Abstract
Extract prepared from aerial part of Pycnocycla spinosa is a relaxant of rat ileum contractions. The objective of this research was to study the spasmolytic activity of P. spinosa root extract for comparison with the aerial part extract. Hydroalcoholic extracts were prepared by percolation method. A portion of rat ileum was removed and suspended under 1 g tension in Tyrode's solution at 37 °C and gassed with O2. Isotonic contractions induced by electrical field stimulation (EFS), and KCl were recorded before and after addition of the extracts. Experiments were performed alongside time-matched vehicle controls. Both EFS responses are blocked by lidocaine (74 μM), indicating that contractile responses are mediated by neuronal mechanism and partially was blocked by atropine. The root extract of P. spinosa (10-320 μg/ml) inhibited both initial rapid (IC50 = 71 ± 11.9 μg/ml) and secondary slow contraction (IC50 = 56 ± 7.8 μg/ml) evoked by EFS (n=6) as well as the KCl response (IC50 = 59 ± 11.7 μg/ml). The aerial part extract had a similar inhibitory effect on both KCl (IC50 = 47 ± 6.3 μg/ml) and EFS responses. This study confirms the inhibitory effects of root extract of P. spinosa on rat ileum contraction. However, the root extract was not more effective than the aerial part extract. Therefore, the aerial parts extract of P. spinosa could be used as a suitable substitute for the root extract.
Keywords: Pycnocycla spinosa, Ileum, Electrical field stimulation, Anti-spasmodic
INTRODUCTION
Plant extracts have been used as medicine for centuries and they are origin of some clinically useful drugs(1). Gastrointestinal is one of the major target organs for herbal medicines particularly in the treatment of gastrointestinal motility. Diarrhea is a common disease, and a major cause of death in infants and children in the tropics(2). In order to combat the problem of diarrhea globally, the World Health Organization in its Diarrheal Disease Control Program, has given a special emphasis on the use of traditional medicine in the control and management of diarrhea(2). Pycnocycla could be a suitable candidate because its extract contained substances with anti-spasmodic and anti-diarrhoeal activities(3–5). Ten different species of Pycnocycla exist and they have close morphologically appearance. P. nodiflora is reported to be used as local herbal medicine for treatment of parasite infestation(6). Roots of P. glauca in the form of aqueous solution are used in the treatment of dysentery. Roots collected before flowering is considered best for preparation of medicine. In fourteen herbal combinations, Pycnocycla roots are added as an important ingredient. In addition to treatment of dysentery, traditional healers use the decoction of roots externally on bleeding piles. This decoction is also considered promising in treatment of bleeding from any part due to injuries. However, P. glauca is not in the list of medicinal herbs(7).
P. spinosa Decne. exBoiss. var. spinosa belongs to the family of Umbelliferae and grow wild throughout Iran. P. spinosa is a vegetation plant with soft cylindrical branched stem and cane leaves tapered to relatively long, straight large spines. It flowers during June-July with greenish-yellow flowers(8–10). The aerial part of P. spinosa contains volatile oils with at least thirty different constituents the quantity of which varies with seasonal variation being more abundant during flowering month(8–10). Isolated essential oil is about 0.1% on dry weight basis and it is very volatile(3). The essential oil is pharma-cologically active and is a strong relaxant of rat isolated ileum and inhibits contraction induced by KCl, acetylcholine and serotonine (5-HT) in vitro with an IC50 of about 20 ng/ml(3). P. spinosa aerial extract is also rich in chemical substances and contains alkaloids, flavonoids and saponine like substances, all of which have anti-spasmodic activity(4). Furthermore, P. spinosa extract shows anti-diarrheal action on castor oil and magnesium sulphate-induced diarrhea with 1 mg oral dose(3,11). The anti-diarrheal activity of P. spinosa extract is most likely related to its anti-spasmodic action as it delays gastrointestinal transit(11). Although, there are several reports on pharmacological effect of aerial extract of P. spinosa on ileum(3–5), uterus(12), bladder(13) and heart rates(14) but so far there is no report on anti-spasmodic of root extract of P. spinosa. Therefore, in this research we have investigated relaxant effect of both aerial and root extract of P. spinosa on rat isolated ileum contraction induced by KCl and nerve stimulation using electrical filed stimulation (EFS) technique.
