Abstract
Doxorubicin is a broad spectrum antibiotic used in the treatment of cancers. Its dose dependent cardiotoxicity is the most serious side effect causing withdrawal of drug from hard chemotherapeutic regimen. Statins are shown to be cytotoxic in concentrations higher than the effective doses for the treatment of hypercholesterolemia (40 mg/day). Co-administration of statins and chemotherapeutic agents suppose to be synergic although there are some controversies in the literature. In this study, cytotoxic effects of doxorubicin alone and in combination with simvastatin on Hela tumor cell line were evaluated. Different concentration of doxorubicin and simvastatin were added to the cultured cells and incubated for 72 h. Cell survival was evaluated using MTT and trypan blue exclusion assays. The results indicated that simvastatin in low concentration (0.25 μM) seems to be growth stimulator although cell viability was reduced in concentrations of ≥2 μM. Doxorubicin alone at all tested concentrations (0.1, 1 and 2 μM) was a cell growth inhibitor. It was also shown that percent cell viability was reduced in a decreasing manner with the following protocols: 1) co-administration of doxorubicin and simvastatin in different concentrations; 2) addition of simvastatin after incubation of cells with doxorubicin and 3) addition of doxorubicin after incubation of cells with simvastatin. It could be concluded that between 3 tested protocols combination of doxorubicin and simvastatin after 72 h incubation, showed the highest cytotoxicity against Hela cells.
Keywords: Doxorubicin, Simvastatin, Cytotoxicity, Hela cell, HPLC, MTT assay
INTRODUCTION
Hypercholesterolemia and increased cancer risk have been associated, particularly with the high fat diets characteristic of western societies. There are association between preexisting hypercholesterolemia and the rapidity and extent of tumor metastasis in these societies. Only a few studies have suggested and explored this determinant of cancer metastases, although it may play a role in a subset of patients who develop cancers. Cholesterol and its derivatives in high concentrations affect cell differentiation and proliferation, cancer progression and enhanc-ement of metastatic potential (1).
Compounds acting as 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase inhibitors can affect development and proli-feration of cells (2,3). In the patients receiving statins for more than 5 years, a decreased risk of cancer has been demonstrated (4).
Simvastatin is a chemically modified deriv-ative of lovastatin. Lovastatin is a natural statin isolated from a strain of Aspergillus terreus which was the first statin approved by the FDA (5).
G and G2/M arrests have been demonstrated in simvastatin treated normal and tumor cells (6). It is possible to devise combinations of drugs which have a different mechanism of action to provide greater benefit than the single agent does individually. If the side effects of the components of the combination are different, these will not be more toxic than when the drugs are given singly. If they have different mechanisms of cytotoxic action, an increase in killing tumor cells is most likely to occur (7). Although some studies on the anti-tumor activity of statins have been performed, the preliminary results of these studies showed that statins alone are not effective anticancer agents. However, when combined with other cytotoxic or cytostatic compouds, obtained data suggest that they may enhance chemotherapeutic effects (3).
Scheme 1.
Chemical structures of simvastatin (1) and lovastatin (2)
In early metastatic cancers, statins are more likely to be effective if given in combination with a cytotoxic agent, especially those combinations that yield synergism in pre-clinical models (2). Gronich and co-workers (8) hypothesized that statins inhibit farnesy-lation of Ras and also production of interleukin 6, a key cytokine in multiple myeloma; therefore they may have anti-proliferative and/or proapoptotic effects in this malignancy. Following these studies, combi-nation therapies indicated that simvastatin and zoledronate had synergistic effects against myeloma cell lines (9). However, this combination sometimes showed antagonists effects against different cells. Drucker et al. showed that incubation of U266 and RPMI 8226, two melanoma cell lines, with simvastatin prior to melphalan increased the cytotoxicity. Although the exposure of cancer cells to other combinations may show different effects; for instance combined simvastatin and dexamethasone in U266 resulted in synergistic amplification, but this combination in RPMI 8226 cells resulted in antagonistic activity (10). Studies have shown that cells have different sensitivity to statins or chemo-therapeutic agents (11,12). Reduction of doxorubicin cardiotoxicity by lipid-lowering agents has been also reported (13). In the light of aforementioned studies and lack of documented studies to show the combination effects of simvastatin and doxorubicin against Hela (Human cervix carcinoma) cells and synergistic effects of combined anticancer regimens, we aimed to perform the current study.increase in killing tumor cells is most likely to occur (7). Although some studies on the anti-tumor activity of statins have been performed, the preliminary results of these studies showed that statins alone are not effective anticancer agents. However, when combined with other cytotoxic or cytostatic compouds, obtained data suggest that they may enhance chemotherapeutic effects (3).
