Abstract
Rosmarinus officinalis L. (Family Lamiaceae) popularly named rosemary, is a common household plant grown around the world, including Iran. Rosemary aerial parts are used as flavoring agent in foods, beverages, and cosmetic preparations and have various traditional uses in ethnomedicine including: analgesic, anti-inflammatory, anti-rheumatic, spasmolytic, carminative and choleretic applications. This study was carried out to investigate the effects of rosemary leaves hydroalcoholic extract (RHE) and essential oil (REO) in a well-defined model of experimental colitis induced by trinitrobenzene sulfonic acid (TNBS) in rats. Different doses of RHE (100, 200 and 400 mg/kg) and REO (100, 200 and 400 μl/kg) were administered orally and intraperitoneally (100, 400 mg/kg and 100, 400 μl/kg) to male Wistar rats (n=6), 6 h after colitis induction and continued for 5 days by intracolonic instillation of 0.25 ml TNBS (80 mg/kg)/ethanol 50% v/v. Wet colon weight/length ratio was measured and tissue damage scores as well as indices of colitis were evaluated both macroscopically and histopathologically. RHE and REO at all test doses used were effective to reduce colon tissue lesions and colitis indices while greater doses were significantly effective to diminish histopathologic parameters irrespective to the route of administration. Administration of oral prednisolone, Asacol® (mesalazine microgranules) and parenteral hydrocortisone acetate were effective to reduce colon tissue injures as well. These data suggest that RHE and REO are both effective to possess anti-colitic activity, and reinforce the use of this plant as a remedy for inflammatory bowel diseases in traditional medicine.
Keywords: Rosmarinus officinalis, Inflammatory bowel diseases, Plant extract, Essential oil, Trinitrobenzene sulfonic acid
INTRODUCTION
Inflammatory bowel disease (IBD) is a widely chronic and multifactorial gastrointestinal (GI) inflammatory condition which is categorized into ulcerative colitis (UC) and Crohn's disease (CD) in the clinic. Etiology and pathophysiology of IBD is still unknown and multifactorial(1). Intestinal mucosal inflammation as a characteristic feature of IBD is induced by increase in the activity of some mucosal immune cells where the T-helper cells play an important role(2,3). Indeed, it is a loss of homeostasis of immune cells that results in intestinal inflammation(4,5). Main current therapies of IBD are amino-salicylate derivatives and corticosteroids administered through various routes but several side effects and/or lack of full effectiveness are common and problematic for regular treatments(6). Therefore, alternative and traditional therapies are vastly considered in the IBD patients for several years(7,8). Rosmarinus officinalis L. (rosemary) is an important medicinal plant from Lamiaceae family originated from Mediterranean region and has been cultivated for long time in Iran(9). It is traditionally used as a spice in foods and beverages and as alternative herbal medicine for GI ailments including flatulence and dyspepsia, and various spasmodic conditions such as renal and biliary colic(10–12). Additionally, various pharmacological studies have demonstrated the analgesic(13), anti-inflammatory(13–15), anti-oxidative(16,17), anti-tumor(18), anti-ulcerogenic(19,20), anti-bacterial(21,22) and hepatoprotective(23) properties of rosemary suggesting that it has a high therapeutic potential for IBD conditions.
In this study, we evaluated the effect of extract and essential oil of rosemary at various doses and via two administration routes in an animal model of experimental colitis in rats.
MATERIALS AND METHODS
Plant material and preparation of extract and essential oil
Leaves of rosemary were collected from the campus of Isfahan University of Medical Sciences (Isfahan, Iran) during August 2009 and authenticated by Pharmacognosy Depart-ment of Isfahan School of Pharmacy and Pharmaceutical Sciences, herbarium voucher No. 1529.
After collecting and identifying, the plant was air-dried; 200 g of the leaves was finely powdered, wetted by enough volume of ethanol:water (70:30) and extracted using percolation apparatus which was maintained for 48 h for a complete extraction. The extract was shaken, filtered and evaporated in a rotary evaporator under reduced pressure until a semisolid extract was obtained(24). Moreover, the concentrated extract was freeze-dried to obtain a dry powdered extract. Test doses were eventually prepared by reconstitution of this dried extract.
