Abstract
The transforming growth factor β (TGFβ) family of proteins are key regulators of growth and differentiation. Members of this family, including multiple TGFβs, activins, bone morphogenetic proteins (BMPs), and growth and differentiation factor 9 (GDF9), are expressed from oocytes or their associated follicular somatic cells (granulosa and thecal cells) with cell-type and stage-dependent specificity. Granulosa cells are the target cells for many of these ligands. Granulosa cell-specific knockout mice for all of the receptor-regulated SMADs, as well as the common regulatory SMAD4, have recently been generated and highlight the importance of this family in most stages of folliculogenesis. These models have also uncovered a novel role for the BMPs in suppression of granulosa cell tumor development and metastasis. This review summarizes the phenotypes of these mouse models and their contribution to our understanding of the complexity of BMP function during follicle development.
Keywords: reproduction, ovary, fertility, SMAD, knockout, bone morphogenetic protein, cancer
1. Introduction
Many of the mechanisms underlying ovarian dysfunction that lead to infertility in women are poorly understood. Complicating our understanding of female reproductive dysfunction is the interdependent relationship between the developing oocyte and its surrounding somatic cells that form the ovarian follicle. The oocyte and follicular somatic cells (granulosa and thecal cells) develop in consort, and each cell type produces a number of growth factors with key paracrine, autocrine, or endocrine functions. Without their associated somatic cells, oocytes cannot develop [1–3]. The co-dependence between ovarian cell types may pose a unique constraint on the development of artificial reproductive technologies (ART), which seek to develop means of growing eggs from earlier developmental stages [4–7]. Thus, understanding growth factor interactions between somatic cells and oocytes is critical not only in understanding normal ovarian physiology, but for the ability to treat or possibly prevent female infertility.
The pool of primordial follicles begins to form midgestation in humans and perinatally in mice, and each follicle will contain an immature non-growing oocyte arrested in early prophase I (Fig. 1). Oocytes are separated into individual follicles by a small number of somatic cells forming a flattened epithelium that will eventually become the granulosa cells. In addition, the oocyte and pre-granulosa cells are separated from the ovarian stroma and other follicles by a basement membrane [8]. Once established, the size of the quiescent primordial follicle pool is thought to limit the reproductive lifespan of the female. Primordial follicles activate by largely unknown mechanisms to enter a growth phase characterized by coupled oocyte and granulosa cell growth, and development of a third somatic cell component, the thecal cells. Activation of oocyte-specific transcription factor cascades can cause unregulated primordial follicle activation and result in premature ovarian failure [9–14]. Whether initial primordial follicle activation is stochastic or regulated, or driven by internal (oocyte) or external (somatic cell) cues, is unclear. While there are excellent mouse models to study oocyte function at early stages of oogenesis and folliculogenesis [15–17], mouse models to analyze granulosa cell function at the transition of pregranulosa cells to cuboidal granulosa cells are lacking. This is due in part to the unavailability of conditional knockout mice to make somatic cell-specific deletions at this stage. Widespread embryonic and perinatal lethality for mice null for components of major developmental signal transduction pathways, including those for the BMPs, are additional confounding issues (Table 1).
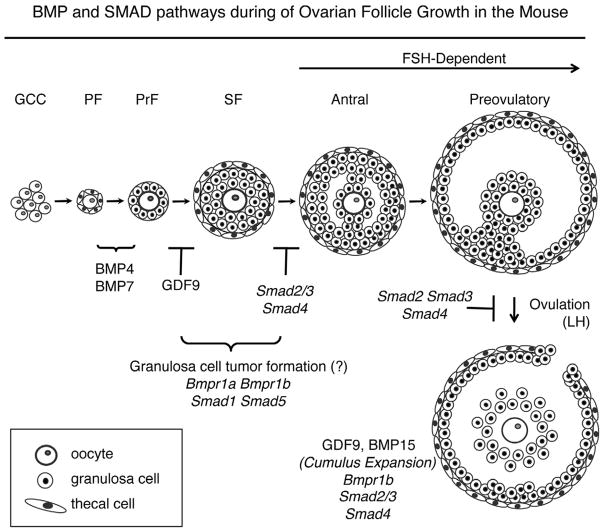
Figure 1.
Ovarian follicle development in the mouse. Oocytes are found as syncytia (germ cell cysts, GCC) in the newborn mouse ovary and GCCs breakdown to form the pool of primordial follicles (PF). Upon activation, primordial follicles transition to primary (PrF) and secondary (SF) stages. BMP4 and BMP7 are implicated in the primordial to primary transition. Follicles in mice null for Gdf9 do not progress past the primary follicle stage. In wild type mice, secondary follicles acquire a fluid-filled space (antrum) upon stimulation by FSH, and undergo cumulus expansion and ovulation under stimulation by LH. Granulosa cell-specific knockouts for Smad4 and Smad2/3 have similar defects at the terminal stages of follicle development. In contrast, granulosa cell-specific knockouts for the BMP type I receptors, Bmp1a and Bmpr1b and downstream SMAD transcription factors, Smad1 and Smad5, develop granulosa cell tumors. The follicle stage at which GCT tumors form is unknown, but based on their expression pattern [78,89], likely occurs prior to antrum formation.
Table 1.
Summary of the general and reproductive phenotypes of mouse knockout (KO) models for the BMP ligands, receptors, and signaling SMADs that are expressed in the mouse ovary. N/D, no data
| Ligands | KO | Overall Phenotype | Reproductive Phenotype | Reference |
|---|---|---|---|---|
| Bmp2 | Embryonic Lethal | Malformation of the amnion/chorion | Fewer primordial germ cells | [97] |
| Bmp3 | Viable and Fertile | Increased bone density | None noted | [98] |
| Bmp3b (Gdf10) | Viable and Fertile | Regulates osteoblast differentiation | None noted | [99] |
| Bmp4 (Bmp2b) | Embryonic Lethal | Failure to form mesoderm | No primordial germ cells | [42,100,101] |
| Bmp5 | Embryonic Lethal | Failure to form mesoderm | N/D | [102] |
| Bmp6 (Vgr1) | Subfertile | Regulates osteoblast function | Fewer oocytes ovulated | [35] |
| Bmp7 (Op1) | Perinatal Lethal | Bone, kidney, eye defects | N/D | [103] |
| Bmp15 (Gdf9b) | Subfertile | Reproductive | Fewer oocytes ovulated; cumulus function | [27] |
| Gdf9 | Infertile | Female infertility | Folliculogenesis blocked after primary stage | [104] |
| Receptors | ||||
| Acvr2a | Perinatal Lethal (25%) | Female infertility | FSH suppression | [105] |
| Acvr2b | Perinatal Lethal | Lateral asymmetry; cardiac defects | N/D | [106] |
| Bmpr2 | Embryonic Lethal | Failure to form mesoderm | N/D | [107] |
| Acvr1a (Alk2) | Embryonic Lethal | Failure to form mesoderm | No primordial germ cells | [108,109] |
| Bmpr1a (Alk3) | Embryonic Lethal | Failure to form mesoderm | N/D | [110] |
| Bmpr1b (Alk6) | Infertile | Brachydactyly; retinal defects | Cumulus expansion defect; irregular estrous cycle | [54,111] |
| Smads | ||||
| Smad1 | Embryonic Lethal | Failure to connect to placenta | Fewer primordial germ cells | [112] |
| Smad2 | Embryonic Lethal | Multiple defects including failed gastrulation and mesoderm development | N/D | [113,114] |
| Smad3 | Subfertile | Defective immune response, colorectal tumors, | Defective follicle development | |
| Smad4 | Embryonic Lethal | Defective visceral endoderm | No primordial germ cells | [115,116] |
| Smad5 | Embryonic Lethal | Gut, cardiac, brain defects | Fewer primordial germ cells | [117,118] |
| Smad6 | Postnatal lethality (majority) | Cardiac defects | N/D | [60] |
| Smad7 | Embryonic lethality (partially penetrant) | Reduced body size, immune defects, cardiac development (in null model) | Smaller litter sizes (hypomorphic allele) | [61,119] |
| Smad9 (Smad8) | Viable and Fertile | Defective vascular remodeling | None noted | [78,79] |
2. Growth and differentiation of murine granulosa cells
Little is known about the origin and initial growth of granulosa cells, or about the signal transduction pathways that control granulosa cell specification and development. Embryologically, cells that give rise to granulosa cells are thought to originate from the embryonic coelomic epithelium, similar to Sertoli cell precursors in the male [18,19]. When a primordial follicle activates, the oocyte begins to increase in size, and pre-granulosa cells transition to a cuboidal shape and undergo mitosis [8] (Fig. 1). Interestingly, primordial follicles that are positive or negative for Ki-67 (a marker of proliferation), have similarly sized oocytes, and while granulosa cells undergo the transition to the cuboidal shape, there is no initial increase in oocyte size [20]. These data suggest that granulosa cell proliferation might occur independently from the oocyte at this early stage [20], though how this is regulated is unclear. Pathologically, granulosa cell growth can also be uncoupled from oocyte growth as demonstrated in the Inha KO mouse, though the resultant phenotype is the development of granulosa cell tumors [21,22].