In early metastatic cancers, statins are more likely to be effective if given in combination with a cytotoxic agent, especially those combi-nations that yield synergism in preclinical models (2). Gronich and co-workers (8) hypothesized that statins inhibit farnesylation of Ras and also production of interleukin 6, a key cytokine in multiple myeloma; therefore they may have antiproliferative and/or proa-poptotic effects in this malignancy. Following these studies, combination therapies indicated that simvastatin and zoledronate had synergistic effects against myeloma cell lines (9). However, this combination sometimes showed antagonists effects against different cells. Drucker et al. showed that incubation of U266 and RPMI 8226, two melanoma cell lines, with simvastatin prior to melphalan increased the cytotoxicity. Although the exposure of cancer cells to other combinations may show different effects; for instance combined simvastatin and dexamethasone in U266 resulted in synergistic amplification, but this combination in RPMI 8226 cells resulted in antagonistic activity (10). Studies have shown that that cells have different sensitivity to statins or chemotherapeutic agents (11,12).
Reduction of doxorubicin cardiotoxicity by lipid-lowering agents has also reported (13). In the light of aforementioned studies and lack of documented study to show the combination effects of simvastatin and doxorubicin against Hela (Human cervix carcinoma) cells, and synergistic effects of combined anticancer regimens We aimed to perform the current study.
MATERIALS AND METHODS
Drugs
Doxorubicin hydrochloride (Adriamycin®) was obtained from Ebewe Pharma (Austria) and pure simvastatin was kindly provided by Sobhan Pharmaceutical Co. (Rasht, Iran).
Preparation and dilution of stock solutions of drugs
Stock solutions of 1 mM doxorubicin hydrochloride and further dilutions (0.1, 1and 2 μM) were prepared in RPMI 1640. Simvastatin stock solution of 1 mM was prepared in DMSO, and other diluted solutions (0.25, 0.5, 1, 2, 5 and 10 μM) were prepared in RPMI1640. The final concentration of DMSO in all experiments was less than 0.5%. The stock solutions were sterilized using 0.22 μ microfilters under laminar flow hood and stored frozen. All dilutions were prepared fresh before addition to the cells, using phosphate buffered solution (PBS) as diluents.
Aqueous doxorubicin hydrochloride solu-tions can be stored in 4 °C for one month, but it is unstable in higher temperature or acidic/alkaline pH (14).
Cell line and culture conditions
Hela cell line was purchased from Pasture Institute (Tehran, Iran), cultured in RPMI-1640 (Gibco, Scotland) in 75 cm2 tissue flask (Nunc, Denmark) and passaged every 3-4 days after trypsinization with trypsin/EDTA. [Each 1 liter of RPMI-1640 was supplemented with 10% fetal bovine serum, 10 ml of penicillin/ streptomycin (50 IU/ ml/50 μg/ml), 10 ml of sodium pyruvate (1 mM), NaHCO3 (2 g) and 10 ml of L-glutamine (2 mM)]. The supplemented medium was then sterilized using 0.22 μ microfilters and stored at 4 °C before use.
Cytotoxicity assay
The cytotoxic/cytostatic effects of simvas-tatin and/or doxorubicin in vitro on Hela cells were tested with a rapid colorimetric assay using MTT and compared with the untreated controls. This assay is based on the metabolic reduction of soluble MTT by mitochondrial enzyme activity of viable tumor cells, into an insoluble colored formazan product, which can be measured spectrophotometrically after dissolving in DMSO (15).
160 μl of cells suspension (3×104 cell/ml) was dispensed into three 96-well U-bottom microplates (Nunc, Denmark) and incubated for 24 h at 37 °C in a fully humidified atmosphere of 5% CO2. Then, in plate 1, serial dilutions of doxorubicin (20 μl; final concentration, 0.1-2 μM) and simvastatin (20 μl; final concentration, 0.25-2 μM) were added to a final volume of 200 μl and incubated for another 72 h.