Essential oil of rosemary was isolated by hydrodistillation of the air-dried powdered leaves of the plant during 3 h in a full glass apparatus, according to the method reported previously(25).
Total phenol assay of the extract
The total phenols in the rosemary extract were determined by the Folin-Ciocalteua method with some modifications(26). Results are given as gallic acid equivalent (GAE)/g of the extract.
Analysis of the essential oil
The essential oil was analyzed by GC/MS on a Hewlett Packard 6890 MS selective detector coupled with Hewlett Packard 6890 gas chromatograph equipped with a cross-linked 5% PHME siloxane HP-5MS capillary column (30 m × 0.25 mm; film thickness 0.25 μm) operated under the same conditions as follows: carrier gas, helium with a flow rate of 2 ml/min; column temperature, 60-275 °C at 4 °C/min; injector and detector temperature, 280 °C; volume injected, 0.1 μl of the oil; split ratio, 1:50. The MS operating parameters were as follows: ionization potential 70 eV, ionization current 2 A, ion source temperature 200 °C, resolution 1000.
Identification of components in the oil was based on the computer matching with the Wiley 275 L. library as well as by comparison of the fragmentation patterns of mass spectra with those reported in the literature(27–29). The relative percentage of the oil constituents was calculated from the peak areas.
Animals
In the present study, male Wistar rats (200 ± 25 g) bred in animal house of Isfahan School of Pharmacy were used. The animals were housed singly in wire-bottomed cages under a uniform condition of light/dark cycle (12/12 h), controlled temperature and humidity, feed with normal rat chow and water ad libitum. All of the experiments were approved by the local Ethics and Research Committee of Isfahan University of Medical Sciences.
Chemicals
Prednisolone and hydrocortisone acetate were purchased from Iran Hormone Pharmaceutical Co. (Tehran, Iran). Asacol® was obtained from Iran Darou Co. (Tehran, Iran). Trinitrobenzene sulfonic acid (TNBS) and Folin-Ciocalteua reagent were purchased from Sigma (St. Louis, MO). All of the organic solvents were of analytical grade and Merck brand (Darmschtdat, Germany).
Test samples including suspension of reference drugs and rosemary extract or emulsion of essential oil were freshly prepared using 0.2% tween 80 in normal saline as a vehicle for oral (p.o.) and intraperitoneal (i.p.) administration.
Grouping
Animals were randomly assigned to sham, control, test, and reference groups of at least 6 rats as following:
Sham groups; treated with vehicle p.o. (5 ml/kg) and i.p. (2 ml/kg) without induction of colitis.
Control groups; treated with vehicle p.o. (5 ml/kg) and i.p. (2 ml/kg), respectively after induction of colitis.
Extract groups; treated orally with rosemary hydroalcoholic extract (RHE) at doses of 100, 200, and 400 mg/kg, and i.p. at doses of 100 and 400 mg/kg. Essential oil groups treated orally with rosemary essential oil (REO) at doses of 100, 200, and 400 μg/kg, and i.p. at doses of 100 and 400 μg/kg.
Reference groups; treated with prednisolone (4 mg/kg, p.o.), Asacol® (mesalazine microgranules, 100 mg/kg, p.o.) and hydrocortisone acetate (20 mg/kg, i.p.), respectively. All the treatments were carried out 6 h after colitis induction and continued daily for 5 days.
Induction of colitis
Rats were fasted for 36 h with free access to water prior to induction of colitis. Following assessment of their health, rats were lightly anesthetized with ether and colitis was then induced by intracolonic instillation of 0.25 ml of TNBS (80 mg/kg in ethanol 50% v/v)(30).