During early folliculogenesis, a glycoprotein rich matrix called the zona pellucida forms between the oocyte and developing granulosa cells, and granulosa cells closest to the oocyte (at later stages termed cumulus cells) remain coupled to the oocyte via transzonal projections [23,24]. Granulosa cells continue to divide, forming multiple layers, and a third cell type, the thecal cells, differentiates and surrounds the basement membrane around the perimeter of the follicle at the secondary follicle stage (Fig. 1). Further growth and differentiation occurs in both the granulosa cells and thecal cells throughout the remainder of folliculogenesis (Fig 1). Finally, under the influence of pituitary gonadotropins [follicle stimulating hormone (FSH) and luteinizing hormone (LH)], ovulation occurs and the remaining cells of the follicle terminally differentiate in a process known as luteinization to form the corpus luteum, a transient progesterone-secreting endocrine organ necessary for the establishment of pregnancy.
3. BMPs in early murine folliculogenesis
There are no studies as of yet that indicate whether the BMPs play a role in the breakdown of germ cell cysts (GCC) or the formation of primordial follicles. Oocytes contained in GCCs prior to their breakdown express oocyte-restricted members of the TGFβ family, Gdf9 and Bmp15 [10], although protein production of GDF9 is not detectable by immunohistochemistry until the early primary follicle stage (3a) [25]) [26]. Gdf9 null ovaries contain follicles arrested at the primary follicle stage (Fig. 1), suggesting that GDF9 function is critical at this stage. However, double mutant female mice containing one copy of Gdf9 but null for Bmp15 (Gdf9+/−Bmp15−/−) are subfertile due to reduced ovulation and fertilization. Interestingly, their ovaries also contain developing follicles with multiple oocytes [27], and this suggests that GCC breakdown may be compromised when the copy number of these oocyte-expressed members of the TGFβ family is reduced.
While BMP15 may act in concert with GDF9 or possibly regulate GDF9 activity [27–29], the mechanism(s) by which it may do so is unclear. In vitro studies have shown that these ligands utilize different signaling pathways; BMP15 signals via the SMAD1/5 pathway, while GDF9 signals through SMAD2/3 [30–34]. In contrast to Gdf9 or Bmp15, deletion of Bmp6, another oocyte-expressed TGFβ-related ligand, has minimal effects on female fertility, while Bmp6 Bmp15 double knockout mice have a phenotype similar to the double heterozygous controls (Bmp6+/− Bmp15+/−)[35]. These latter data along with additional published studies [31,36], suggest that BMP15 and BMP6 likely do not have the same role in granulosa cell function. Furthermore, based on the similarity between the double heterozygous to the homozygous Bmp6 Bmp15 mutant mice, it has also been suggested that deletion of Bmp6 may in fact rescue some of the fertility defects demonstrated in Bmp15 KO females, though this remains speculative [35].
There is sufficient data to suggest that the BMP family plays a likely role at the primordial to primary follicle transition (Fig. 1). BMP4 and BMP7 are produced from the ovarian stroma and thecal cells [37,38]. Studies using in vitro cultures of isolated postnatal rat ovaries show that BMP4 treatment promotes the development of primary follicles, while treatment of ovaries with a BMP4 neutralizing antibody show progressive lose of oocytes in primordial follicles [39]. In vivo, passive immunization in postnatal mice against BMP4 results in fewer primary follicles than control mice, though also in this study, there was an increase in the number of primordial follicles and no associated apoptosis [40]. BMP7 increases the percentage of primary follicles (as well as secondary and antral) but reduces primordial follicles, when injected into the ovarian bursa of adult rats [41]. However, early follicle development cannot be studied in embryos null for either Bmp4 or Bmp2 because they die midgestation and either lack germ cells (Bmp4) [42], or have reduced numbers (Bmp2) [43]. Bmp7 null mice die perinatally [44,45]. Overexpression of Bmp15 in oocytes causes accelerated follicle growth, with decreased numbers of primary follicles and increases in secondary follicles, increases in the mitotic index of granulosa cells, and though adult mice have normal litter sizes, they also display an earlier onset of acyclicity [46].
While preantral follicles grow independent of extraovarian factors [47], the pituitary gonadotrophins, FSH and LH, are required for continuation of antral stage growth and ovulation, respectively [15,48,49] (Fig. 1). The TGFβ family, including the BMPs, modulates the effects of both FSH and LH. BMP4 and BMP7 promote FSH-induced estrogen synthesis, while inhibiting progesterone production [38]. Some BMPs can also modulate gonadotropin action by regulating expression of their receptors; BMP15 inhibits FSH receptor (Fshr) expression [46,50], while BMP7 and BMP2 increase it [51,52]. In contrast, BMP6 has no effect of Fshr expression [50]. BMPs appear to suppress expression of the LH receptor (Lhcgr), including mouse BMP15 [53], human BMP7 [52], and human BMP2 [51]. BMPs also play a role during ovulation and cumulus cell function, and mice with null mutations in the BMP type I receptor, Bmpr1b (Alk6) have defects in cumulus expansion [54]. The BMP system appears to be downregulated during ovulation, further suggesting that their important role may be as regulators of luteinization [55,56]. Bmp2 expression, in particular, is dynamically regulated during later stages of follicle development [55]; it is highly expressed in mural granulosa cells to the preovulatory stage and suppressed during ovulation, but re-expressed during luteolysis of corpora lutea, thus suggesting that BMP2 might also play a critical role in terminal granulosa cell differentiation and as a luteinization inhibitor [55]. Interestingly, Bmp2 expression is repressed in cumulus cells and periantral granulosa cells [55]. This differential expression pattern of mural versus cumulus is typical of genes regulated by oocyte-expressed factors such as GDF9 and BMP15 [57–59].