MATERIALS AND METHODS
Plant material
Aerial parts of P. spinosa were collected in June from Isfahan University campus and identified in the Biology Department at Isfahan University. A voucher specimen (No. A24) was authenticated and then deposited in the herbarium of the School of Pharmacy and Pharmaceutical Sciences (Isfahan, Iran). The aerial part of the plant was dried in shade. The total hydroalcoholic extract was obtained by percolation(15).
Experimental procedure
Male Wistar rats, 200-250 g, were killed by a blow on the head, followed by exsanguination. Central portion of ileum was then removed and placed in Tyrode's solution containing (composition in mM): NaCl, 136.9; KCl, 2.68; CaCl2, 1.8; MgCl2, 1.05; NaHCO3, 11.9; NaH2PO4, 0.42 and glucose 5.55) at room temperature. The ileum was trimmed and separated into 2-3 cm long segment before the contents were gently squeezed out.
The tissue segments were suspended in Tyrode's solution at 37 °C in an organ bath (Harvard Apparatus) and bubbled with O2. The tissue was washed several times and left for at least 15 min to allow the tissue to relax to a stable baseline. From a resting tension of 1 g, isotonic contractions, elicited by EFS or KCl, were recorded using a Harvard transducer and displayed on a Harvard Universal Oscillograph pen recorder.
EFS was delivered (Stimulator, designed in School of Pharmacy and Pharmaceutical Science) through parallel platinum wire electrodes (10 cm long, 1 cm apart) in trans of 1 s rectangular pulses (frequency 50 Hz, 6 V output) at 5 min intervals. KCl (80 mM) was directly added into organ bath.
The effects of drugs/extracts on FES induced contractions were studied using single dose regimen, each concentration of extract remaining in contact with the tissue at least for 10 min before its effect was evaluated. The tissue then was exposed to the next drug/extract concentration for another 10 min and so on. Relaxant effect of the drugs/extracts on KCl-induced contraction was studied using cumulative addition. When appropriate, expe-riments were conducted in parallel with time matched controls using tissue from the same animal adding vehicle in lieu of extract.
Measurements and statistical analysis
Contractions were measured as maximum changes in tension from pre-contraction baseline and expressed as percentage of initial values before addition of drugs or extracts. Mean and standard error of mean (SEM) values were calculated for each group of results and inter-group comparisons were made with one way analysis of variance (ANOVA) and further compared with their time matched controls using unpaired Student's t-test. Differences considered statis-tically significant for P<0.05.
Drugs and solutions
The hydroalcoholic extracts was prepared as 10 mg/ml stock solution in dimethylsulfoxide (DMSO, Germany). Nifedipine (Sigma, Germany) was made as 100 mM stock solution in DMSO. Atropine sulfate (Merck, Germany) was made up as 10 mM stock solution in distilled water. Ten folds serial dilution was prepared in DMSO or distilled water for experiment use. KCl was made up in distilled water as 2 M stock solution. Lidocaine hydro-chloride (vial from Pasture Institute, Iran) was diluted in distilled water. Other chemicals were purchased from Merck (Germany).
RESULTS
Rat isolated ileum suspended in the organ bath starts to show spontaneous contractile activity which tends to subside by washing the tissue with fresh Tyrode's solution. KCl (80 mM) caused a rapid contraction in rat ileum followed by a maintained tonic contraction. Both root and aerial extracts of P. spinosa concentration dependently inhibited contrac-tion induced by 80 mM KCl with IC50 = 59 ± 11.7 μg/ml (n=5) and IC50 = 47 ± 6.3 μg/ml (n=6) respectively (Fig. 1). There were no statistically significant differences in percen-tage inhibition of responses when equivalent concentrations of aerial and root extracts were compared with each other.
Fig. 1.
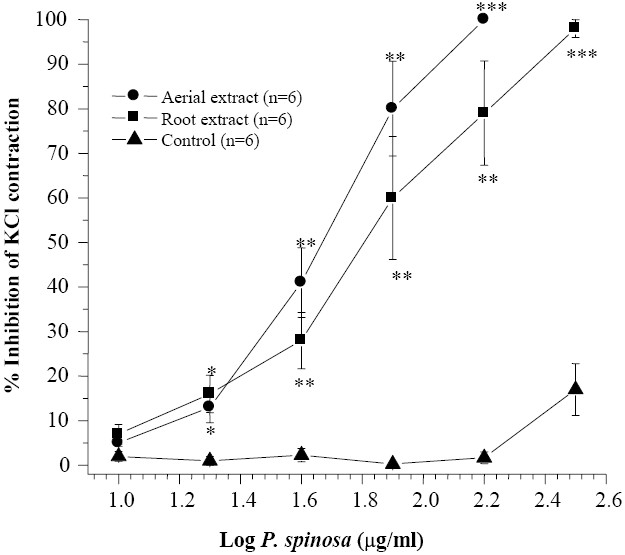
Effect of Pycnocycla spinosaextracts on tonic contractions developed in rat isolated ileum by KCl (80 mM). Time-matched control tissues treated with vehicle in equivalent volume. Results are given as mean and vertical bars indicate SEM (n=6). Stars shows statistically significant differences between the test and control groups at corresponding points. *P<0.05, **P<0.01, ***P<0.001 (Student's t-test).