Evaluation of the colonic damage
Rats were euthanized using over-dose ether anesthesia at the day of six. The abdomen was opened and the colon, 8 cm in length and 3 cm proximal to the anus, was excised and incised longitudinally and washed with normal saline. Wet colon was then weighed and weight/ length ratio was determined for each specimen. Macroscopic mucosal damage was evaluated using the validated grading scale according to Morris et al.(31). Scores were: 0 = no ulcer, 1 = mucosal erythema only, 2 = mild mucosal edema, slight bleeding or slight erosion, 3 = moderate edema, bleeding ulcers or erosions, 4 = severe ulceration, erosions, edema and tissue necrosis and perforation. Ulcer area was determined using 3M® scaled surgical transparent tape, which was fixed on a light and transparent sheet. Each cell on the tape was 1 mm2 in area and the number of cells was counted for measuring the ulcerated areas for each specimen. Ulcer index was measured by summing the ulcer score and the ulcer area for each colon(32,33).
For histological examination, colon tissues were individually fixed in 10% formalin, and dehydrated, paraffin embedded, processed, sectioned in 4 μm thick slices, and stained with haematoxylin and eosin (H&E). Inflammation severity and extent as well as crypt damage were evaluated on H&E-stained and coded sections using a modification of a validated scoring system described by Cooper et al.(34) and Dieleman et al.(35). Total colitis index was measured by summing 3 subscores (inflammation severity, inflammation extent, crypt damage). A pathologist unaware of treatments recorded macroscopic and histological injuries. It was implemented by using a Zeiss®microscope equipped with a Sony®color video camera for digital imaging.
Statistical analysis
Analysis of data was performed by SPSS (version 17.0) statistical software. Non-parametric data were analyzed by Kruskal-Wallis and Mann-Whitney U tests. Results were expressed as mean ± standard deviation (SD). Differences between groups were determined using one-way analysis of variance (ANOVA) with Scheffe as post hoc test. The minimal level of significance was identified at P< 0.05.
RESULTS
Analysis of the extract
The hydroalcoholic extract yielded 32.4% w/w. The total phenols determined by the Folin-Ciocalteua method showed 49.8 mg GAE/g extract of the plant.
Analysis of the essential oil
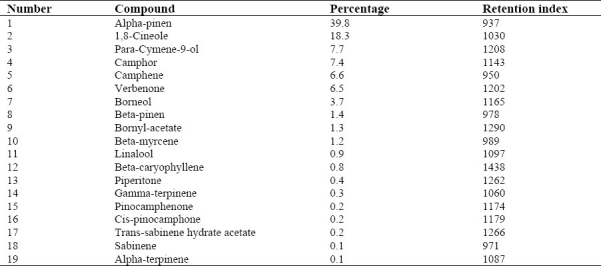
The plant leaves yielded 1.71% of a pale-yellowish essential oil with a fresh pleasant odor. Nineteen constituent were characterized accounting for 97.1% of the total oil components detected, which are listed in Table 1 with their percentage composition and retention indices.
Table 1.
Volatile oil constituents of the essential oil of Rosmarinus officinalis L.
Macroscopic assessment
As it is shown in Fig. 1 and Table 2, macroscopic tissue damage parameters mani-fested severe inflammation; hemorrhage, ulcer, and necrosis as well as thickening of colon wall in colitis control groups compared to sham groups in which no changes were observed.
Fig. 1.