4. The role of the SMADs in ovarian function
The canonical signaling pathway for the TGFβ family is through the intracellular SMAD transcription factors. There are three general subcategories of SMADs: (1) the receptor-associated SMADs, which are phosphorylated upon formation of specific serine-threonine kinase receptor complexes [SMAD1, SMAD2, SMAD3, SMAD5, SMAD9 (formerly referred to as SMAD8)]; (2) the common mediator SMAD4, which forms part of the heterotrimeric transcription factor complex along with the phosphorylated R-SMADs; and (3) the inhibitory SMADs, SMAD6 and SMAD7, which act to negatively regulate SMAD signaling by competing for SMAD4 or by blocking receptor phosphorylation of the R-SMADs. KO mice for the R-SMADs and Smad4 are embryonic lethal except for Smad3 and Smad9 (Table 1). The majority of Smad6 null mice die postnatally with cardiac defects [60], while mice homozygous for a Smad7 hypomorphic allele have reduced body size and small litter sizes [61].
The first conditional SMAD deletion generated in the mouse ovary was for Smad4 [62] (Table 2). This mouse was created using cre-loxP technology [63], with cre recombinase expression from the endogenous Amhr2 locus [64]. Amhr2cre shows expression in the embryonic Müllerian duct and ovarian stroma [64,65], as well as in granulosa cells of the postnatal and adult mouse ovary with variable expression in thecal cells [65,66]. Herein, these mice are referred to as “ovarian” or “granulosa cell” conditional knockout mice. The expectation for these mice was that Smad4 deletion would result in ovarian tumors because SMAD4 [also called deleted in pancreatic carcinoma 4, (DPC4)] is a well-known tumor suppressor in humans [67]. However no ovarian tumors ever develop in these mice [62]. Instead, Smad4 conditional knockout mice (termed Smad4 cKOs) are initially subfertile, but half of the females are infertile by six months of age [62].
Table 2.
Summary of granulosa cell phenotypes in female mice with SMAD conditional (cKO) deletion in granulosa cells of the ovary. Mice were generated either as heterozygotes with one floxed allele and one null allele (Smadflox/−), or homozygotes of floxed alleles (Smadflox/flox), with or without cre recombinase, as indicated in the original references, except for Smad9, which was generated as a homozygous null (Smad9−/−). Recombination of floxed alleles was carried out using mice with cre recombinase expression from the Amhr2 promoter [64].
| Conditional KO | Fertility | Ovarian Defects | Reference |
|---|---|---|---|
| Smad1 cKO | Normal | None noted | [78] |
| Smad2 cKO | Normal | None noted | [68] |
| Smad3 cKO | Normal | None noted | [68] |
| Smad4 cKO | Subfertile, then 50% infertile by 6 months | Reduced antral follicles and ovulation rates, cumulus cell defects, increased luteinization, increased serum progesterone | [62] |
| Smad5 cKO | Normal | None noted | [78] |
| Smad9 KO | Normal | None noted | [78] |
| Smad2 Smad3 dKO | Subfertile, then infertile at 5 months | Reduced antral follicles and ovulation rates, cumulus cell defects | [68] |
| Smad1 Smad9 dKO (formerly Smad1 Smad8) | Normal | None noted | Unpublished data, S.Pangas |
| Smad5 Smad9 dKO (formerly Smad5 Smad8) | Normal | None noted | [78] |
| Smad1 Smad5 dKO | Subfertile, then infertile at 4–6 months | Granulosa cell tumors | [78] |
| Smad1 Smad5 Smad9 tKO (formerly Smad1 Smad5 Smad8) | Subfertile, then infertile at 4–6 months | Granulosa cell tumors | [78] |
The ovarian phenotype of Smad4 cKO mice is complex, in part due to the breadth of TGFβ family functions in granulosa cells, which includes activity of TGFβ isoforms, GDF9, activin isoforms, BMP15, and other BMPs. It is interesting, however, that the phenotype of the Smad4 cKO in many ways closely resembles the phenotype of the Smad2 Smad3 double cKO mice (dKO) that were subsequently generated with Amhr2cre [68] (Table 2). SMAD2 and SMAD3 signal for TGFβ, activin, and GDF9 and may be referred to as AR-SMADs [69]. Conditional deletion of either Smad2 or Smad3, when generated as single mutations, has little effect on ovarian function [68] (Table 2). Therefore double conditional (Smad2 Smad3 dKO) knockouts were produced [68]. These mice show progressive defects in litter size, resulting in infertility by 5 months of age. Similar to Smad4 cKO mice, the Smad2 Smad3 dKO mice show fewer antral follicles, luteinized follicles with trapped oocytes, reduced ovulation rates, and impaired cumulus expansion [62,68]. Interestingly, one of the differences between Smad4 cKO and Smad2 Smad3 dKO mice is that progesterone levels increase in the former by 12 weeks of age [62], but not in the latter at the same age [68]. Deletion in granulosa cells of the BMP type I receptors, Bmpr1a and Bmpr1b, which signal though Smad1 and Smad5, also do not show an increase in progesterone [70]. One possible explanation may be that both the SMAD2/3 and SMAD1/5 pathways act similarly with respect to suppression of progesterone production. Thus, only when all SMAD signaling is suppressed (i.e. in Smad4 cKO cells) is progesterone increased. This is supported by data that show that although they utilize different SMADs, GDF9 (or activin) and BMP15 have been shown to suppress basal, or in some cases FSH-induced, expression of the same genes involved in granulosa cells luteinization and progesterone production including Lhcgr, Star, and Cyp11a1 [26,50,71–75]. However, the mechanisms by which they do so need not be identical. A full comparison of the defects in Smad4 deficient and Smad2 Smad3 dKO would be necessary to understand the similarities and differences between these mouse models.
5. SMADs in granulosa cell tumor development
SMAD1, SMAD5, and SMAD9 signal in association with ligands using the type I receptors ACVRL1 (ALK1), ACVR1A (ALK2), BMP1A (ALK3), and BMP1B (ALK6). Typically, these are BMP receptors, though they are also used by anti-Müllerian hormone (AMH) and some GDFs [69]. SMAD1, SMAD5 and SMAD9 may be referred to as the BR-SMADs. TGFβ may also utilize SMAD1 or SMAD5 in a limited number of cell types [76,77]. As with Smad2 or Smad3 single cKOs using Amhr2cre, no phenotype is observed in mice with Amhr2cre-driven granulosa cell deletion of Smad1 or Smad5 when generated as single mutations. [78] (Table 2). In addition, no phenotype is detected when either of these mutations is generated in a Smad9 homozygous null background [78]. However, mice with conditional mutations in granulosa cells using Amhr2cre to delete both Smad1 and Smad5 (Smad1 Smad5 dKO) or the combination of Smad1 Smad5 dKO in a Smad9 homozygous null background (Smad1 Smad5 Smad9 tKO), have a phenotype distinct from Smad4 cKO or Smad2 Smad3 dKO. For clarity, these mouse models for the SMADs with Amhr2cre are referred to as BR-SMAD cKOs (for Smad1 Smad5 deletion, both with or without Smad9), while Smad2 Smad3 deletion is referred to as AR-SMAD cKOs.
