Abstract
In everyday life, our body generates free radicals and other reactive oxygen species which are derived either from the endogenous metabolic processes (within the body) or from external sources. Many clinical and pharmacological studies suggest that natural antioxidants can prevent oxidative damage. Among the natural antioxidant products, Pycnogenol® (French Pinus pinaster bark extract) has been received considerable attention because of its strong free radical-scavenging activity against reactive oxygen and nitrogen species. P. pinaster bark extract (PBE) contains polyphenolic compounds (these compounds consist of catechin, taxifolin, procyanidins of various chain lengths formed by catechin and epicatechin units, and phenolic acids) capable of producing diverse potentially protective effects against chronic and degenerative diseases. This herbal medication has been reported to have cardiovascular benefits, such as vasorelaxant activity, angiotensin-converting enzyme inhibiting activity, and the ability to enhance the microcirculation by increasing capillary permeability. Moreover, effects on the immune system and modulation of nitrogen monoxide metabolism have been reported. This article provides a brief overview of clinical studies describing the beneficial and health-promoting effects of PBE.
Keywords: Pycnogenol, P. pinaster, Pine bark extract, Antioxidant activity, Anti-inflammatory effects, Cardiovascular system
INTRODUCTION
Herbal supplements and remedies have been used around the world because of their beneficial effects on human health(1). Pycnogenol®, a standardized extract of the bark of the French P. pinaster (Pinus pinaster Ait. Subsp. atlantica), has been used worldwide as a herbal remedy, nutrition and supplemental food in many kinds of degenerative diseases. Bark extract of French P. pinaster has been reported to have cardio-vascular and cholesterol lowering benefits, the ability to enhance microcirculation by increasing capillary permeability, significant free radical scavenging activity against reactive oxygen and nitrogen species, the potential to regenerate the ascorbyl radical and to protect endogenous vitamin E and glutathione from oxidative stress, and the potential to protect erythrocytes in G6PD deficiency(2). It also protects the nerve cells against beta-amyloid, or glutamate induced toxicity, accelerates wound healing processes, reduces scar formation, decreases histamine release from mast cells, and inhibits pro-inflammatory cytokine actions(3,4). Anti-inflammatory effects in asthma patients and reduction of attention-deficit disorder and attention-deficit hyperactivity disorder symptoms in children have been observed(5,6). This article provides an overview of pharmacological and clinical studies indicating the pharmaceutical and nutraceutical effects of P. pinaster bark extract (PBE).
The specifications of PBE are described comprehensively in the USP 30-Dietary supplements(7). PBE contains numerous phenolic compounds such as polyphenolic monomers, procyanidins, and phenolic acids (derivatives of benzoic and cinnamic acids) which have received considerable attentions because of their anti-inflammatory, anti-mutagenic, antimetastatic, anticarcinogenic, and high antioxidant activities(8,9). Proanthocyanidins are known as oligomeric proanthocyanidins or condensed tannins which represent a group of condensed flavan-3-ols, such as procyanidins, prodelphinidins and propelargonidins. They are mixtures of oligomers and polymers composed of flavan-3-ol units linked mainly through C4-C8 bond, but the C4-C6 linkage also exists. The most widely studied proanthocyanidins are based on flavan-3-ol (-)-epicatechin and (+)-catechin (Fig. 1). Procyanidins are biopolymers of catechin and epicatechin subunits with a chain length of up to dodecamers. Moreover, (epi)afzelechin or (epi)gallocatechin are the subunits of propelargonidin or prodelphinidin, respectively(10)
Fig. 1.
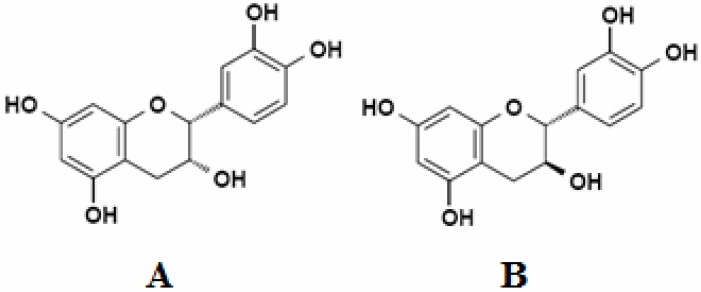
Flavan-3-ols: epicatechin (A) and catechin (B)
Antioxidant and free radical scavenging activities
Inactivation of the superoxide and hydroxyl radical, and inhibition of singlet oxygen formation are two important beneficial effects of PBE(9,11–13). PBE was reported to exert protective effects against lipid peroxidation, thiobarbituric acid reactive products generation, and oxidative hemolysis induced by peroxide hydrogen(14). Furthermore, it prevents accumulation of oxidatively damaged proteins and may reduce the risk of several neurodegenerative diseases such as Parkinson's, Alzheimer's, and Huntington's diseases(15). In vitro studies indicated that PBE inhibited peroxidation of low-density lipoprotein cholesterol (LDL), lipid peroxida-tion in phospholipid liposomes, lipid peroxidation caused by t-butylhydroperoxide, and UVB-induced lipid peroxidation in cells(12,16,17).
Pycnogenol® exhibited a concentration-dependent inhibition of oxidative burst triggered by zymosan in J774 murine macrophage and LDL oxidation. J774 is a murine macrophages cell line established from a tumor which arose in a female BALB/c mouse. Its growth is inhibited by dextran sulfate, purified protein derivative, and bacterial lipopolysaccharides. J774 cells have been used for numerous biological and biochemical investigations aimed at under-standing the physiology of monocytes-macrophages. Pycnogenol® also minimized the stand cleavage (measured by agarose gel electrophoresis) in pBR322 plasmid DNA caused by hydroxyl radicals produced by iron/ascorbic acid(18). Plasmid vector pBR322, a well-established multipurpose cloning vector in laboratories worldwide, is designed and constructed for the efficient cloning and selection of recombinant DNA molecules in Escherichia coli(19,20). Pycnogenol® showed synergetic antioxidant effects with vitamin C and E, and lutein for prevention of lipid peroxidation in liposome and porcine retinal homogenate, respectively(21,22). The antioxidant and anti-inflammatory effects of PBE have been proven by in vitro and in vivo studies and clinical trials summarized in (Fig. 2) (2,4,9,11,23–27).
Fig. 2.