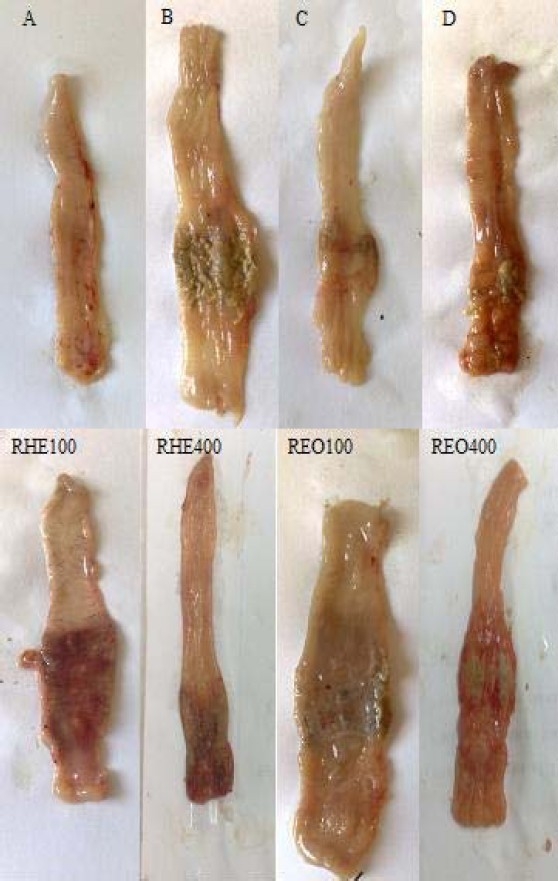
Macroscopic presentation of TNBS-induced colitis in rats. A) Normal colon treated with vehicle, 2 ml/kg; B) Control colitis treated with vehicle, 2 ml/kg; C) Prednisolone treated colitis, 4 mg/kg; D) Asacol® treated colitis, 100 mg/kg; RHE100, RHE400 (Rosemary hydroalcoholic extract treated colitis, 100 and 400 mg/kg); REO100, REO400 (Rosemary essential oil treated colitis, 100 and 400 μl/kg)
Table 2.
Effects of Rosmarinus officinalis hydroalcoholic L. extract and essential oil on the macroscopic parameters of TNBS-induced colitis in rats
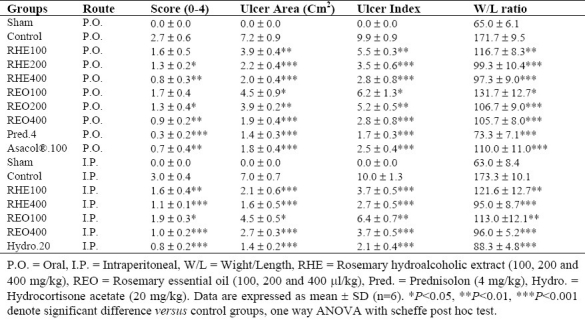
All the treatments with increasing doses of RHE and REO (with exception of lowest doses) were effective to reduce weight/length ratio as well as ulcer index (ulcer area + ulcer severity) in colon samples compared to control groups (P <0.05) (Table 2). RHE and REO also diminished ulcer features after both routes of oral and parenteral administration.
On the other hand, prednisolone and hydrocortisone acetate significantly (P<0.001) diminished macroscopic scores in colitis rats. Asacol® had desirable effect on macroscopic features of colitis lesions (P<0.01) after oral administration.
Histological assessment
No histological damage was observed in sham groups. In control groups, microscopic assess-ments revealed highly severe inflammation and infiltration of white blood cells in mucus and sub-mucosal layers (Fig. 2).
Fig. 2.
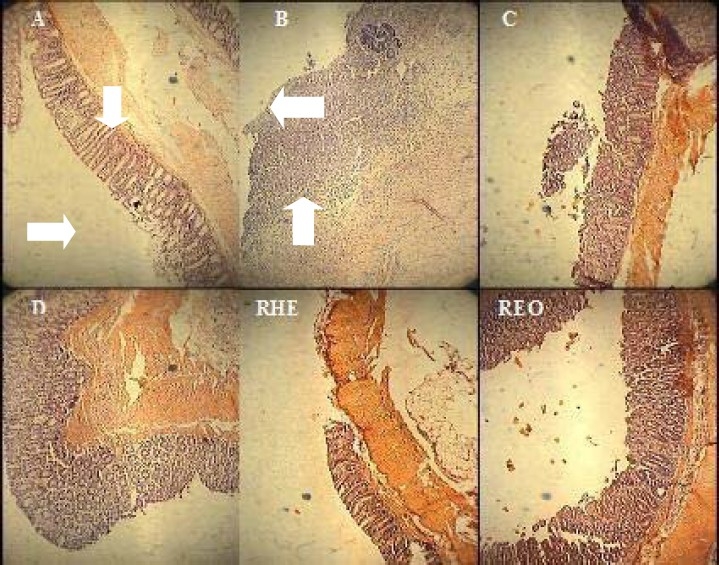
Microscopic presentation of TNBS-induced colitis in rats. A (Normal colon treated with vehicle, 2ml/kg, mucus layer and crypts are normal and leucocyte infiltration is absent); B (Control colitis treated with vehicle, 2ml/kg, mucusal and submucusal inflammation as well as crypt damage and leucocyte infiltration are evident); C (Prednisolon treated colitis, 4mg/kg); D (Asacol® treated colitis, 100mg/kg); RHE400 (Rosemary hydroalcoholic extract treated colitis, 400mg/kg); REO400 (Rosemary essential oil treated colitis, 400μl/kg). H&E staining with low power (×10) magnification
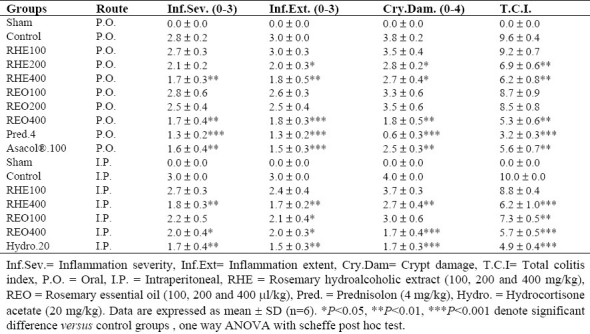
Prednisolone and Asacol® by oral administration and hydrocortisone acetate by i.p. injection were all effective to reduce inflammation severity and extent as well as crypt damage and total colitis index in injurious colons (Table 3). Similar findings were obtained for greater doses of both the essential oil (400 μl/kg) and extract (400 mg/kg) after i.p. administration. In oral route, RHE as well as REO with different increasing doses had a significant effect on inflammatory parameters in comparison with control groups (Table 3).