Female BR-SMAD cKOs are initially subfertile, but most become infertile as young adults (Table 2). Litter sizes are the same between the Smad1 Smad5 dKO and the Smad1 Smad5 Smad9 tKO, which suggests that Smad9 is not redundant with Smad1 or Smad5 in granulosa cells. This hypothesis is in line with embryological data that also do not show a genetic interaction between Smad1 and Smad5 with Smad9 [79]. BR-SMAD cKO female mice develop granulosa cell tumors by 8 weeks of age, and showed an increasing incidence of peritoneal metastases with age. Because the onset of granulosa cell tumor development mirrors the time frame for the development of infertility, it is unclear if the fertility defects are simply secondary to tumor development or whether there are additional underlying defects related to loss of BMP signaling in granulosa cells. Cumulus cell defects might be expected, particularly because female mice null for Bmpr1b (Alk6), which phosphorylates the BR-SMADs, are sterile in part due to impaired cumulus expansion [54].
The target genes for BR-SMADs implicated in tumorigenesis of granulosa cells are unknown. Partly this is due to incomplete knowledge of direct BR-SMAD target genes regulated by BMPs in normal folliculogenesis. However, gene expression arrays have been carried out for WT granulosa cells compared to Smad1 Smad5 dKO tumors, and indicate that a set of genes known to be BMP-regulated in other tissues are also altered in Smad1 Smad5 dKO tumors, including gremlin-1 (Grem1) and inhibitor of differentiation-1 (Id1) [57,78,80]. These were confirmed as likely downstream target genes for BMP signaling by treatment of WT granulosa cells with BMP4 [78]. A role of Grem1 in the ovary has been suggested [57,81], but Grem1 KO mice die perinatally [82], and thus, conditional knockout mice will have to be generated to study its role in postnatal folliculogenesis. IDs function as negative regulators of basic helix-loop-helix transcription factors, and mediate cell growth, differentiation, and cancer development [83–85]. Very little is known about their role in reproduction or reproductive cancers. In sheep ovaries, all four ID genes are expressed in granulosa and thecal cells, and BMPs and activin differentially regulate (up and down, respectively) their expression [86]. Currently, any role of the Id genes in mouse granulosa cell differentiation or their contribution to BR-SMAD cKO tumor development is unknown.
Because TGFβ has been shown to signal via SMAD1 and SMAD5 in other cell types [76,87] (though this has not been demonstrated in granulosa cells), and loss of function in TGFβ signaling is implicated in tumor development in epithelial cells, it is possible that deletion of Smad1 and Smad5 causes tumors in granulosa cells due to disruption of TGFβ signaling though SMAD1 and SMAD5. However, mice with deletion of the BMP type I receptors, Bmpr1a and Bmpr1b, in granulosa cells (herein termed BMP-RI cKO), which were subsequently generated after the BR-SMAD cKOs, also develop granulosa cell tumors [70]. Similar gene expression changes occur in both the BR-SMAD and BR-RI cKO mouse models. These data firmly establish that it is loss of signaling via the BMP receptor-SMAD pathway that is critical in granulosa cell tumor development in these mouse models. However, there are some differences between the BR-SMAD cKO and the BMP-RI cKO. In contrast to the development of tumors in the BR-SMAD cKO in young adult mice, BMP-RI cKO do not develop granulosa cell tumors until late in age (+16 months) and rarely show metastases [70]. Because an additional BMP type I receptor [Acvr1a (Alk2)], which also phosphorylates SMAD1 and SMAD5, is expressed in granulosa cells and is increased when Bmpr1a and Bmpr1b are deleted, residual BR-SMAD signaling may be sufficient to delay tumorigenesis in the BMP-RI cKO [70]. Interestingly, Id1 is one of the genes that does not change in the BMP-R1 cKO tumors. Thus, it is tempting to speculate that changes in Id1 status may be linked to the development of metastases in granulosa cell tumors of BR-SMAD cKO (which have reduced levels of Id1) and the granulosa cell tumors of BMP-R1 cKO, in which it is unchanged.
6. Questions regarding BR-SMAD loss and additional signaling pathways leading to granulosa cell tumorigenesis
Granulosa cell tumors in humans are classified into either adult (AGCT) or juvenile (JGCT) forms, with JGCT being the more rare type. Recent studies have identified a mutation in FOXL2 associated with the majority of AGCT but not in JGCT [88], and the etiology of JGCT is still unknown. Because of the relative rarity of JGCT, it is difficult to obtain a large patient population or tissues for analysis. However, histologic and hormone analysis of BR-SMAD cKO tumors suggest these tumors are more similar to JGCT than to AGCT [89] and as such, allow for a model to study this rare disease.
There are a number of mouse models that develop granulosa cell tumors, including mice with deletion of the inhibin α subunit (Inha KO) [22], mice with granulosa cell overexpression of β-catenin with and without deletion of Pten [90,91], mice with chronic overexpression of LH [92], transgenic mice with granulosa cell expression of SV40 T-antigen [93], as well as a number of spontaneous mutations [94]. It is not yet clear if these mouse models share a common mechanism that leads to GCT. Analysis of gene expression changes in BR-SMAD cKO tumors indicated that significant changes were found for ligands, receptors, and downstream target genes related to the TGFβ pathway [78]. This led us to hypothesize that part of the phenotype of the BR-SMAD cKO is due to dysregulation of TGFβ signaling via SMAD2 and SMAD3 when the BR-SMADs are deleted [78]. This is supported by immunohistochemical data that show BR-SMAD cKO tumors contain high levels of nuclear and phosphorylated SMAD2 and SMAD3, indicative of an active signaling pathway. Similar phospho-SMAD2 and SMAD3 immunostaining is also found in granulosa cell tumors from Inha KO mice as well as samples of human JGCTs [89], suggesting that AR-SMADs are active in GCT, though their function within the tumor is currently not known.
TGFβ signaling in other human cancers is also known to upregulated the transcription factors glioma associated oncogene (Gli1) and Gli2, via a SMAD3 dependent pathway [95], and high Gli2 expression is correlated with increased invasiveness and bone metastases in mice [96]. Interestingly, Gli1 and Gli2 are upregulated in response to TGFβ1 treatment in granulosa cells [70], and both are significantly increased in BMP-R1 cKO and BR-SMAD cKO tumors [55](S.A. Pangas, unpublished data). GLI1 and GLI2 serve as transcription factors for the hedgehog signaling pathway, and may play an as yet uncharacterized role in JGCT [70]. Besides TGFβ and hedgehog signaling, there is likely other signaling that is misregulated when the BR-SMADs are deleted. Future studies will be necessary to determine what changes contribute to the infertility in BR-SMAD cKO mice or are involved in GCT development and tumor dissemination.