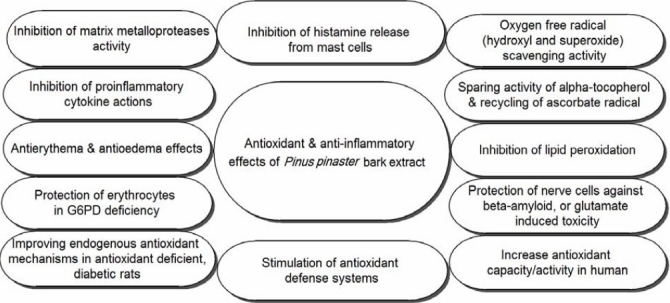
Antioxidant and anti-inflammatory effects of Pinus pinasterbark extract
Ahn and coworkers have investigated the putative antioxidant activity of PBE against CCl4-induced hepatic oxidative damage in Sprague-Dawley rats. A single oral dose of CCl4 (1.25 mL/kg) produced significantly increased levels of serum aminotransferase and alanine aminotransferase activities, increased malondialdehyde concentration, reduced glutathione content, and decreased catalase, superoxide dismutase and glutathione -S-transferase activities in the hepatic tissues. Results of this study showed that administration of Pycnogenol® (10 or 20 mg/kg) improved CCl4-induced hepatic injury, as evidenced by the decline of serum aminotransferase and alanine aminotransferase activities in a dose dependent manner, reduction of malondialdehyde concentration, and enhancement of glutathione levels and catalase, superoxide dismutase and glutathione-S-transferase activities in hepatic tissues, indicating that administration of Pycnogenol® efficiently prevents the CCl4-induced oxidative damage in rats(28). In another study, after administration of Pycnogenol®, the glutathione (GSH) and GSH-disulphide reductase (GSSG-R) ratio, the activities of endogenous antioxidant enzymes (SOD, CAT, GSH-Px, GSSG-R), and the activity of γ-glutamyl transpeptidase and enzyme in the pathway of glutathione synthesis were increased in streptozotocin-induced diabetic rats(27). Moreover, it was reported that the consumption of Pycnogenol® alone or in combination with beta-carotene may lead to an increase in activity of GSSG-R. Decreased retinal γ-GT activity in diabetic rats and elevated activity of superoxide dismutase in diabetic retina due to the oxidative stress were normalized by Pycnogenol® and beta-carotene combination(29,30).
Polyphenols containing PBE were found to have scavenging activity against reactive oxygen and nitrogen species. Decreased nitrogen monoxide (NO) generation due to the scavenging activity against reactive oxygen species and NO, inhibition of iNOS activity, and inhibition of iNOS-mRNA expression were noticed. PBE was shown to have scavenging activities against reactive oxygen species in macrophages and blocks the activation of NF-kappaB and activator protein-1, and inhibits the expression of pro-inflammatory cytokine IL-1, vascular cell adhesion molecule-1 and intracellular adhesion molecule-1(31). It has also been reported that PBE inhibited TNF-α secretion, nuclear factor (NF)-kappaB-dependent gene expression, and MMP-9 release from lipopolysaccharide-stimulated monocytes (mouse leukemic macrophage cell line, RAW 264.7)(32–34).
Anti-inflammatory effects
Several in vitro studies have revealed that PBE has anti-inflammatory effects and inhibits the initiation of inflammation by preventing the release of pro-inflammatory mediators regulated by oxidative stress. PBE inhibits the pro-inflammatory mediators in keratinocytes (epidermal cells), leukocytes, and endothelial cells(32,35,36). Furthermore, an in vitro study has shown that PBE and its metabolites inhibit the release of tissue destroying enzymes (matrix metalloprotein-ases) collagenase, elastase, and gelatinase from inflammatory cells(33). It has also reported that after oral intake of PBE, the enzymatic activity of Cyclo-oxygenase (COX-1 and COX-2) in serum samples of human volunteers was inhibited. Cyclo-oxygenase is responsible for formation of biological mediators, such as prostaglandins, prosta-cyclin, and thromboxane. Pharmacological Inhibition of this enzyme can provide relief from the symptoms of inflammation and pain(37).
One of the major pro-oxidant challenge, exposure to UV radiation, may lead to the expression of many pro-inflammatory genes including tumor necrosis factor-α (TNF-α), IL-1 α, IL-1β, IL-8, and IL-6 (all these cytokines contain nuclear factor-kappaB binding sites in the 5 flanking region of their encoding gene)(2). Application of Pycnogenol® topically could be used for significant and dose-dependent protection from solar-simulated UV radiation (SSUV)-induced acute inflammation, photo-carcinogenesis, and immune-suppression when applied after sunburn and daily irradiation(38). In one study, 21 Caucasian volunteers received Pycnogenol® (1.1 mg/kg body weight) orally. As a result, the protective effect of Pycnogenol® against UV-light induced skin damages has been proven(32). It was demonstrated that PBE suppressed the expression of pro-inflammatory cytokines and decreased the expression of mast cell related tryptase and stem cell factor(39). The inhibition of NF-kappaB activation in lipopolysaccharide-stimulated monocytes is one of the reasons for anti-inflammatory effects of Pycnogenol®(40). PBE inhibited iNOS and iNOS-mRNA expression in murine macrophages, activated by the bacterial wall components lipopolysaccharide and interferon (INF-γ)(23).
In the most industrialized countries, osteoarthritis of the main joints (severe bone and joint abnormalities) is a diffuse social problem affecting the quality of human lives. Among the herbal antioxidative supplement, Pycnogenol® has beneficial effects on symptoms of osteoarthritis(41–43). A double-blind, placebo-controlled study reported that Pycnogenol® lowered pain and stiffness and improved symptoms of knee osteoarthritis (flexibility of osteoarthritic joints)(41).
Cardiovascular system
Zibadi and coworkers have reported that PBE resulted in improvement of type 2 diabetes controls and reduction of the risk factor of cardiovascular disease and antihypertensive medicine use in subjects with type 2 diabetes. In another study, they investigated the impact of Pycnogenol®(30 mg/kg) on left ventricular function and myocardial extracellular matrix composition in old C57BL/6N mice following induction of cardiac remodeling by chronic nitric oxide synthase blockade by NG-nitro-L-arginine methyl ester (L-NAME) administration. Consequently, cardiac gene expression patterns for pro-MMP-2, -9, and -13, and MMP-9 activity were significantly decreased in L-NAME-exposed mice which was associated with a significant increase in cardiac tissue inhibitor of metalloproteinase (TIMP)-4 expression after using Pycnogenol® (44,45). Consumption of Pycnogenol® (100 mg over a period of 12 weeks) decreased endothelin-1 concentrations and increased the concentrations of 6-keto prostaglandin F1a in plasma. Therefore, use of polyphenols rich PBE could help to reduce the dose of the calcium antagonist nifedipine and to improve endothelial function of hypertensive patients(46). A randomized, double blind, placebo controlled, prospective, 16-week crossover study was conducted to determine the role of Pycnogenol® (200 mg/day) in modifying blood pressure in mildly hypertensive patients. Significant decrease in serum thromboxane concentration and the systolic blood pressure was observed during Pycnogenol® supplemen-tation(47).