Table 3.
Effects of Rosmarinus officinalis hydroalcoholic L. extract and essential oil on the histopathologic parameters of TNBS- induced colitis in rats
DISCUSSION
It has been described that TNBS model of experimental colitis is beneficial for the screening of drugs with anticolitic activity and has several similarities to pathological and clinical features of the human ulcerative colitis(36,37).
The results of the current study indicated that administration route did not exert a significant influence on the therapeutic efficacy and activity of hydroalcoholic extract and essential oil of rosemary. Two suggestions might be presented to describe this finding. Firstly, it is supposed that treatment of animals with oral administration of rosemary fractions for a period of 5 days provided a suitable condition for systemic absorption and availability of active plant constituents comparable with parenteral route. Secondly, in agreement with previous studies carried out on rosemary fractions, rosmarinic acid and ursolic acid as the main anti-inflammatory consti-tuents of RHE have high lipid solubility similar to volatile oil constituents of REO which could be absorbed readily from GI tract and even after applying on the skin(10,12).
Therefore, the same results obtained after oral and intraperitoneal administration seems to be reasonable. It has also been demonstrated that rosmarinic acid can increase the produc-tion of prostaglandin E2 and reduce the production of leucotriene B4 in human polymorphonuclear leucocytes(12). Rosmarinic acid is also an inhibitor of the complement system activity. The beneficial activity of rosmarinic acid could also be attributed to its antioxidant property(38).
The rosemary extract and essential oil inhibited the ulcerative lesion index both macroscopically and pathologically at their highest doses (400 mg/kg and 400 μl/kg) used. At the lowest dose of extract and essential oil (100 mg/kg and 100 μl/kg), lesion improve-ment was negligible in pathologic evaluation in spite of a significant healing in macroscopic parameters suggesting that ultimate anti-ulcerogenic activity had at least in part a relationship with the amount of doses used. Indeed, doses greater than 400 mg/kg (or 400 μl/kg) are needed to explore the exact role of the extent of administered doses and dose-effect relationship for the anticolitic effect of RHE and REO.
