of the control region of the argECBH bipolar operon in Escherichia coli. Gene. 1979 Mar;5(3):207–231. doi: 10.1016/0378-1119(79)90079-9. [DOI] [PubMed] [Google Scholar]
- Crabeel M., Messenguy F., Lacroute F., Glansdorff N. Cloning and expression of argF (ar3), the yeast structural gene for ornithine carbamoyltransferase, in Saccharomyces cerevisiae and in Escherichia coli [proceedings]. Arch Int Physiol Biochim. 1980 Feb;88(1):B21–B22. [PubMed] [Google Scholar]
- Dubois E., Hiernaux D., Grennon M., Wiame J. M. Specific induction of catabolism and its relation to repression of biosynthesis in arginine metabolism of Saccharomyces cerevisiae. J Mol Biol. 1978 Jul 15;122(4):383–406. doi: 10.1016/0022-2836(78)90417-5. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg C. P., Degelmann A., Kustermann-Kuhn B., Royer H. D. Characterization of 2-mum DNA of Saccharomyces cerevisiae by restriction fragment analysis and integration in an Escherichia coli plasmid. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2072–2076. doi: 10.1073/pnas.73.6.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison H. T., Hartwell L. H., McLaughlin C. S. Temperature-sensitive yeast mutant defective in ribonucleic acid production. J Bacteriol. 1969 Sep;99(3):807–814. doi: 10.1128/jb.99.3.807-814.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux J. C., Urrestarazu L. A., Wiame J. M. Arginine metabolism in Saccharomyces cerevisiae: subcellular localization of the enzymes. J Bacteriol. 1978 Mar;133(3):1096–1107. doi: 10.1128/jb.133.3.1096-1107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain C., Stalon V., Glansdorff N., Gigot D., Piéard A., Crabeel M. Structural and regulatory mutations allowing utilization of citrulline or carbamoylaspartate as a source of carbamoylphosphate in Escherichia coli K-12. J Bacteriol. 1976 Oct;128(1):39–48. doi: 10.1128/jb.128.1.39-48.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenguy F., Cooper T. G. Evidence that specific and "general" control of ornithine carbamoyltransferase production occurs at the level of transcription in Saccharomyces cerevisiae. J Bacteriol. 1977 Jun;130(3):1253–1261. doi: 10.1128/jb.130.3.1253-1261.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenguy F. Regulation of arginine biosynthesis in Saccharomyces cerevisiae: isolation of a cis-dominant, constitutive mutant for ornithine carbamoyltransferase synthesis. J Bacteriol. 1976 Oct;128(1):49–55. doi: 10.1128/jb.128.1.49-55.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenguy F., Wiame J. -M. The control of ornithinetranscarbamylase activity by arginase in Saccharomyces cerevisiae. FEBS Lett. 1969 Apr;3(1):47–49. doi: 10.1016/0014-5793(69)80093-1. [DOI] [PubMed] [Google Scholar]
- Penninckx M., Simon J. P., Wiame J. M. Interaction between arginase and L-ornithine carbamoyltransferase in Saccharomyces cerevisiae. Purification of S. cerevisiae enzymes and evidence that these enzymes as well as rat-liver arginase are trimers. Eur J Biochem. 1974 Nov 15;49(2):429–442. doi: 10.1111/j.1432-1033.1974.tb03848.x. [DOI] [PubMed] [Google Scholar]
- Petes T. D., Broach J. R., Wensink P. C., Hereford L. M., Fink G. R., Botstein D. Isolation and analysis of recombinant DNA molecules containing yeast DNA. Gene. 1978 Sep;4(1):37–49. doi: 10.1016/0378-1119(78)90013-6. [DOI] [PubMed] [Google Scholar]
- Ramos F., Thuriaux P., Wiame J. M., Bechet J. The participation of ornithine and citrulline in the regulation of arginine metabolism in Saccharomyces cerevisiae. Eur J Biochem. 1970 Jan;12(1):40–47. doi: 10.1111/j.1432-1033.1970.tb00818.x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Paterson B. M. Efficient cap-dependent translation of polycistronic prokaryotic mRNAs is restricted to the first gene in the operon. Nature. 1979 Jun 21;279(5715):696–701. doi: 10.1038/279696a0. [DOI] [PubMed] [Google Scholar]
- Sargent M. G. Rapid fixed-time assay for penicillinase. J Bacteriol. 1968 Apr;95(4):1493–1494. doi: 10.1128/jb.95.4.1493-1494.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfner M., Yep D., Messenguy F., Fink G. R. Integration of amino acid biosynthesis into the cell cycle of Saccharomyces cerevisiae. J Mol Biol. 1975 Aug 5;96(2):273–290. doi: 10.1016/0022-2836(75)90348-4. [DOI] [PubMed] [Google Scholar]