In plates 2 and 3 serial dilutions of each drug (simvastatin or doxorubicin, 40 μl) were added. After an incubation period of 24 h, the medium was aspirated and the cells were washed in PBS. Then, serial dilutions of other drug (40 μl) were added and supplemented with culture medium to a final volume of 200 μl, and incubated for 48 h. Doxorubicin and simvastatin were used individually as positive controls (40 μl in each well), and the cells treated only with solvent were considered as negative controls.
To evaluate cell survival, 20 μl of MTT solution (5 mg/ml in PBS) was added to each well and incubated for 3 h. Then the media was replaced with 150 μl of DMSO, and complete solubilization of formazan crystals was achieved by repeated pipetting of the solution. Absorbance was then determined at 540 nm by an ELISA plate reader. Each drug concentration was assayed in 4 or 8 wells and repeated 3 times. The cytotoxic/cytostatic effect of doxorubicin and/or simvastatin was expressed as the relative viability (% control) and calculated as shown below. Percentage of cell survival in the negative control was assumed as 100. Relative viability = (experimental absorbance - background absorbance)/ (absorbance of untreated controls-background absorbance) × 100 %
Statistical analysis
The mean, standard deviation (SD) and coefficient of variation (CV) were determined for concentrations of each drug. SPSS was used to perform statistical test. Analysis of variance followed by “Post Hoc” test was used to distinguish the differences among groups. Significance was assumed at P<0.05.
RESULTS
For Hela cell line, a linear relationsphip between the number of cells and absorbance was was observed (r2 = 0.9687).
Effects of simvastatin on Hela cell survival
To determine the sensitivity of Hela cells to the cytotoxic/cytostatic effects of simvastatin, the cells were seeded into the microplates and incubated with various concentrations of simvastatin as mentioned under materials and methods. As shown in Fig. 1, simvastatin in low concentration (0.25 μM) after 72 h seems to be growth stimulator (cell viability 112%, P<0.05), although in concentration of (2 μM) and more (5 and 10 μM), cell viability reduced at least by 20%, (P<0.05). As a whole, simvastatin showed relatively low cytotoxic effects on this cell line. The IC50 (50% of growth inhibition) of simvastatin and doxorubicin on Hela cells were determined at 9.14 μM and 0.374, respectively. It is reported that sensitivity to statins is related to the type of cell line used (2).
Fig. 1.

Viability of Hela cells exposed to different concentrations of simvastatin after 72 h incubation. The cytotoxicity was determined by MTT assay. Data are expressed as the percentage of inhibition compared with negative control in which cell survival was assumed 100 % (means ± SD, n=24).
The influence of pre-incubation of the cells on antiproliferative effects of simvastatin
As shown in Fig. 2, pre-incubation of the cells before addition of simvastatin did not show any significant difference in cell viabi-lity. Percent cell survival in low concentrations of simvastatin (0.25, 0.5, 1 and 2 μM) indicated no significant reduction in cell viability compared to control (100%), but in higher concentrations (5 and 10 μM) cell viability was decreased sharply after 24 h of incubation.
Fig. 2.

Cytotoxic effect of simvastatin (Sim) on Hela cells pre-incubated either 24 or 48 h. Simvastatin at concentrations of 5 and 10 μM showed significant cytotoxic effect compare to negative control (ANOVA, P<0.05, n=24), but no significant differences was seen in cell survival between 24 and 48 h pre-incubation.
The influence of simvastatin on the cytotoxic effects of doxorubicin
It has been shown that statins possibly inhibit cell cycle progression in G1 phase (16). The current study indicates that combination of simvastatin and doxorubicin with different concentrations reduced percent cell survival significantly after 72 h incubation (P<0.05, Fig. 3).
Fig. 3.
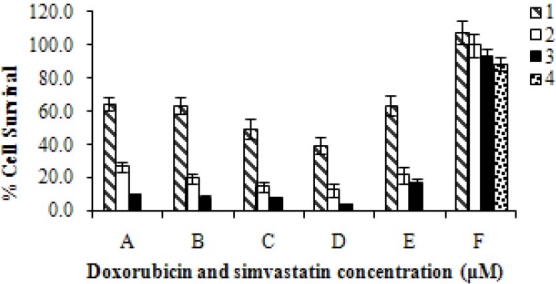
The effects of co-administration of doxorubicin (D) and simvastatin (S) in different concentrations on the viability of Hela cells after 72 h incubation, using MTT assay. The graph bars represent the mean viability of 4 separate sets of experiments including 6 wells and SD between experiments is indicated (ANOVA, P<0.05, n=24). All sets of experiments showed significant differences with simvastatin alone.