Our results are in accordance with previous studies reporting antiulcerogenic activity of rosemary hydroalcoholic extract in which the activity was independent of the administration routes but dependent on the dose which probably mediated through mucosal cytoprotective mechanisms related to non-protein sulfydryl groups which is enhanced against the ulcerogenic agents(19,39). This was attributed to the antioxidant compounds found in the crude extract of rosemary(38). Juhas et al.(14) reported that REO in part per million (PPM) doses given by daily food intake were also effective to alleviate TNBS-induced colitis in mice. Moreover, REO with three different doses was effective to suppress the extent of mice paw edema induced by carrageenan and the activity was dependent on the dose and the time of the treatment.
In the present study, all macroscopic and microscopic lesion parameters were improved after administration of the reference drugs. Asacol® administered orally was similarly effective (compared to glucocorticoides) in TNBS- induced colitis suggesting involvement of an important radical scavengering related mechanism. Moreover, Asacol® can inhibit the cyclooxygenase and 5-lipoxygenase pathways of arachidonic acid metabolism similar to corticosteroids, in which the latter mechanism seems to be more important in IBD pathology(40).
According to GC/MS analysis of REO, the most present component was alpha-pinene which is known as an anti-inflammatory agent. Moreover, alpha-pinene has an inhibitory effect on the nuclear translocation of nuclear factor-kappa B (NF-kappa B)(41) that play an important role in colitis pathology(42). Also 1, 8-cineole, another component of rosemary essential oil, has anti-inflammatory and analgesic activities(43). Therefore, it is probable that alpha-pinene and 1,8-cineole are, at least partially, responsible for anti-ulcerative and anti-inflammatory effects found in this study.
CONCLUSION
In conclusion, our results suggest an advan-tageous therapeutic activity for Rosmarinus officinalis extract and essential oil as an anti-inflammatory medicinal plant for IBD conditions. This reinforces the use of this plant as a remedy for IBD conditions and the therapy prevention of recurrence, in traditional medicine. More studies are strongly recommended to establish the mechanisms are involved and the active constituents which are really responsible for its beneficial pharmacologic actions.
ACKNOWLEDGEMENT
This is a research project fully sponsored by Research Council of Isfahan University of Medical Sciences, Isfahan, Iran.
REFERENCES
- 1.Sellin JH, Pasricha PJ. Pharmacotherapy of inflammatory bowel diseases. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilmans the pharmacological basis of therapeutics. 10th ed. New York: McGraw-Hill companies; 2006. pp. 1009–11. [Google Scholar]
- 2.Sartor RB. Pathogenesis and immune mechanism of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:5–11. [PubMed] [Google Scholar]
- 3.Bouma C, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 4.Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078–2086. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 5.Murata Y, Ishiguru Y, Itoh J, Munakata A, Yoshida Y. The role of proinflammatory and immuno-regulatory cytokines in the pathogenesis of ulcerative colitis. J Gasteroenterol. 1995;30:56–60. [PubMed] [Google Scholar]
- 6.McQuaid KR. Drugs used in the treatment of gastrointestinal disease. In: Katzung BG, editor. Basic and clinical pharmacology. 10th ed. New York: McGraw Hill Companies; 2007. pp. 1029–35. [Google Scholar]
- 7.Jagtap AG, Shirke SS, Phadke AS. Effect of polyherbal formulation on experimental models of inflammatory bowel diseases. J Ethnopharmacol. 2004;90:195–204. doi: 10.1016/j.jep.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 8.Yuan G, Wahlqvist ML, He G, Yang M, Li D. Natural products and anti-inflammatory activity. Asia Pac J Clin Nutr. 2006;15:143–152. [PubMed] [Google Scholar]