7. Conclusion
There are a large number of related BMP ligands expressed in the ovary that have cell-type, as well as follicle-stage, specificity. Because the same conserved developmental signal transduction pathways that drive embryogenesis also control ovarian folliculogenesis, many of the TGFβ and BMP-related mouse knockout models cannot be used because of embryonic or perinatal lethality. All of the receptor-related SMADs and the common regulatory SMAD4 have been conditionally deleted in granulosa cells of the ovary, with varying effects on female fertility. Granulosa cell deletion of the AR-SMADs (Smad2 Smad3 dKO) or Smad4 (Smad4 cKO) generates the most similar phenotype. Ovary-specific receptor and SMAD conditional knockouts also demonstrate that the BMP ligands are important for maintenance of female fertility, but also surprisingly for the suppression of granulosa cell tumor growth. The mechanism(s) by which the BR-SMADs do so are still unknown. Data from mouse and human JGCT suggest that the TGFβ pathway is active in these tumors, and thus is a potential target for inhibition. While it is necessary to understand the genes regulated by the SMADs, it is also important to study the interaction of the SMADs with other signal transduction pathways, such as the hedgehog pathway, that control granulosa cell growth and differentiation. These are key steps for uncovering candidate genes underlying female infertility and possibly cancer development in the ovary as well as other tissues.
We review BMP signaling via the SMAD pathway in female reproductive biology.
We emphasize the phenotype of SMAD ovarian conditional knockout models.
BMPs regulate stage-specific ovarian follicle growth and tumor suppression.
Acknowledgments
Transgenic mouse studies in the Pangas Laboratory involving the BMP and SMAD signaling pathways are supported by a Burroughs Wellcome Career Award in Biomedical Sciences and National Institutes of Health grant CA138628.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eppig JJ. Intercommunication between mammalian oocytes and companion somatic cells. Bioessays. 1991;13:569–74. doi: 10.1002/bies.950131105. [DOI] [PubMed] [Google Scholar]
- 2.Myers M, Pangas SA. Regulatory roles of transforming growth factor beta family members in folliculogenesis. Wiley Interdiscip Rev Syst Biol Med. 2010;2:117–25. doi: 10.1002/wsbm.21. [DOI] [PubMed] [Google Scholar]
- 3.Matzuk MM, Burns K, Viveiros MM, Eppig J. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- 4.Eppig JJ, O'Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 5.Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng. 2003;9:1013–21. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- 6.Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, Hirshefeld-Cytron J, Kiesewetter SE, Shea LD, Woodruff TK. In Vitro Oocyte Maturation and Preantral Follicle Culture from the Luteal Phase Baboon Ovary Produce Mature Oocytes. Biol Reprod. 2011;84:689–97. doi: 10.1095/biolreprod.110.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, Picton HM, Plancha C, Shea LD, Stouffer RL, Telfer EE, Woodruff TK, Zelinski MB. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 16:395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirshfield AN. Development of follicles in the mammalian ovary. International Review of Cytology. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- 9.Pangas SA, Choi Y, Ballow DJ, Zhao Y, Westphal H, Matzuk MM, Rajkovic A. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc Natl Acad Sci U S A. 2006;103:8090–5. doi: 10.1073/pnas.0601083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305:1157–9. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- 11.Soyal SM, Amleh A, Dean J. FIGα, a germ cell-specific transcription factor required for ovarian follicle formation. Development. 2000;127:4645–54. doi: 10.1242/dev.127.21.4645. [DOI] [PubMed] [Google Scholar]
- 12.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–8. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 13.Pangas SA, Rajkovic A. Transcriptional regulation of early oogenesis: in search of masters. Hum Reprod Update. 2006;12:65–76. doi: 10.1093/humupd/dmi033. [DOI] [PubMed] [Google Scholar]
- 14.Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–3. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- 15.Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120:963–72. doi: 10.1172/JCI41350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun QY, Liu K, Kikuchi K. Oocyte-specific knockout: a novel in vivo approach for studying gene functions during folliculogenesis, oocyte maturation, fertilization, and embryogenesis. Biol Reprod. 2008;79:1014–20. doi: 10.1095/biolreprod.108.070409. [DOI] [PubMed] [Google Scholar]
- 17.Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawyer HR, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP. Formation of ovarian follicles during fetal development in sheep. Biol Reprod. 2002;66:1134–50. doi: 10.1095/biolreprod66.4.1134. [DOI] [PubMed] [Google Scholar]
- 19.Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol. 1998;203:323–33. doi: 10.1006/dbio.1998.9068. [DOI] [PubMed] [Google Scholar]
- 20.Da Silva-Buttkus P, Jayasooriya GS, Mora JM, Mobberley M, Ryder TA, Baithun M, Stark J, Franks S, Hardy K. Effect of cell shape and packing density on granulosa cell proliferation and formation of multiple layers during early follicle development in the ovary. J Cell Sci. 2008;121:3890–900. doi: 10.1242/jcs.036400. [DOI] [PubMed] [Google Scholar]
- 21.Myers M, Middlebrook BS, Matzuk MM, Pangas SA. Loss of inhibin alpha uncouples oocyte-granulosa cell dynamics and disrupts postnatal folliculogenesis. Dev Biol. 2009;334:458–67. doi: 10.1016/j.ydbio.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matzuk M, Finegold M, Su J, Hsueh A, Bradley A. α-Inhibin is a tumor-suppressor gene with gonadal specificity in mice. Nature. 1992;360:313–319. doi: 10.1038/360313a0. [DOI] [PubMed] [Google Scholar]
- 23.Combelles CM, Carabatsos MJ, Kumar TR, Matzuk MM, Albertini DF. Hormonal control of somatic cell oocyte interactions during ovarian follicle development. Mol Reprod Dev. 2004;69:347–55. doi: 10.1002/mrd.20128. [DOI] [PubMed] [Google Scholar]
- 24.Zhao M, Dean J. The zona pellucida in folliculogenesis, fertilization and early development. Rev Endocr Metab Disord. 2002;3:19–26. doi: 10.1023/a:1012744617241. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17:555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- 26.Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol. 1999;13:1035–1048. doi: 10.1210/mend.13.6.0310. [DOI] [PubMed] [Google Scholar]
- 27.Yan C, Wang P, DeMayo J, DeMayo F, Elvin J, Carino C, Prasad S, Skinner S, Dunbar B, Dube J, Celeste A, Matzuk M. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- 28.McIntosh CJ, Lun S, Lawrence S, Western AH, McNatty KP, Juengel JL. The proregion of mouse BMP15 regulates the cooperative interactions of BMP15 and GDF9. Biol Reprod. 2008;79:889–96. doi: 10.1095/biolreprod.108.068163. [DOI] [PubMed] [Google Scholar]