Rat ileum contracted rapidly to EFS reaching a peak within 10 s followed by partial relaxation which was then followed by a slower second peak with variable magnitude and then relaxed towards the baseline. Lidocaine (74 μM and 740 μM) reduced the initial response by 73 ± 6.5% and 84 ± 4.9%, respectively while completely abolished the secondary response to EFS with 740 μM lidocaine concentration in the bath (n=6). However, the tissue still contracted when KCl (80 mM) was added to the bath.
Nifedipine (0.5 nM to 128 nM) concen-tration dependently reduced both initial and secondary induced responses to EFS and at its highest used concentration removed the secondary contraction to EFS while still 31% of initial response remained (Fig. 2). Atropine (200 nM to 12.8 μM) at concentration which blocks effect of muscarinic agonist on rat ileum(16) progressively attenuated both initial and secondary contractions induced by EFS up to a maximum of 72 ± 9.8% and 36 ± 7.6%, respectively (Fig. 3). Increasing atropine concentration had no further inhibitory effect on either initial or secondary contraction induced by EFS.
Fig. 2.
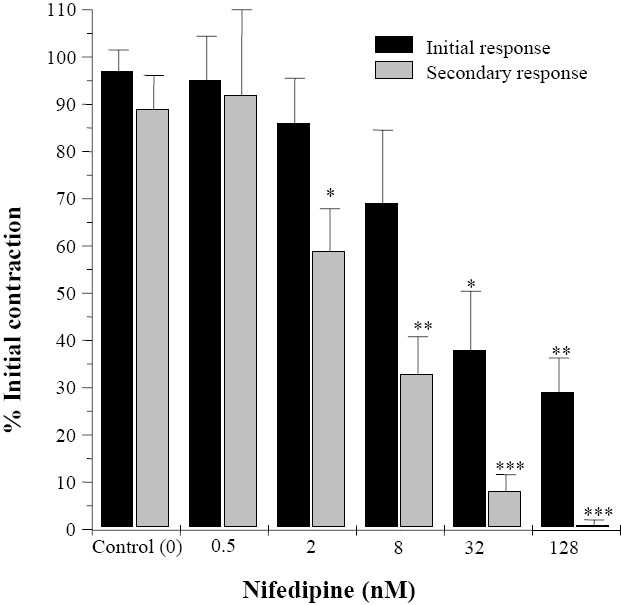
Effects of nifedipine on the biphasic contractions of rat ileum induced by electrical field stimulation. Results are given as mean and vertical bars indicate SEM (n=6). Stars show statistically significant differences between nifedipine and the initial controls. *P<0.05, **P<0.01, ***P<0.001 (Student's t-test).
Fig. 3.
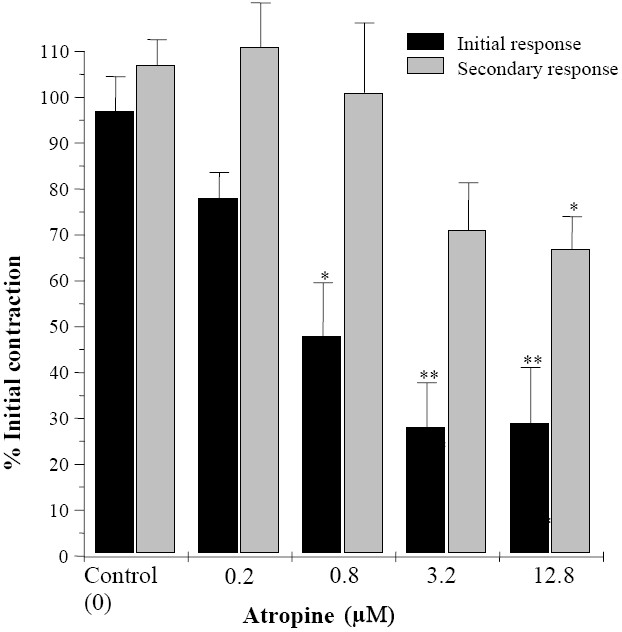
Effects of atropine on biphasic contractions of rat ileum induced by electrical field stimulation. Results are given as mean and vertical bars indicate SEM (n=6). Stars show statistically significant differences between atropine and the initial controls. *P<0.05, **P<0.01, (Student's t-test).