Cytotoxic effects of combination of simvas-tatin and doxorubicin on Hela cells, using different protocol of treatment
The results of the present study showed that using fixed concentration of doxorubicin (0.1, 1 and 2 μM) in the presence of various concentrations of simvastatin, each time with different protocol of treatment, different pattern of cell survival would be obtained (Fig. 4 A, B and C). Combination of doxorubicin and simvastatin in the highest tested concentrations (2 μM and 10 μM, respec-tively) killed 97% of the cells (data not shown).
Figure 4.

The effects of combination of: A) Doxorubicin (0.1 μM); B) Doxorubicin (1 μM) and C) Doxorubicin (2 μM) with different concentration of simvastatin (0.25, 0.5, 1 and 2 μM). ■Cells were incubated for 72 h at the presence of combination of doxorubicin and simvastatin. □Cells were incubated for 24 h at the presence of doxorubicin, and then the media was aspirated and different concentrations of simvastatin were added in fresh media and incubated for another 48 h.  Cells were incubated at the presence of different concentrations of simvastatin for 24 h, the media was then aspirated and doxorubicin was added in fresh media and incubated for another 48 h.
Cells were incubated at the presence of different concentrations of simvastatin for 24 h, the media was then aspirated and doxorubicin was added in fresh media and incubated for another 48 h.
DISCUSSION
Statins are extensively prescribed for the treatment of hypercholesterolemia. Our results showed that simvastatin posses some anti-tumor effects in experimental models, although induced cell proliferation in lower concentrations. These results are in agreement with previous reports indicating that statins in low concentrations stimulate cell growth, whose mechanism is still unclear (17). According to our results, combination of doxorubicin and simvastatin in the highest tested concentrations killed most of the cells. These results are consistent with those of other researchers who showed that different combinations of chemotherapeutic agents with statins were effective against myeloma cells (8–10).
According to Werner and his colleagues (18) , synergic effect of simvastatin and doxorubicin on human rhabdomy sarcoma cells was experimented through measuring the activation of caspases. They showed that simvastatin induced apoptosis in this cell line by inducing caspase 3, 9 activation through translocation form the cytosol in to the nucleus, and translocation of Bax from the cytosol in to mitochondria,. They indicated that simvastatin in concentration of 1 μM was enough to initiate a consecutive activation of caspase 3, 9 within 48 h. On the other hand, in co-administration of 2 compounds, doxorubicin was capable of increasing the potency of simvastatin to trigger caspase 3, 9 activity; conversely, simvastatin facilitated doxorubicin-mediated apoptotic stimuli. Therefore, the combination of two drugs sensitized Rhabdomy cells for apoptosis by enhancing caspase 3, 9 activity, synergically (18).
In another study, it has been shown that lovastatin may increase the vulnerability of tumor cells to the action of other chemo-therapeutic agents by specifically targeting drug-resistant P-glicoprotein expressing tumor cells. This observation may be considered as a possible explanation for the increase in cytotoxicity of doxorubicin in combination with lovastatin, although an augmentation of apoptosis induced by both agents cannot be ruled out (11). Our results also showed that incubation of the cells with simvastatin after 24 h incubation with doxorubicin seems to be more effective protocol than the other way around (14-76% cytotoxicity vs. 12-61%). This can be explained by the known cytotoxic effect of doxorubicin against Hela cells and possible cytostatic effects of simvastatin.
CONCLUSION
According to our study it can be concluded that a) Cell survival was reduced between 40 to 83% after 72 h incubation with doxorubicin alone (0.1, 1 and 2 μM) in a dose dependent manner. b) Co-administration of doxorubicin and simvastatin with different concentrations after 72 h reduced the cell viability by 35-97% (P<0.05). c) Incubation of the cells with doxorubicin-, 24 h prior to addition of simvastatin, was more effective than the other way around. Finally, it could be concluded that co-administration of these drugs, in longer period, might help apoptosis more than each drug alone or incubation of one compound prior to the other one.
ACKNOWLEDGMENT
This study was financially supported by the research council of Isfahan University of Medical Scinces, Isfahan, Iran.