PBE was found to cause endothelium-dependent vaso-relaxation and protect against contractile responses to epinephrine and norepinephrine in intact isolated rat aortic rings due to an increase in production of NO by endothelial cells. Nitrogen monoxide has beneficial vaso-protective effects such as inhibition of platelet aggregation and platelet and leukocyte adhesion, decrease of lipid oxidation, and also reduction of vascular smooth muscle cell proliferation(48–51). Sivoòová and coworkers determined the fluidity of erythrocyte membrane using 1-[4-trimethyl - aminophenyl] - 6 - phenyl- 1, 3, 5 -hexatriene, 1, 6-diphenyl-1, 3, 5-hexatriene, and 12-(9-anthroyloxy) stearic acid fluorescence anisotropy. It was observed that after Pycnogenol® action (50-300 μg/ml), the anisotropy values of TMA-DPH (N,N,N-Trimethyl-4-(6-phenyl-1, 3, 5-hexatrien-1-yl) phenylammonium p-toluenesulfonate) and DPH were decreased in a dose-dependent manner compared with the untreated erythrocyte membranes. The results have shown that the fluidity of erythrocyte was increased predominantly at the membrane surface(14). Araghi-niknam and coworkers reported that Pycnogenol® (100-150 mg) reduced smoke-enhanced thromboxane B formation and the platelet aggregation in response to cigarette smoking in vivo(52).
Venous disorders
PBE showed preventive effects against edema during long flights. The assessment of flight-edema was performed by evaluating an analogue scale, the rate of ankle swelling by strain-gauge derived rate of ankle swelling, and by assessing the ankle circumference variation(53). PBE can also be used for prevention and treatment of chronic venous insufficiency (CVI) and related venocapillary disturbances. Chronic venous insufficiency causes venous hypertensive microangiopathy leading to venous ulcerations(54). PBE was effective in improvement of the legs heaviness and subcutaneous edema and in reduction of venous pressure in patients with CVI. These clinical effects of PBE might be due to the stabilizing the collagenous subendothelial basal membrane or scavenging the free radicals or by the combination of these activities(55). A controlled comparative study on 40 patients with diagnosed CVI revealed that Pycnogenol® (360 mg/day over a period of 4 weeks) reduced the circumference of the lower limbs and improved the symptoms of CVI(56). Oral administration of Pycnogenol® (150 mg/day) for 8 weeks in patients with severe venous hypertension (chronic venous insufficiency, ankle swelling) and history of venous ulcerations indicated a decrease in skin flux and capillary filtration, improvement in the level of microangiopathy and the symptomatic score; and reduction in edema(57). Moreover, the results of the study on 16 patients with venous ulcerations (in the period of 6 weeks) indicated that oral and local treatment with Pycnogenol® might have important role in improvement of venous ulcers(54). It was also reported that combined systemic and topical application of Pycnogenol® could be used in order to treatment of diabetic ulcers(58).
Edema is very common in hypertensive patients under treatment. Belcaro et al. have investigated the effects of oral consumption of Pycnogenol® on adverse effects of antihypertensive drugs. As a result, Pycnogenol® helped to prevent and reduce long-term damage in the microcirculation, to control edema, and to reduce the dose of anti-hypertensive drugs (e.g., Nifedipine) in hypertensive patients(59).
Cholesterol lowering effects
Supplementation with PBE rich in polyphenols has favorable effects on two risk factors for coronary artery disease, i.e., reducing total cholesterol and LDL-cholesterol levels and increasing high-density lipoprotein (HDL)-cholesterol levels, resulting in a better atherosclerotic index(60). It was reported that PBE increased plasma antioxidant capacity (determined by oxygen radical absorbance capacity) and altered plasma lipoprotein profile (especially, reduction of bad cholesterol LDL in human volunteers)(61). The result of a randomized, double blind, placebo controlled study demonstrated that Pycnogenol ®significantly lowered LDL and increased HDL in 155 menopausal woman during a treatment period of 6 months(62). In another independent placebo study, significant increase of plasma antioxidant activity was observed after administration of Pycnogenol®. The total cholesterol decreased from 5.41 to 4.98 mmol/L which was associated with a decrease in LDL-cholesterol levels from 3.33 to 2.78 mmol/L(60).
Diabetic syndrome
Pycnogenol® was given in addition to diabetic and hypertensive medications may improve blood sugar and cardiovascular risk factors, and allow patients to lower antihypertensive medication(44). Nocun and coworkers have investigated the antithrombotic effect of long term Pycnogenol® intake in diabetes associated with enhanced thromboxane synthesis leading to severe vascular complications. The levels of thromboxane B2 (the main plasma thromboxane A2 metabolite) were assessed in animal model of insulin-dependent diabetes by enzyme-linked immunosorbent assay. The result of this study suggested that Pycnogenol® lowered platelet hyperactivity and had antithrombotic effects when administered alone or as supplementation of anti-platelet therapy in type 1 diabetes pharmacologic model(63). Oligomeric procyanidins of PBE inhibited dietary carbohydrate absorption by inhibition of α-glucosidase (IC50 about 5 μg/mL) which might contribute in glucose-lowering effects of Pycnogenol® observed in clinical trials with diabetic patients(63,64).
Results of a prospective, controlled study on patients with diabetic microangiopathy received oral Pycnogenol® (150/day for 4 weeks) demonstrated that Pycnogenol® was microangiopathy, and in prevention of diabetic ulcerations by controlling the level of microangiopathy(65). In addition, combined local and systemic application of Pycnogenol® might help to improve the lower limb ulcers in patients with diabetic microangiopathy(58).
Diabetes mellitus is an endocrine disorder, and its complications such as dyslipidemia (elevated levels of total cholesterol, LDL, and triglycerides and a low concentration of HDL) and hyperglycemia are a major cause of disability and hospitalization, posing a significant financial burden(66). Chronic hyperglycemia of uncontrolled diabetes mellitus increases reactive oxygen species (oxidative stress) and decreases enzymatic antioxidant defenses responsible for retinopathy and cataract formation. This chronic disease may lead to microvascular pathologies, especially in the eye, kidney, and peripheral nerve(67). Pycnogenol ®either alone or in combination with other antioxidants (e.g., beta-carotene and α-lipoic acid) have increased retinal glutathione peroxidase and glutathione reductase activities in diabetic animals, and can be used as an effective antioxidant and anti-hyperglycemic therapy for diabetic retinopathy caused by oxidative stress(29,68).