- 9.Ghannadi AR. Iranian herbal pharmacopeia. Tehran: Iranian Ministry of Health & Medical Education Publications; 2002. Rosemary (Rosmarinus officinalis L.). In: Iranian herbal pharmacopeia scientific committee; pp. 334–339. [Google Scholar]
- 10.Al-Sereitia MR, Abu-Amerb KM, Sena P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian J Exp Biol. 1999;37:124–131. [PubMed] [Google Scholar]
- 11.Gilani AH, Rahman AU. Trends in ethnopharma-cology. J Ethnopharmacol. 2005;100:43–49. doi: 10.1016/j.jep.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Nusier MK, Bataineh HN, Daradkah HM. Adverse effects of rosemary (Rosmarinus officinalis) on reproductive function in adult male rats. Exp Biol Med. 2007;232:809–813. [PubMed] [Google Scholar]
- 13.Takaki I, Bersani-Amado LE, Vendroscolo A, Sartoretto SM, Diniz SP, Bersani-Amado CA, et al. Anti-inflammatory and antinociceptive effects of Rosmarinus officinalis L. essential oil in experi-mental animal models. J Med Food. 2008;11:741–746. doi: 10.1089/jmf.2007.0524. [DOI] [PubMed] [Google Scholar]
- 14.Juhas S, Bukovska A, Cikos S, Czikkova S, Fabian D, Koppel J. Anti-inflammatory effects of Rosmarinus officinalis essential oil in mice. Acta Vet Brno. 2009;78:121–127. [Google Scholar]
- 15.Altinier G, Sosa S, Aquino RP, Mencherini T, Della Loggia R, Tubaro A. Charachterization of topical antiinflammatory compounds in Rosmarinus officinalis L. J Agric Food Chem. 2007;55:1718–1723. doi: 10.1021/jf062610+. [DOI] [PubMed] [Google Scholar]
- 16.Inatani R, Nakatani N, Fuwa H. Antioxidative effect of the constituents of rosemary (Rosmarinus officinalis) and their derivatives. Agric Biol Cehm. 1983;47:521–528. [Google Scholar]
- 17.Stefanovits-Banyai E, Tulok MH, Hegedus A, Renner C, Varga IS. Antioxidant effect of various rosemary (Rosmarinus officinalis L.) clones. Acta Biol Szeged. 2003;47:111–113. [Google Scholar]
- 18.Cheung S, Tai J. Anti-proliferative and antioxidant properties of Rosmarinus officinalis. Oncol Rep. 2007;17:1525–1531. [PubMed] [Google Scholar]
- 19.Dias PC, Foglio MA, Possenti A, Carvalho JE. Antiulcerogenic activity of crude hydroalcoholic extract of Rosmarinus officinalis L. J Ethno-pharmacol. 2000;69:57–62. doi: 10.1016/s0378-8741(99)00133-6. [DOI] [PubMed] [Google Scholar]
- 20.Alkofahi A, Atta AH. Pharmacological screening of the anti-ulcerogenic effects of some Jordanian medicinal plants in rats. J Ethnopharmacol. 1999;67:341–345. doi: 10.1016/s0378-8741(98)00126-3. [DOI] [PubMed] [Google Scholar]
- 21.Oluwatuy M, Kaatz GW, Gibbons S. Antibacterial and resistance modifying activity of Rosmarinus officinalis. Phytochemistry. 2004;65:3249–3254. doi: 10.1016/j.phytochem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Ghannadi A, Sajjadi SE, Abedi D, Yousefi J, Daraei-Ardekani R. The in vitro activity of seven Iranian plants of the Lamiaceae family against Helicobacter pylori. Niger J Nat Prod Med. 2004;8:40–42. [Google Scholar]
- 23.Galistco M, Suarez A, Del Pilar Montilla M, Del Pilar Utrilla M, Jimenez J, Gil A, Faus MJ, et al. Antihepatotoxic activity of Rosmarinus tomentosus in a model of acute hepatic damage induced by thioacetamide. Phytother Res. 2000;14:522–526. doi: 10.1002/1099-1573(200011)14:7<522::aid-ptr660>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 24.Sadraei H, Ghannadi A, Tak-Bavani M. Effects of Zataria multiflora and Carum carvi essential oils and hydroalcoholic extracts of Passiflora incarnata, Berberis integerrima and Crocus sativus on rat isolated uterus concentrations. Intern J Aromather. 2003;13:121–127. [Google Scholar]
- 25.Ghannadi AR, Sajjadi SE, Beigihassan A. Composition of the essential oil of Ferula ovina (Bioss.) Bioss. from Iran. Daru. 2002;10:165–167. [Google Scholar]