- 29.McNatty KP, Moore LG, Hudson NL, Quirke LD, Lawrence SB, Reader K, Hanrahan JP, Smith P, Groome NP, Laitinen M, Ritvos O, Juengel JL. The oocyte and its role in regulating ovulation rate: a new paradigm in reproductive biology. Reproduction. 2004;128:379–86. doi: 10.1530/rep.1.00280. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Rajanahally S, Edson MA, Matzuk MM. Stable expression and characterization of N-terminal tagged recombinant human bone morphogenetic protein 15. Mol Hum Reprod. 2009;15:779–88. doi: 10.1093/molehr/gap062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilchrist RB, Ritter LJ, Myllymaa S, Kaivo-Oja N, Dragovic RA, Hickey TE, Ritvos O, Mottershead DG. Molecular basis of oocyte-paracrine signalling that promotes granulosa cell proliferation. J Cell Sci. 2006;119:3811–21. doi: 10.1242/jcs.03105. [DOI] [PubMed] [Google Scholar]
- 32.Kaivo-Oja N, Bondestam J, Kamarainen M, Koskimies J, Vitt U, Cranfield M, Vuojolainen K, Kallio JP, Olkkonen VM, Hayashi M, Moustakas A, Groome NP, ten Dijke P, Hsueh AJ, Ritvos O. Growth differentiation factor-9 induces Smad2 activation and inhibin B production in cultured human granulosa-luteal cells. J Clin Endocrinol Metab. 2003;88:755–62. doi: 10.1210/jc.2002-021317. [DOI] [PubMed] [Google Scholar]
- 33.Roh JS, Bondestam J, Mazerbourg S, Kaivo-Oja N, Groome N, Ritvos O, Hsueh AJ. Growth differentiation factor-9 stimulates inhibin production and activates Smad2 in cultured rat granulosa cells. Endocrinology. 2003;144:172–8. doi: 10.1210/en.2002-220618. [DOI] [PubMed] [Google Scholar]
- 34.Moore RK, Otsuka F, Shimasaki S. Molecular basis of bone morphogenetic protein-15 signaling in granulosa cells. J Biol Chem. 2003;278:304–310. doi: 10.1074/jbc.M207362200. [DOI] [PubMed] [Google Scholar]
- 35.Sugiura K, Su YQ, Eppig JJ. Does bone morphogenetic protein 6 (BMP6) affect female fertility in the mouse? Biol Reprod. 2010;83:997–1004. doi: 10.1095/biolreprod.110.086777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otsuka F, Moore RK, Shimasaki S. Biological function and cellular mechanism of bone morphogenetic protein-6 in the ovary. J Biol Chem. 2001;276:32889–95. doi: 10.1074/jbc.M103212200. [DOI] [PubMed] [Google Scholar]
- 37.Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004;25:72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- 38.Shimasaki S, Zachow RJ, Li D, Kim H, Iemura SI, Ueno N, Sampath K, Chang RJ, Erickson GF. A functional bone morphogenetic protein system in the ovary. Proc Natl Acad Sci USA. 1999;96:7282–7287. doi: 10.1073/pnas.96.13.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nilsson EE, Skinner MK. Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol Reprod. 2003;69:1265–72. doi: 10.1095/biolreprod.103.018671. [DOI] [PubMed] [Google Scholar]
- 40.Tanwar PS, O'Shea T, McFarlane JR. In vivo evidence of role of bone morphogenetic protein-4 in the mouse ovary. Anim Reprod Sci. 2008;106:232–40. doi: 10.1016/j.anireprosci.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Lee WS, Otsuka F, Moore RK, Shimasaki S. Effect of bone morphogenetic protein-7 on folliculogenesis and ovulation in the rat. Biol Reprod. 2001;65:994–9. doi: 10.1095/biolreprod65.4.994. [DOI] [PubMed] [Google Scholar]
- 42.Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ying Y, Zhao GQ. Cooperation of endoderm-derived BMP2 and extraembryonic ectoderm- derived BMP4 in primordial germ cell generation in the mouse. Dev Biol. 2001;232:484–92. doi: 10.1006/dbio.2001.0173. [DOI] [PubMed] [Google Scholar]
- 44.Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–20. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- 45.Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- 46.McMahon HE, Hashimoto O, Mellon PL, Shimasaki S. Oocyte-specific overexpression of mouse bone morphogenetic protein-15 leads to accelerated folliculogenesis and an early onset of acyclicity in transgenic mice. Endocrinology. 2008;149:2807–15. doi: 10.1210/en.2007-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar TR, Palapattu G, Wang P, Woodruff TK, Boime I, Byrne MC, Matzuk MM. Transgenic models to study gonadotropin function: the role of follicle-stimulating hormone in gonadal growth and tumorigenesis. Mol Endocrinol. 1999;13:851–865. doi: 10.1210/mend.13.6.0297. [DOI] [PubMed] [Google Scholar]
- 48.Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 49.Nagaraja AK, Agno JE, Kumar TR, Matzuk MM. Luteinizing hormone promotes gonadal tumorigenesis in inhibin-deficient mice. Mol Cell Endocrinol. 2008;294:19–28. doi: 10.1016/j.mce.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Otsuka F, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15 inhibits follicle-stimulating hormone (FSH) action by suppressing FSH receptor expression. J Biol Chem. 2001;276:11387–92. doi: 10.1074/jbc.M010043200. [DOI] [PubMed] [Google Scholar]
- 51.Shi J, Yoshino O, Osuga Y, Koga K, Hirota Y, Nose E, Nishii O, Yano T, Taketani Y. Bone Morphogenetic Protein-2 (BMP-2) Increases Gene Expression of FSH Receptor and Aromatase and Decreases Gene Expression of LH Receptor and StAR in Human Granulosa Cells. Am J Reprod Immunol. 2011;65:421–427. doi: 10.1111/j.1600-0897.2010.00917.x. [DOI] [PubMed] [Google Scholar]
- 52.Shi J, Yoshino O, Osuga Y, Nishii O, Yano T, Taketani Y. Bone morphogenetic protein 7 (BMP-7) increases the expression of follicle-stimulating hormone (FSH) receptor in human granulosa cells. Fertil Steril. 2010;93:1273–9. doi: 10.1016/j.fertnstert.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 53.Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, O'Brien MJ, Matzuk MM, Shimasaki S, Eppig JJ. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development. 2007;134:2593–603. doi: 10.1242/dev.006882. [DOI] [PubMed] [Google Scholar]
- 54.Yi SE, LaPolt PS, Yoon BS, Chen JY, Lu JK, Lyons KM. The type I BMP receptor BmprIB is essential for female reproductive function. Proc Natl Acad Sci U S A. 2001;98:7994–9. doi: 10.1073/pnas.141002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erickson GF, Shimasaki S. The spatiotemporal expression pattern of the bone morphogenetic protein family in rat ovary cell types during the estrous cycle. Reprod Biol Endocrinol. 2003;1:9–28. doi: 10.1186/1477-7827-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- 57.Pangas SA, Jorgez CJ, Matzuk MM. Growth differentiation factor 9 regulates expression of the bone morphogenetic protein antagonist gremlin. J Biol Chem. 2004;279:32281–6. doi: 10.1074/jbc.M403212200. [DOI] [PubMed] [Google Scholar]