The findings of an in vitro study revealed that Pycnogenol® increased the effects of other antioxidants such as Co-enzyme Q10 when combined together, and could be useful in order to protect retina of the eye from the damage caused by oxidative stress and free radicals(25,69).
Allergy and asthma
A randomized, placebo-controlled, double-blind study on 60 subjects (6-18 years, in a period of 3 weeks) reported the significant efficacy of Pycnogenol® in improvement of pulmonary functions and reduction of asthma symptoms in children(6). A placebo-controlled, crossover study also indicated that Pycnogenol® reduced asthma symptoms and might be a valuable nutraceutical in the management of chronic asthma (chronic eosinophilic bronchitis and inflammatory process in the lung)(70). Sharma and coworkers reported that Pycnogenol® inhibited the release of histamine from rat peritoneal mast cells and its inhibitory effects was favorably comparable to sodium cromoglycate(71).
Menstrual disorders, pregnancy associated pain and endometriosis
A multicenter, randomized, double-blind, placebo-controlled study in four hospitals in Japan indicated that Pycnogenol® significantly lowered menstrual pain and the quantity of required analgesic medication in dysmenorrhea (gynecological medical condition with severe uterine pain during menstruation)(72). It was also reported that PBE reduced menstrual cramps, abdominal pain and tenderness in gynecological disorders such as endometriosis and dysmenorrhea, and could be used as a therapeutic alternative to gonadotropin releasing hormone agonist (Gn-RHa) in the treatment of endometriosis(73,74). A randomized, double-blind, placebo-controlled study indicated that Pycnogenol® reduced climacteric symptoms without unwanted effects in peri-menopausal women(62). Polyphenolic compounds rich PBE has beneficial effects in a series of painful conditions such as stiff shoulder, herniated disc, and pregnancy associated pain (lower back pain, hip joint pain, pain in the inguinal region, and pain due to varices or calf cramps)(75). The analgesic and antispasmodic effects of medicinal herbs may have suitable effects on menstrual cramps and premenstrual syndrome(76). Two polyphenolic components of PBE (caffeic and protocatechuic acids) have non-specific antispasmodic action on smooth muscle in several organs of the rat. It seems that this antispasmodic effect may contribute in beneficial effects of PBE in premenstrual syndrome(77).
Attention deficit hyperactivity disorder (ADHD)
Polyphenols rich extract like PBE reduces hyperactivity of children, catecholamine excretion, and oxidative stress(5,78). A randomized, double-blind, placebo controlled study on ADHD children indicated that the treatment with Pycnogenol® caused decrease of dopamine and trend of adrenaline and noradrenaline decrease, and increased GSH/GSSG ratio(5). In another randomized, double-blind, placebo-controlled study, the influence of administered Pycnogenol® or placebo on the level of reduced GSH and oxidized GSSG glutathione in children suffering from ADHD (the neuro-developmental disorder with impulsivity, distractibility and hyperactivity) and on total antioxidant status were investigated. As a result, significant decrease in GSSG and increase in GSH levels, as well as improvement of GSH/GSSG ratio in comparison with placebo group were reported. Moreover, Pycnogenol® improved the antioxidant status of ADHD children(79). Chovanovα and coworkers investigated the effect of polyphenolic extract of pine bark on the level of oxidized purines represented by 8-oxo-7, 8-dihydroguanine (8-oxoG) and on the total antioxidant status in children with attention deficit/hyperactivity disorder(80). They found that French PBE protected DNA against oxidation, normalized total antioxidant status and improved attention of ADHD children.
Antimicrobial and antiviral activity
PBE rich in procyanidins inhibited not only the binding of human immunodeficiency virus type-1 (HIV-1) to host cells but also inhibited HIV viral replication and T-cell interaction in cell culture experiments(81). PBE was found to induce expression of an intracellular antioxidant protein and manganese superoxide dismutase, and inhibition of phosphorylation of the ribosomal S6 protein. It seems that these biochemical alterations induced by Pycnogenol® play an important role in its antiviral effects. PBE is a promising agent for inhibi-tion of encephalomyocarditis viral replication, prevention of development of viral myocarditis, and improvement of inflamma-tion and myocardial necrosis. It was reported that Pycnogenol® (100 mg/kg) had beneficial effects on viral myocarditis by inhibition of viral replication and by suppression of pro-inflammatory cytokines, genes related to cardiac remodeling, and mast cell-related genes in the heart muscle of mice (gene expressions of tumor necrosis factor, type-1 procollagen, stem cell factor, and mast cell tryptase)(81,82).
PBE was shown to have antimicrobial activity against various pathogenic prokaryotes (gram negative and positive bacteria), and eukaryotes (fungi and yeasts)(83,84). For instance, growth and adherence of Helicobacter pylori (the gram negative, microaerophilic bacterium) to mucosal cells of the stomach were inhibited by Pycnogenol®, in vitro. In another study, antimicrobial effects of Pycnogenol® against 23 pathogenic microorganisms (e.g., Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeroginosa, Bacillus cereus, Enterococcus faecalis, Candida albicans, Aspergillus oryzae, and Salmonella sp.) have been investigated. Consequently, Pycnogenol® inhibited the growth of all tested microorganisms in minimum inhibitory dosages (MID) ranging from 20 to 250 μg/mL(84).
CONCLUSION
PBE rich in polyphenolic compounds has been shown to cause endothelium-dependent vasorelaxation and decrease the amount of circulating inflammatory substances in the blood stream. Intake of PBE is useful in order to reduce the risk of heart disease and is effective in the treatment of chronic venous insufficiency and retinal micro-hemorrhages venous disorders. Pycnogenol®, a dietary antioxidant with low acute and chronic toxicity, can be used in order to ameliorate excessive oxidative stress in several cell systems by doubling the intracellular synthesis of anti-oxidative enzymes and by acting as a potent scavenger of free radicals. Furthermore, PBE plays an important role in the regeneration and protection of vitamin C and E. Anti-inflammatory and antioxidant activities of PBE have been demonstrated in vitro and in vivo. PBE also protects the nerve cells against beta-amyloid, or glutamate induced toxicity and the erythrocytes in G6PD deficiency. Protection against UV-radiation-induced erythema, anti-inflammatory effects in asthma patients, and reduction of attention-deficit disorder and ADHD symptoms in children have been reported in clinical studies following oral intake of Pycnogenol®. Immunomodulation has been observed in both animal models and patients with Lupus erythematosus. Reduction of smoke-enhanced thromboxane B formation and platelet aggregation in response to cigarette smoking has also been reported. PBE inhibits angiotension-converting enzyme which is associated with a mild antihypertensive effect. Pycnogenol®relieves menstrual abdominal pain which is attributed to the antispasmodic of some phenolic acids on smooth muscle. Results from pharmacological and clinical studies have demonstrated that PBE can be used as a source of natural antioxidants in the food and pharmaceutical industries. However, more investigations are needed to confirm and extend the previous observations.