- 26.Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods. Am J Enol Viticult. 1977;28:49–55. [Google Scholar]
- 27.Sandra P, Bicchi C. Capillary gas chromatography in essential oil analysis. Heidelberg: Dr. A. Huethig. 1987 [Google Scholar]
- 28.Mclafferty FW, Stauffer DB. New York: John Wiley & Sons Inc; 1991. The important peak index of the registry of mass spectral data. [Google Scholar]
- 29.Adams RP. Carol Stream: Allured Publishing Co; 1995. Identification of essential oil components by gas chromatography/mass spectroscopy. [Google Scholar]
- 30.Esmaily H, Hosseini-Tabatabaei A, Rahimian R, Khorasani R, Baeeri M, Barazesh-Morgani A, et al. On the benefits of silymarine in murine colitis by improving balance of destructive cytokines and reduction of toxic stress in the bowel cells. Cent Eur J Biol. 2009;4:204–213. [Google Scholar]
- 31.Morris G, Beck P, Herridge M, Depew W, Szewczuck M, Wallace J. Hapten induced model of inflammation and ulceration in rat colon. Gastro-enterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 32.Minaiyan M, Ghannadi A, Karimzadeh A. Antiulcerogenic effects of ginger (Zingiber officinale Roscoe) on cysteamine induced duodonal ulcer in rats. Daru. 2006;14:97–101. [Google Scholar]
- 33.Minaiyan M, Ghannadi A, Mahzouni P, Nabi-Meibodi M. Antiulcerogenic effects of ginger (rhizome of Zingiber officinale Roscoe) hydro-alcoholic extract on acetic acid-induced acute colitis in rats. Res Pharm Sci. 2008;3:79–86. [Google Scholar]
- 34.Cooper H, Murthy S, Shah R, Sedergran D. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 35.Dieleman L, Palmen M, Akol H, Bloemena E, Pena A, Meuwissen S. Chronic experimental colitis induced by dextran sulfate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immuno. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jurjus AR, Khoury NN, Reimund JM. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Method. 2004;50:81–92. doi: 10.1016/j.vascn.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Torres MI, Garcia-Martin M, Fernandez MI, Nietro N, Gil A, Rios A. Experimental colitis induced by trinitrobenzene sulfonic acid: an ultrastructural and histochemical study. Dig Dis Sci. 1999;44:2523–2529. doi: 10.1023/a:1026651408998. [DOI] [PubMed] [Google Scholar]
- 38.Ramirez P, Senorans FJ, Ibanez E, Reglero G. Separation of rosemary antioxidant compounds by superficial fluid chromatography on coated packed capillary columns. J Chromatogr A. 2004;1057:241–245. doi: 10.1016/j.chroma.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 39.Repetto MG, Liesuy SF. Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz J Med Biol Res. 2002;35:523–534. doi: 10.1590/s0100-879x2002000500003. [DOI] [PubMed] [Google Scholar]
- 40.Varshosaz J, Emami J, Fassihi A, Tavakoli N, Minaiyan M, Ahmadi F. Effectiveness of budesonide-succinate-dextran conjugate as a novel prodrug of budesonide against acetic acid-induced colitis in rats. Int J Colorectal Dis. 2010;25:1159–1165. doi: 10.1007/s00384-010-1026-2. [DOI] [PubMed] [Google Scholar]
- 41.Zhou JY, Tang FD, Mao GG, Bian RL. Effects of alpha-pinene on nuclear translocation of NF-kappa B in THP-1 cells. Acta Pharmacol Sci. 2004;25:480–484. [PubMed] [Google Scholar]
- 42.Kawada M, Arihiro A, Mizoguchi E. Insights from advances in research of chemically induced experimental models of human infllamatory bowel disease. World J Gastroenterol. 2007;13:5581–5593. doi: 10.3748/wjg.v13.i42.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santos FA, Rao VSN. Anti-inflammatory and Antinociceptive Effects of 1,8-Cineole a Terpenoid Oxide Present in many Plant Essential Oils. Phytother Res. 2000;14:240–244. doi: 10.1002/1099-1573(200006)14:4<240::aid-ptr573>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]