REFERENCES
- 1.Mehta N, Hordines J, Volpe C, Doerr R, Cohea SA. Cellular effects of hypercholesterolemia in modulation of cancer growth and metastasis: a review of the evidence. Surg Oncol. 1997;6:179–185. doi: 10.1016/s0960-7404(97)00027-3. [DOI] [PubMed] [Google Scholar]
- 2.Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003;9:10–19. [PubMed] [Google Scholar]
- 3.Hindler K, Cleeland CS, Rivera E, Collard CD. The role of statins in cancer therapy. Oncologist. 2006;11:306–315. doi: 10.1634/theoncologist.11-3-306. [DOI] [PubMed] [Google Scholar]
- 4.Sleijfer S, van der Gaast A, Planting AS, Stoter G, Verweij J. The potential of statins as part of anti-cancer treatment. Eur J Cancer. 2005;41:516–522. doi: 10.1016/j.ejca.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Dulak J, Jozkowicz A. Anti-angiogenic and anti-inflammatory effects of statins: relevance to anti-cancer therapy. Curr Cancer Drug Targets. 2005;5:579–594. doi: 10.2174/156800905774932824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jakobisiak M, Golab J. Potential anti-tumor effects of statins. Int J Oncol. 2003;23:1055–1069. [PubMed] [Google Scholar]
- 7.Gillies HC, Rogers HJ, Spector RG, Trounce JR. 2nd ed. London: Edward Arnold; 1986. A textbook of clinical pharmacology; pp. 770–775. [Google Scholar]
- 8.Gronich N, Drucker L, Shapira H, Radnay J, Yarkoni S, Lishner M. Simvastatin induces death of multiple myeloma cell lines. J Investig Med. 2004;52:335–344. doi: 10.1136/jim-52-05-34. [DOI] [PubMed] [Google Scholar]
- 9.Schmidmaier R, Simsek M, Baumann P, Emmerich B, Meinhardt G. Synergistic antimyeloma effects of zoledronate and simvastatin. Anticancer Drugs. 2006;17:621–629. doi: 10.1097/01.cad.0000215058.85813.02. [DOI] [PubMed] [Google Scholar]
- 10.Drucker L, Afensiev F, Radnay J, Shapira H, Lishner M. Co-administration of simvastatin and cytotoxic drugs is advantageous in myeloma cell lines. Anticancer Drugs. 2004;15:79–84. doi: 10.1097/00001813-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Holmberg M, Sandberg C, Nygren P, Larsson R. Effects of lovastatin on a human myeloma cell line: increased sensitivity of a multidrug-resistant subline that expresses the 170 kDa P-glycoprotein. Anticancer Drugs. 1994;5:598–600. doi: 10.1097/00001813-199410000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Feleszko W, Mlynarczuk I, Balkowiec-Iskra EZ, Czajka A, Switaj T, Stoklosa T, et al. Lovastatin potentiates anti-tumor activity and attenuates cardiotoxicity of doxorubicin in three tumor models in mice. Clin Cancer Res. 2000;6:2044–2052. [PubMed] [Google Scholar]
- 13.Iliskovic N, Singal PK. Lipid lowering: an important factor in preventing adriamycin-induced heart failure. Am J Pathol. 1997;150:727–739. [PMC free article] [PubMed] [Google Scholar]
- 14.Budavari S, O’Neil MJ, Smith A, Heckelman PE, Kinneary JF. 13th ed. Whitehouse station: Merck Research Laboratories; The Merck index: an encyclopedia of chemicals, drugs and biologicals; p. 3472. [Google Scholar]
- 15.Denizlt F, Lang R. Rapid colorimetric assay for cell growth and survival. Modification to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 16.Jakobisiak M, Bruno S, Skierski JS, Darzynkiewicz Z. Cell cycle-specific effects of lovastatin. Proc Natl Acad Sci USA. 1991;88:3628–3632. doi: 10.1073/pnas.88.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abd El-latif MI, Murota H, Terao M, Katayama I. Effects of a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor and low-density lipoprotein on proliferation and migration of keratinocytes. Br J Dermatol. 2010;163:128–137. doi: 10.1111/j.1365-2133.2010.09694.x. [DOI] [PubMed] [Google Scholar]
- 18.Werner M, Sacher J, Hohenegger M. Mutual amplification of apoptosis by statin-induced mitochondrial stress and doxorubicin toxicity in human rhabdomyosarcoma cells. Br J Pharmacol. 2004;143:715–724. doi: 10.1038/sj.bjp.0705928. [DOI] [PMC free article] [PubMed] [Google Scholar]