Previous studies suggested that bark extract obtained from P. brutia, P. nigra, P. sylvestris, and P. pinea which contained high amounts of polyphenolic compounds are potential alternatives to the French P. pinaster in dietary supplements, cosmetic products, and food/beverages. We demonstrated that Iranian P. pinaster and P. eldarica bark extract could be used as effective sources of polyphenolic compounds, especially catechin and taxifolin. Therefore, these two Iranian Pinus species other than French P. pinaster can also possess remarkable antioxidant activities, and might be potential alternatives for food and pharmaceutical applications.
ACKNOWLEDGMENT
This review was part of the thesis of Pharmacognosy department, School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences.
REFERENCES
- 1.Zolfaghari B, Ghannadi A. The principles and therapeutic uses of phytomedicinals- what every physician must know. Res Med Sci J. 2000;6:1–6. [Google Scholar]
- 2.Gulati OP. The nutraceutical Pycnogenol: its role in cardiovascular health and blood glucose control. Biom Rev. 2005;16:49–57. [Google Scholar]
- 3.Blazso G, Gabor M, Schonlau F, Rohdewald P. Pycnogenol® accelerates wound healing and reduces scar formation. Phytother Res. 2004;18:579–581. doi: 10.1002/ptr.1477. [DOI] [PubMed] [Google Scholar]
- 4.Peng QL, Buz’Zard AR, Lau B. Research report: Pycnogenol® protects neurones from amyloid β peptide-induced apoptosis. Mol Brain Res. 2002;104:55–65. doi: 10.1016/s0169-328x(02)00263-2. [DOI] [PubMed] [Google Scholar]
- 5.Dvorakova M, Jezova D, Blazicek P, Trebaticka J, Skodacek I, Suba J, et al. Urinary catecholamines in children with attention deficit hyperactivity disorder (ADHD): modulation by a polyphenolic extract from pine bark (Pycnogenol®) Nutr Neurosci. 2007;10:151–157. doi: 10.1080/09513590701565443. [DOI] [PubMed] [Google Scholar]
- 6.Lau BH, Riesen SK, Truong KP, Lau EW, Rohdewald P, Barreta RA. Pycnogenol as an adjunct in the management of childhood asthma. J Asthma. 2004;8:825–832. doi: 10.1081/jas-200038433. [DOI] [PubMed] [Google Scholar]
- 7.The United States Pharmacopeia, United States Pharmacopeial Convention, Inc. official from. 2007. Maritime Pine Bark Extract – USP. 30:965–966. [Google Scholar]
- 8.Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther. 2002;40:158–168. doi: 10.5414/cpp40158. [DOI] [PubMed] [Google Scholar]
- 9.Packer L, Rimbach G, Virgili F. Antioxidant activity and biologic properties of a procyanidin-rich extract from the pine (Pinus maritima) bark, Pycnogenol. Free Rad Biol Med. 1999;27:704–724. doi: 10.1016/s0891-5849(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 10.Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, et al. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and Thiolytic degradation. J Agric Food Chem. 2003;51:7513–7521. doi: 10.1021/jf034815d. [DOI] [PubMed] [Google Scholar]
- 11.Blazso G, Gabor M, Sibbel R, Rohdewald P. An anti-inflammatory and superoxide radical scavenging activities of a procyanidins containing extract from the bark of Pinus pinaster sol. and its fractions. Pharm Parmacol Lett. 1994;3:217–220. [Google Scholar]
- 12.Gouchang Z. Ultraviolet radiation-induced oxidative stress in cultured human skin fibroblasts and antioxidant protection. Biol Res Rep Univ. 1993;33:1–86. [Google Scholar]
- 13.Noda Y, Anzai K, Mori A, Kohno M, Shinmie M, Packer L. Hydroxyl and superoxide radical scavenging activities of natural source antioxidants using the computerized JES FR30 ESR spectrometer system. Biochem Mol Biol Int. 1997;42:35–44. doi: 10.1080/15216549700202411. [DOI] [PubMed] [Google Scholar]
- 14.Sivoòová M, Waczulíková I, Kilanczyk E, Hrnèiarová M, Bryszewska M, Klajnert B, et al. The effect of Pycnogenol on the erythrocyte membrane fluidity. Gen Physiol Biophys. 2004;23:39–51. [PubMed] [Google Scholar]
- 15.Voss P, Horakova L, Jakstadt M, Kiekebusch D, Grune T. Ferritin oxidation and proteasomal degradation: Protection by antioxidants. Free Radic Res. 2006;40:673–683. doi: 10.1080/10715760500419357. [DOI] [PubMed] [Google Scholar]
- 16.Wei Z, Peng Q, Lau BHS. Pycnogenol enhances endothelial cell antioxidant defenses. Redox Rep. 1997;3:219–224. doi: 10.1080/13510002.1997.11747113. [DOI] [PubMed] [Google Scholar]
- 17.Rong Y, Li L, Shah V, Lau BHS. Pycnogenol protects vascular endothelial cells from t-butyl hydroperoxide induced oxidant injury. Biotech Ther. 1995;5:117–126. [PubMed] [Google Scholar]
- 18.Nelson AB, Lau BHS, Ide N, Rong Y. Pycnogenol inhibits macrophage oxidative burst, lipoprotein oxidation, and hydroxyl radical-induced DNA damage. Drug Dev Ind Pharm. 1998;24:139–144. doi: 10.3109/03639049809085598. [DOI] [PubMed] [Google Scholar]
- 19.Balbás P, Soberón X, Merino E, Zurita M, Lomeli H, Valle F, et al. Plasmid vector pBR322 and its special-purpose derivatives--a review. Gene. 1986;50:3–40. doi: 10.1016/0378-1119(86)90307-0. [DOI] [PubMed] [Google Scholar]
- 20.Balbás P, Bolívar F. Back to basics: pBR322 and protein expression systems in E.coli. Methods Mol Biol. 2004;267:77–90. doi: 10.1385/1-59259-774-2:077. [DOI] [PubMed] [Google Scholar]
- 21.Nakanishi-Ueda T, Kamegawa M, Ishigaki S, Tsukahara M, Yano S, Wada K, et al. Inhibitory effect of lutein and Pycnogenol® on lipid peroxidation in porcine retinal homogenate. J Clin Biochem Nutr. 2006;38:204–210. [Google Scholar]