- 58.Su YQ, Wu X, O'Brien MJ, Pendola FL, Denegre JN, Matzuk MM, Eppig JJ. Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence for an oocyte-granulosa cell regulatory loop. Dev Biol. 2004;276:64–73. doi: 10.1016/j.ydbio.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 59.Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002;16:1154–1167. doi: 10.1210/mend.16.6.0859. [DOI] [PubMed] [Google Scholar]
- 60.Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA, Jr, Falb D, Huszar D. A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet. 2000;24:171–4. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- 61.Li R, Rosendahl A, Brodin G, Cheng AM, Ahgren A, Sundquist C, Kulkarni S, Pawson T, Heldin CH, Heuchel RL. Deletion of exon I of SMAD7 in mice results in altered B cell responses. J Immunol. 2006;176:6777–84. doi: 10.4049/jimmunol.176.11.6777. [DOI] [PubMed] [Google Scholar]
- 62.Pangas SA, Li X, Robertson EJ, Matzuk MM. Premature luteinization and cumulus cell defects in ovarian-specific Smad4 knockout mice. Mol Endocrinol. 2006;20:1406–22. doi: 10.1210/me.2005-0462. [DOI] [PubMed] [Google Scholar]
- 63.Yu Y, Bradley A. Engineering chromosomal rearrangements in mice. Nat Rev Genet. 2001;2:780–90. doi: 10.1038/35093564. [DOI] [PubMed] [Google Scholar]
- 64.Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet. 2002;7:7. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- 65.Jorgez CJ, Klysik M, Jamin SP, Behringer RR, Matzuk MM. Granulosa cell-specific inactivation of follistatin causes female fertility defects. Mol Endocrinol. 2004;18:953–67. doi: 10.1210/me.2003-0301. [DOI] [PubMed] [Google Scholar]
- 66.Jeyasuria P, Ikeda Y, Jamin SP, Zhao L, De Rooij DG, Themmen AP, Behringer RR, Parker KL. Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Mol Endocrinol. 2004;18:1610–9. doi: 10.1210/me.2003-0404. [DOI] [PubMed] [Google Scholar]
- 67.Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–3. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 68.Li Q, Pangas SA, Jorgez CJ, Graff JM, Weinstein M, Matzuk MM. Redundant roles of SMAD2 and SMAD3 in ovarian granulosa cells in vivo. Mol Cell Biol. 2008;28:7001–11. doi: 10.1128/MCB.00732-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–82. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 70.Edson MA, Nalam RL, Clementi C, Franco HL, Demayo FJ, Lyons KM, Pangas SA, Matzuk MM. Granulosa cell-expressed BMPR1A and BMPR1B have unique functions in regulating fertility but act redundantly to suppress ovarian tumor development. Mol Endocrinol. 2010;24:1251–66. doi: 10.1210/me.2009-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Myers M, van den Driesche S, McNeilly AS, Duncan WC. Activin A reduces luteinisation of human luteinised granulosa cells and has opposing effects to human chorionic gonadotropin in vitro. J Endocrinol. 2008;199:201–12. doi: 10.1677/JOE-08-0302. [DOI] [PubMed] [Google Scholar]
- 72.Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM. Molecular characterization of the follicle defects in the growth differentiation factor-9-deficient ovary. Molecular Endocrinology. 1999;13:1018–1034. doi: 10.1210/mend.13.6.0309. [DOI] [PubMed] [Google Scholar]
- 73.Shi FT, Cheung AP, Klausen C, Huang HF, Leung PC. Growth differentiation factor 9 reverses activin A suppression of steroidogenic acute regulatory protein expression and progesterone production in human granulosa-lutein cells. J Clin Endocrinol Metab. 2010;95:E172–80. doi: 10.1210/jc.2010-0477. [DOI] [PubMed] [Google Scholar]
- 74.Otsuka F, Yao Z, Lee T, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15. IDENTIFICATION OF TARGET CELLS AND BIOLOGICAL FUNCTIONS. J Biol Chem. 2000;275:39523–8. doi: 10.1074/jbc.M007428200. [DOI] [PubMed] [Google Scholar]
- 75.Yamamoto N, Christenson LK, McAllister JM, Strauss JF., 3rd Growth differentiation factor-9 inhibits 3'5'-adenosine monophosphate- stimulated steroidogenesis in human granulosa and theca cells. J Clin Endocrinol Metab. 2002;87:2849–56. doi: 10.1210/jcem.87.6.8551. [DOI] [PubMed] [Google Scholar]
- 76.Wrighton KH, Lin X, Yu PB, Feng XH. Transforming Growth Factor {beta} Can Stimulate Smad1 Phosphorylation Independently of Bone Morphogenic Protein Receptors. J Biol Chem. 2009;284:9755–63. doi: 10.1074/jbc.M809223200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF– beta type I receptors. Embo J. 2002;21:1743–53. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez C, Wang D, Martin JF, Jamin SP, Behringer RR, Robertson EJ, Matzuk MM. Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol. 2008;28:248–57. doi: 10.1128/MCB.01404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arnold SJ, Maretto S, Islam A, Bikoff EK, Robertson EJ. Dose-dependent Smad1, Smad5 and Smad8 signaling in the early mouse embryo. Dev Biol. 2006;296:104–118. doi: 10.1016/j.ydbio.2006.04.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miyazono K, Miyazawa K. Id: a target of BMP signaling. Sci STKE. 2002;2002:PE40. doi: 10.1126/stke.2002.151.pe40. [DOI] [PubMed] [Google Scholar]
- 81.Hussein TS, Froiland DA, Amato F, Thompson JG, Gilchrist RB. Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J Cell Sci. 2005;118:5257–68. doi: 10.1242/jcs.02644. [DOI] [PubMed] [Google Scholar]
- 82.Khokha MK, Hsu D, Brunet LJ, Dionne MS, Harland RM. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat Genet. 2003;34:303–7. doi: 10.1038/ng1178. [DOI] [PubMed] [Google Scholar]
- 83.Yokota Y, Mori S. Role of Id family proteins in growth control. J Cell Physiol. 2002;190:21–8. doi: 10.1002/jcp.10042. [DOI] [PubMed] [Google Scholar]
- 84.Ruzinova MB, Benezra R. Id proteins in development, cell cycle and cancer. Trends Cell Biol. 2003;13:410–8. doi: 10.1016/s0962-8924(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 85.Perk J, Iavarone A, Benezra R. Id family of helix-loop-helix proteins in cancer. Nat Rev Cancer. 2005;5:603–14. doi: 10.1038/nrc1673. [DOI] [PubMed] [Google Scholar]
- 86.Hogg K, Etherington SL, Young JM, McNeilly AS, Duncan WC. Inhibitor of differentiation (Id) genes are expressed in the steroidogenic cells of the ovine ovary and are differentially regulated by members of the transforming growth factor-beta family. Endocrinology. 151:1247–56. doi: 10.1210/en.2009-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goumans MJ, Liu Z, ten Dijke P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–27. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- 88.Shah SP, Kobel M, Senz J, Morin RD, Clarke BA, Wiegand KC, Leung G, Zayed A, Mehl E, Kalloger SE, Sun M, Giuliany R, Yorida E, Jones S, Varhol R, Swenerton KD, Miller D, Clement PB, Crane C, Madore J, Provencher D, Leung P, DeFazio A, Khattra J, Turashvili G, Zhao Y, Zeng T, Glover JN, Vanderhyden B, Zhao C, Parkinson CA, Jimenez-Linan M, Bowtell DD, Mes-Masson AM, Brenton JD, Aparicio SA, Boyd N, Hirst M, Gilks CB, Marra M, Huntsman DG. Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J Med. 2009;360:2719–29. doi: 10.1056/NEJMoa0902542. [DOI] [PubMed] [Google Scholar]