- 22.Sivonová M, Zitnanová I, Horáková L, Strosová M, Muchová J, Balgavý P, et al. The combined effect of pycnogenol with ascorbic acid and trolox on the oxidation of lipids and proteins. Gen Physiol Biophys. 2006;25:379–396. [PubMed] [Google Scholar]
- 23.Virgili F, Kim D, Packer L. Procyanidins extracted from pine bark protect á-tocopherol in ECV 304 endothelial cells challenged by activated RAW 264.7 macrophages: role of nitric oxide and peroxynitrite. FEBS Lett. 1998;431:315–318. doi: 10.1016/s0014-5793(98)00778-9. [DOI] [PubMed] [Google Scholar]
- 24.Virgili F, Kobuchi H, Noda Y, Cossins E, Packer L. Procyanidins from Pinus maritima bark: Antioxidant activity, effects on the immune system and modulation of nitrogen monoxide metabolism. In: Packer L, Hiramatsu M, Yoshikawa T, editors. Antioxidant Food Supplements in Human Health. Vol. 21. USA: Academic Press; 1999. pp. 323–342. [Google Scholar]
- 25.Chida M, Suzuki K, Nakanishi-Ueda T, Ueda T, Yasuhara H, Koide R, et al. In vitro testing of antioxidants and biochemical end-points in bovine retinal tissue. Ophthalmol Res. 1999;31:407–415. doi: 10.1159/000055565. [DOI] [PubMed] [Google Scholar]
- 26.Sharma SC, Sharma S, Gulati O. Pycnogenol® prevents haemolytic injury in G6PD deficient human erythrocytes. Phytother Res. 2003;17:671–674. doi: 10.1002/ptr.1334. [DOI] [PubMed] [Google Scholar]
- 27.Maritim A, Dene BA, Sanders RA, Watkins J. Effect of Pycnogenol® treatment on oxidative stress in streptozoitin-induced diabetic rats. J Biochem Mol Toxicol. 2003;17:193–199. doi: 10.1002/jbt.10078. [DOI] [PubMed] [Google Scholar]
- 28.Ahn TH, Yang YS, Lee JC, Moon CJ, Kim SH, Jun W, et al. Ameliorative effects of pycnogenol on carbon tetrachloride-induced hepatic oxidative damage in rats. Phytother Res. 2007;21:1015–1019. doi: 10.1002/ptr.2146. [DOI] [PubMed] [Google Scholar]
- 29.Berryman AM, Maritim AC, Sanders RA, Watkins JB. Influence of treatment of diabetic rats with combinations of Pycnogenol®, beta-carotene, and alpha-lipoic acid on parameters of oxidative stress. J Biochem Mol Toxicol. 2004;18:345–352. doi: 10.1002/jbt.20046. [DOI] [PubMed] [Google Scholar]
- 30.Dene BA, Maritim AC, Sanders RA, Watkins JB. Effects of antioxidant treatment on normal and diabetic rat retinal enzyme activities. J Ocul Pharmacol Ther. 2005;21:28–35. doi: 10.1089/jop.2005.21.28. [DOI] [PubMed] [Google Scholar]
- 31.Cho KJ, Yun CH, Yoon DY, Cho YS, Rimbach G, Packer L, et al. Effect of bioflavonoids extracted from the bark of Pinus maritime on proinflammatory cytokine interleukin-1 production in lipopolysaccharide-stimulated RAW 264.7. Toxicol Appl Pharmacol. 2000;168:64–71. doi: 10.1006/taap.2000.9001. [DOI] [PubMed] [Google Scholar]
- 32.Saliou C, Rimbach G, Moini H, McLaughlin L, Hosseini S, Lee J, et al. Solar ultraviolet-induced erythema in human skin and nuclear factor-kappa-B-dependent gene expression in keratiocytes are modulated by a French maritime pine bark extract. Free Radic Biol Med. 2001;30:154–160. doi: 10.1016/s0891-5849(00)00445-7. [DOI] [PubMed] [Google Scholar]
- 33.Grimm T, Schafer A, Hogger P. Antioxidant activity and inhibition of matrix metalloproteinases by metabolites of maritime pine bark extract (Pycnogenol) Free Rad Biol Med. 2004;36:811–822. doi: 10.1016/j.freeradbiomed.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Park YC, Rimbach G, Saliou C, Valacchi G, Packer L. Activity of monomeric, dimeric, and trimeric flavonoids on NO production, TNFalpha secretion, and NF-kappaB-dependent gene expression in RAW 264.7 macrophages. FEBS Lett. 2004;465:93–97. doi: 10.1016/s0014-5793(99)01735-4. [DOI] [PubMed] [Google Scholar]
- 35.Bayeta E, Lau BHS. Pycnogenol inhibits generation of inflammatory mediators in macrophages. Nutr Res. 2001;20:249–259. [Google Scholar]
- 36.Peng Q, Wei Z, Lau BHS. Pycnogenol® inhibits tumor necrosis factor-α-induced nuclear factor kappa B activation and adhesion molecule expression in human vascular endothelial cells. Cell Mol Life Sci. 2000;57:834–841. doi: 10.1007/s000180050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schäfer A, Chovanová Z, Muchová J, Sumegová K, Liptáková A, Duracková Z, et al. Inhibition of COX-1 and COX-2 activity by plasma of human volunteers after ingestion of French maritime pine bark extract (Pycnogenol) Biomed Pharmacother. 2006;60:5–9. doi: 10.1016/j.biopha.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Sime S, Reeve V. Protection from inflammation, immunosuppression and carcinogenesis induced by UV radiation in mice by topical Pycnogenol®. Photochem Photobiol. 2004;79:193–198. doi: 10.1562/0031-8655(2004)079<0193:pfiiac>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Matsumori A, Higuchi H, Shimada M. French maritime pine bark extract inhibits viral replication and prevents development of viral myocarditis. J Card Fail. 2007;13:785–791. doi: 10.1016/j.cardfail.2007.06.721. [DOI] [PubMed] [Google Scholar]
- 40.Grimm T, Chovanova Z, Muchova J, Sumegova K, Liptava A, Durackova Z, et al. Inhibition of NF-kappaB activation and MMP-9 secretion by plasma of human volunteers after ingestion of maritime pine bark extract (Pycnogenol) J Inflamm. 2006;3:1. doi: 10.1186/1476-9255-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belcaro G, Cesarone MR, Errichi S, Zulli C, Errichi BM, Vinciguerra G, et al. Treatment of osteoarthritis with Pycnogenol® .The SVOS (San Valentino Osteo-Arthrosis Study). Evaluation of Signs, Symptoms, Physical Performance and Vascular Aspects. Phytother Res. 2007;22:518–523. doi: 10.1002/ptr.2376. [DOI] [PubMed] [Google Scholar]
- 42.Cisar P, Jany R, Waczulikova I, Sumegova K, Muchova J, Vojtassak J, et al. Effect of pine bark extract (Pycnogenol®) on symptoms of knee osteoarthritis. Phytother Res. 2008;22:1087–1092. doi: 10.1002/ptr.2461. [DOI] [PubMed] [Google Scholar]
- 43.Farid R, Mifteizi Z, Mirheidari M, Yasdi S, Torghabeh H, Esmaelli H, et al. Pycnogenol® supplementation reduces pain and stiffness and improves physical function in adults with knee osteoarthritis. Nutr Res. 2007;27:692–697. [Google Scholar]
- 44.Zibadia S, Rohdewald PJ, Park D, Watson RR. Reduction of cardiovascular risk factors in subjects with type 2 diabetes by Pycnogenol supplementation. Nutr Res. 2008;28:315–320. doi: 10.1016/j.nutres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Zibadi S, Yu Q, PJ R, Larson DF, Watson RR. Impact of Pycnogenol on cardiac extracellular matrix remodeling induced by L-NAME administration to old mice. Cardiovasc Toxicol. 2007;7:10–18. doi: 10.1007/s12012-007-0001-9. [DOI] [PubMed] [Google Scholar]
- 46.Liu X, Wei J, Tan F, Zhou S, Wuürthwein G, Rohdewald P. Pycnogenol, French maritime pine bark extract, improves endothelial function of hypertensive patients. Life Sci. 2004;74:855–862. doi: 10.1016/j.lfs.2003.07.037. [DOI] [PubMed] [Google Scholar]
- 47.Hosseini S, Lee J, Sepulveda RT, Rohdewald P, Watson RR. A randomized, double-blind, placebo-controlled, prospective, 16 week crossover study to determine the role of Pycnogenol in modifying blood pressure in mildly hypertensive patients. Nutr Res. 2001;21:1251–1260. [Google Scholar]
- 48.Iwasaki A, Matsumori A, Yamada T, Shioi T, Wang W-Z, Ono K, et al. Pimobendan inhibits the production of proinflammatory cytokines and gene expression of inducible nitric oxide synthase in a murine model of viral myocarditis. J Am Coll Cardiol. 1999;33:1400–1407. doi: 10.1016/s0735-1097(98)00692-5. [DOI] [PubMed] [Google Scholar]
- 49.Matsumori A, Nunokawa Y, Sasayama S. Pimobendan inhibits the activation of transcription factor NF-kB: a mechanism which explains its inhibition of cytokine production and inducible nitric oxide synthase. Life Sci. 2000;67:2513–2519. doi: 10.1016/s0024-3205(00)00834-1. [DOI] [PubMed] [Google Scholar]
- 50.The EPOCH Study Group. Effects of pimobendan on adverse cardiac events and physical activities in patients with mild to moderate chronic heart failure-the effects of pimobendan on chronic heart failure study (EPOCH study) Circ J. 2002;66:149–157. doi: 10.1253/circj.66.149. [DOI] [PubMed] [Google Scholar]
- 51.Fitzpatrick DF, Bing B, Rohdewald PJ. Endothelium-dependent vascular effects of pycnogenol. J card pharm. 1998;32:509–515. doi: 10.1097/00005344-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Araghi-niknam M, Hosseini S, Larson D, Rohdewald P, Watson RR. Pine bark extract reduces platelet aggregation. Integrative Medicine. 1999;2:73–77. doi: 10.1016/s1096-2190(00)00002-0. [DOI] [PubMed] [Google Scholar]
- 53.Cesarone MR, Belcaro G, Rohdewald P, Pellegrini L, Ippolito E, Scoccianti M, et al. Prevention of edema in long flights with Pycnogenol. Clin Appl Thrombosis/Hemostasis. 2005;11:1–6. doi: 10.1177/107602960501100307. [DOI] [PubMed] [Google Scholar]
- 54.Belcaro G, Cesarone MR, Errichi BM, Ledda A, Di Renzo A, Stuard S, et al. Venous Ulcers: microcirculatory improvement and faster healing with local use of Pycnogenol®. Angiology. 2005;56:1–7. doi: 10.1177/000331970505600607. [DOI] [PubMed] [Google Scholar]
- 55.Petrassi C, Mastromarino A, Spartera C. Pycnogenol in chronic venous insufficiency. Phytomedicine. 2000;7:383–388. doi: 10.1016/S0944-7113(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 56.Koch R. Comparative study of Venostasin and Pycnogenol in chronic venous insufficiency. Phytother Res. 2002;16:1–5. doi: 10.1002/ptr.1010. [DOI] [PubMed] [Google Scholar]
- 57.Cesarone MR, Belcaro G, Rohdewald P, Pellegrini L, Ledda A, Vinciguerra G, et al. Rapid relief of signs/symptoms in chronic venous microangiopathy with pycnogenol: a prospective, controlled study. Angiology. 2006;57:569–576. doi: 10.1177/0003319706291392. [DOI] [PubMed] [Google Scholar]
- 58.Belcaro G, Cesarone MR, Errichi BM, Ledda A, Di Renzo A, Stuard S, et al. Diabetic Ulcers: Microcirculatory Improvement and Faster Healing with Pycnogenol. Clin Appl Thrombosis/ Hemostasis. 2006;12:1–6. doi: 10.1177/1076029606290133. [DOI] [PubMed] [Google Scholar]
- 59.Belcaro G, Cesarone MR, Ricci A, Cornelli U, Rodhewald P, Ledda A, et al. Control of edema in hypertensive subjects treated with calcium antagonist (Nifedipine) or angiotensin-converting enzyme inhibitors with Pycnogenol. Clin and Appl Thrombosis/Hemostasis. 2006;12:440–444. doi: 10.1177/1076029606292248. [DOI] [PubMed] [Google Scholar]
- 60.Durackova Z, Trebaticka B, Novotny V, Zitnanova I, Breza J. Lipid metabolism and erectile function improvement by Pycnogenol®, extract from the bark of Pinus pinaster in patients suffering from erectile Dysfunction - a pilot study. Nutr Res. 2003;23:1189–1198. [Google Scholar]
- 61.Devaraj S, Vega-López S, Kaul N, Schönlau F, Rohdewald P, Jialal I. Supplementation with a pine bark extract rich in polyphenols increases plasma antioxidant capacity and alters plasma lipoprotein profile. Lipids. 2002;37:931–934. doi: 10.1007/s11745-006-0982-3. [DOI] [PubMed] [Google Scholar]
- 62.Yang H-M, Liao M-F, Zhu SY, Liao MN, Rohdewald P. A randomized, double-blind, placebo-controlled trial on the effect of Pycnogenol® on the climacteric syndrome in peri-menopausal women. Acta Obstet Gynecol Scand. 2007;86:978–985. doi: 10.1080/00016340701446108. [DOI] [PubMed] [Google Scholar]