- 89.Middlebrook BS, Eldin K, Li X, Shivasankaran S, Pangas SA. Smad1-Smad5 Ovarian Conditional Knockout Mice Develop a Disease Profile Similar to the Juvenile Form of Human Granulosa Cell Tumors. Endocrinology. 2009 doi: 10.1210/en.2009-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lague MN, Paquet M, Fan HY, Kaartinen MJ, Chu S, Jamin SP, Behringer RR, Fuller PJ, Mitchell A, Dore M, Huneault LM, Richards JS, Boerboom D. Synergistic effects of Pten loss and WNT/CTNNB1 signaling pathway activation in ovarian granulosa cell tumor development and progression. Carcinogenesis. 2008;29:2062–72. doi: 10.1093/carcin/bgn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boerboom D, Paquet M, Hsieh M, Liu J, Jamin SP, Behringer RR, Sirois J, Taketo MM, Richards JS. Misregulated Wnt/beta-catenin signaling leads to ovarian granulosa cell tumor development. Cancer Res. 2005;65:9206–15. doi: 10.1158/0008-5472.CAN-05-1024. [DOI] [PubMed] [Google Scholar]
- 92.Keri RA, Lozada KL, Abdul-Karim FW, Nadeau JN, Nilson JH. Luteinizing hormone induction of ovarian tumors: oligogenic differences between mouse strains dictates tumor disposition. PNAS. 2000;97:383–387. doi: 10.1073/pnas.97.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kananen K, Markkula M, Rainio E, Su JG, Hsueh AJ, Huhtaniemi IT. Gonadal tumorigenesis in transgenic mice bearing the mouse inhibin alpha-subunit promoters/simian virus T-antigen fusion gene: characterization of ovarian tumors and establishment of gonadotropin-responsive granulosa cell lines. Mol Endocrinol. 1995;9:616–627. doi: 10.1210/mend.9.5.7565808. [DOI] [PubMed] [Google Scholar]
- 94.Beamer WG, Shultz KL, Tennent BJ, Azumi N, Sundberg JP. Mouse model for malignant juvenile ovarian granulosa cell tumors. Toxicol Pathol. 1998;26:704–10. doi: 10.1177/019262339802600518. [DOI] [PubMed] [Google Scholar]
- 95.Dennler S, Andre J, Alexaki I, Li A, Magnaldo T, ten Dijke P, Wang XJ, Verrecchia F, Mauviel A. Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981–6. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- 96.Alexaki VI, Javelaud D, Van Kempen LC, Mohammad KS, Dennler S, Luciani F, Hoek KS, Juarez P, Goydos JS, Fournier PJ, Sibon C, Bertolotto C, Verrecchia F, Saule S, Delmas V, Ballotti R, Larue L, Saiag P, Guise TA, Mauviel A. GLI2-mediated melanoma invasion and metastasis. J Natl Cancer Inst. 2011;102:1148–59. doi: 10.1093/jnci/djq257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 98.Daluiski A, Engstrand T, Bahamonde ME, Gamer LW, Agius E, Stevenson SL, Cox K, Rosen V, Lyons KM. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet. 2001;27:84–8. doi: 10.1038/83810. [DOI] [PubMed] [Google Scholar]
- 99.Zhao R, Lawler AM, Lee SJ. Characterization of GDF-10 expression patterns and null mice. Dev Biol. 1999;212:68–79. doi: 10.1006/dbio.1999.9326. [DOI] [PubMed] [Google Scholar]
- 100.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 101.Dunn NR, Winnier GE, Hargett LK, Schrick JJ, Fogo AB, Hogan BL. Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev Biol. 1997;188:235–47. doi: 10.1006/dbio.1997.8664. [DOI] [PubMed] [Google Scholar]
- 102.Pfendler KC, Yoon J, Taborn GU, Kuehn MR, Iannaccone PM. Nodal and bone morphogenetic protein 5 interact in murine mesoderm formation and implantation. Genesis. 2000;28:1–14. doi: 10.1002/1526-968x(200009)28:1<1::aid-gene10>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 103.Jena N, Martin-Seisdedos C, McCue P, Croce CM. BMP7 null mutation in mice: developmental defects in skeleton, kidney, and eye. Exp Cell Res. 1997;230:28–37. doi: 10.1006/excr.1996.3411. [DOI] [PubMed] [Google Scholar]
- 104.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 105.Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. 1995;374:356–360. doi: 10.1038/374356a0. [DOI] [PubMed] [Google Scholar]
- 106.Oh SP, Li E. The signaling pathway mediated by the type IIB activin receptor controls axial patterning and lateral asymmetry in the mouse. Genes Dev. 1997;11:1812–26. doi: 10.1101/gad.11.14.1812. [DOI] [PubMed] [Google Scholar]
- 107.Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol. 2000;221:249–58. doi: 10.1006/dbio.2000.9670. [DOI] [PubMed] [Google Scholar]
- 108.Gu Z, Reynolds EM, Song J, Lei H, Feijen A, Yu L, He W, MacLaughlin DT, van den Eijnden-van Raaij J, Donahoe PK, Li E. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development. 1999;126:2551–61. doi: 10.1242/dev.126.11.2551. [DOI] [PubMed] [Google Scholar]
- 109.de Sousa Lopes SM, Roelen BA, Monteiro RM, Emmens R, Lin HY, Li E, Lawson KA, Mummery CL. BMP signaling mediated by ALK2 in the visceral endoderm is necessary for the generation of primordial germ cells in the mouse embryo. Genes Dev. 2004;18:1838–49. doi: 10.1101/gad.294004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–37. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- 111.Yi SE, Daluiski A, Pederson R, Rosen V, Lyons KM. The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development. 2000;127:621–30. doi: 10.1242/dev.127.3.621. [DOI] [PubMed] [Google Scholar]
- 112.Tremblay KD, Dunn NR, Robertson EJ. Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development. 2001;128:3609–21. doi: 10.1242/dev.128.18.3609. [DOI] [PubMed] [Google Scholar]
- 113.Nomura M, Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;393:786–790. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- 114.Waldrip WR, Bikoff EK, Hoodless PA, Wrana JL, Robertson EJ. Smad2 signaling in extraembryonic tissues determines anterior-posterior polarity of the early mouse embryo. Cell. 1998;92:797–808. doi: 10.1016/s0092-8674(00)81407-5. [DOI] [PubMed] [Google Scholar]
- 115.Sirard C, de la Pompa JL, Elia A, Itie A, Mirtsos C, Cheung A, Hahn S, Wakeham A, Schwartz L, Kern SE, Rossant J, Mak TW. The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev. 1998;12:107–19. doi: 10.1101/gad.12.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang X, Li C, Xu X, Deng C. The tumor suppressor SMAD4/DPC4 is essential for epiblast proliferation and mesoderm induction in mice. Proc Natl Acad Sci U S A. 1998;95:3667–72. doi: 10.1073/pnas.95.7.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chang H, Matzuk MM. Smad5 is required for mouse primordial germ cell development. Mech Dev. 2001;104:61–7. doi: 10.1016/s0925-4773(01)00367-7. [DOI] [PubMed] [Google Scholar]
- 118.Chang H, Zwijsen A, Vogel H, Huylebroeck D, Matzuk MM. Smad5 is essential for left-right asymmetry in mice. Dev Biol. 2000;219:71–78. doi: 10.1006/dbio.1999.9594. [DOI] [PubMed] [Google Scholar]
- 119.Chen Q, Chen H, Zheng D, Kuang C, Fang H, Zou B, Zhu W, Bu G, Jin T, Wang Z, Zhang X, Chen J, Field LJ, Rubart M, Shou W, Chen Y. Smad7 is required for the development and function of the heart. J Biol Chem. 2009;284:292–300. doi: 10.1074/jbc.M807233200. [DOI] [PMC free article] [PubMed] [Google Scholar]