- 63.Nocun M, Ulicna O, Muchova J, Durackova Z, Watala C. French maritime pine bark extract (Pycnogenol®) reduces thromboxane generation in blood from diabetic male rats. Biomed Pharmacother. 2007;62:168–172. doi: 10.1016/j.biopha.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 64.Schäfer A, Högger P. Oligomeric procyanidins of French maritime pine bark extract (Pycnogenol® ) effectively inhibit alpha-glucosidase. Diabetes Res Clin Pract. 2007;77:41–46. doi: 10.1016/j.diabres.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 65.Cesarone MR, Belcaro G, Rohdewald P, Pellegrini L, Ledda A, Vinciguerra G, et al. Improvement of diabetic microangiopathy with Pycnogenol® : prospective, controlled Study. Angiology. 2006;57:431–436. doi: 10.1177/0003319706290318. [DOI] [PubMed] [Google Scholar]
- 66.Movahedian A, Zolfaghari B, Sajjadi SE, Moknatjou R. Antihyperlipidemic effect of Peucedanum pastinacifolium extract in streptozotocin-induced diabetic rats. Clinics (Sao Paulo) 2010;65:629–933. doi: 10.1590/S1807-59322010000600011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schönlau F, Rohdewald P. Pycnogenol® for diabetic retinopathy: A review. Int Ophthalmol. 2002;24:161–171. doi: 10.1023/a:1021160924583. [DOI] [PubMed] [Google Scholar]
- 68.Kamuren ZT, McPeek CG, Sanders RA, Watkins JB. Effects of low-carbohydrate diet and Pycnogenol® treatment on retinal antioxidant enzymes in normal and diabetic rats. J Ocul Pharmacol Ther. 2006;22:10–18. doi: 10.1089/jop.2006.22.10. [DOI] [PubMed] [Google Scholar]
- 69.Ueda T, Ueda T, Armstrong D. Preventive effect of natural and synthetic antioxidants on lipid peroxidation in the mammalian eye. Ophthalmic Res. 1996;28:184–192. doi: 10.1159/000267901. [DOI] [PubMed] [Google Scholar]
- 70.Hosseini S, Pishnamazi S, Sadrzadeh MH, Farid F, Farid R, Watson R. Pycnogenol® in the management of asthma. J Med Food. 2001;4:201–209. doi: 10.1089/10966200152744472. [DOI] [PubMed] [Google Scholar]
- 71.Sharma SC, Sharma S, Gulati OP. Pycnogenol® inhibits the release of histamine from mast cells. Phytother Res. 2003;17:66–69. doi: 10.1002/ptr.1240. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki N, Uebaba K, Kohama T, Moniwa N, Kanayama N, Koike K. French maritime pine bark extract significantly lowers the requirement for analgesic medication in dysmenorrhea. a multicenter, randomized, double-blind, placebo-controlled study. J Reprod Med. 2008;53:338–346. [PubMed] [Google Scholar]
- 73.Kohama T, Herai K, Loue M. Effect of French Martime Pine Bark Extract on endometriosis as compared with Leuprorelin acetate. J Reprod Med. 2007;52:703–708. [PubMed] [Google Scholar]
- 74.Kohama T, Suzuki N, Ohno S, Inoue M. Analgesic efficacy of French maritime pine bark extract in dysmenorrhea – An open clinical trial. J Reprod Med. 2004;49:828–832. [PubMed] [Google Scholar]
- 75.Kohama T, Inoue M. Pycnogenol® alleviates pain associated with pregnancy. Phytother Res. 2006;20:232–234. doi: 10.1002/ptr.1840. [DOI] [PubMed] [Google Scholar]
- 76.Zolfaghari B, Barile E, Capasso R, Izzo AA, Sajjadi SE, V L. The sapogenin atroviolacegenin and its diglycoside atroviolaceoside from Allium atroviolaceum. J Nat Prod. 2006;69:191–195. doi: 10.1021/np0503350. [DOI] [PubMed] [Google Scholar]
- 77.Ortiz de Urbina JJ, Martin ML, Sevilla MA, Montero MJ, Carron R, Roman LS. Antispasmodic activity on rat smooth muscle of polyphenol compounds caffeic and protocathechic acids. Phytother Res. 1990;4:71–76. [Google Scholar]
- 78.Trebaticka J, Kopasova S, Hradecna Z, Cinovsky K, Skodacek I, Suba J, et al. Treatment of ADHD with French maritime pine bark extract, Pycnogenol®. Eur Child Adolesc Psychiatry. 2006;15:329–335. doi: 10.1007/s00787-006-0538-3. [DOI] [PubMed] [Google Scholar]
- 79.Dvorakova M, Sivonova M, Trebaticka J, Skodacek I, Waczulikova I, Muchova J, et al. The effect of polyphenolic extract from pine bark, Pycnogenol® , on the level of glutathione in children suffering from attention deficit hyperactivity disorder (ADHD) Redox Rep. 2006;11:163–172. doi: 10.1179/135100006X116664. [DOI] [PubMed] [Google Scholar]
- 80.Chovanova Z, Muchova J, Sivonova M, Dvorakova M, Zitnanova I, Waczulikova I, et al. Effect of polyphenolic extract, Pycnogenol® , on the level of 8-oxoguanine in children suffering from attention deficit/hyperactivity disorder. Free Radic Res. 2006;40:1003–1010. doi: 10.1080/10715760600824902. [DOI] [PubMed] [Google Scholar]
- 81.Feng WY, Tanaka R, Inagaki Y, Saitoh Y, Chang MO, Amet T, et al. Pycnogenol® , a procyanidin-rich extract from French maritime pine, inhibits intracellular replication of HIV-1 as well as its binding to host cells. Jpn J Infect Dis. 2008;61:279–285. [PubMed] [Google Scholar]
- 82.Matsumori A, Higuchi H, Shimada M. French maritime pine bark extract inhibits viral replication and prevents development of viral myocarditis. J Card Fail. 2007;13:785–791. doi: 10.1016/j.cardfail.2007.06.721. [DOI] [PubMed] [Google Scholar]
- 83.Rohdewald P, Beil W. In vitro inhibition of Helicobacter pylori growth and adherence to gastric mucosal cells by Pycnogenol®. Phytother Res. 2007;22:685–688. doi: 10.1002/ptr.2409. [DOI] [PubMed] [Google Scholar]
- 84.Torras MAC, Faura CA, Schönlau F, Rohdewald P. Short Communication: Anti-microbial activity of Pycnogenol®. Phytother Res. 2005;19:647–648. doi: 10.1002/ptr.1662. [DOI] [PubMed] [Google Scholar]