Relaxant effect of root and aerial extract of P. spinosa at concentration ranges which inhibited the KCl responses were also examined on biphasic contractions induced by EFS. The root extract of P. spinosa (10 μg/ml to 320 μg/ml) concentration dependently inhibited both initial (IC50 = 71 ± 11.9 μg/ml) and secondary contraction (IC50 = 56 ± 7.8 μg/ml) responses to EFS. At its highest used concentration the root extract almost removed the EFS response (Fig. 4). The aerial extract of P. spinosa at similar range of concentration (10-160 μg/ml) had a similar pattern of inhibition to EFS responses (Fig. 5). The IC50 value of aerial extract for initial and secondary contractile response to EFS were IC50 = 70 ± 22 μg/ml and IC50 = 61 ± 9.3 μg/ml respectively. Inhibitory effects of both extracts are more pronounced on the secondary contraction of EFS response than the initial contraction (Fig 4 and 5).
Fig. 4.
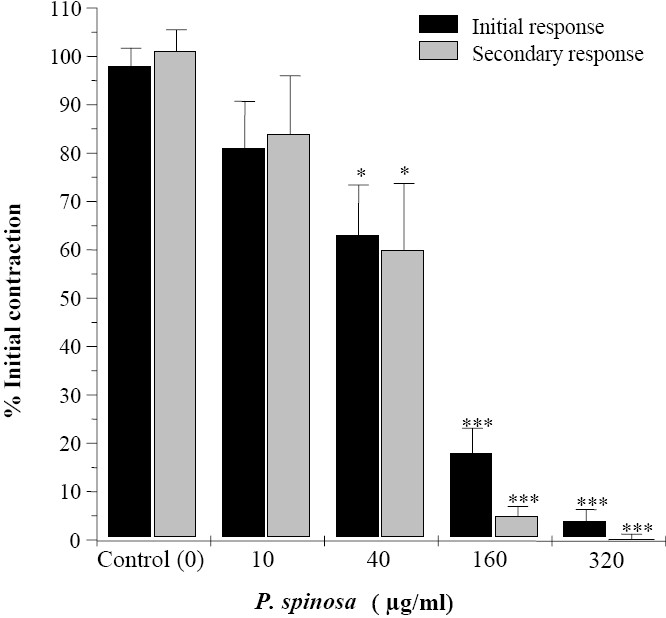
Inhibitory effect of Pycnocyla spinosa root extract on biphasic contractions of rat ileum induced by electrical field stimulation. Results are given as mean and vertical bars indicate SEM (n=6). Stars show statistically significant differences between extract and the initial controls. *P<0.05, ***P<0.001 (Student's t-test).
Fig. 5.
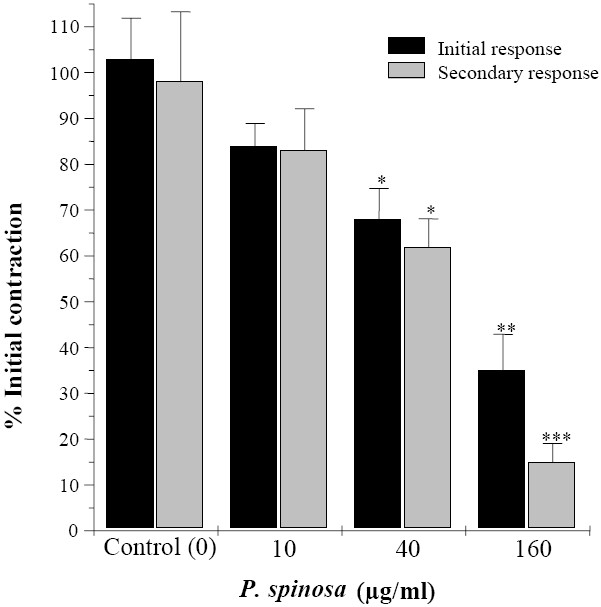
Inhibitory effects of Pycnocyla spinosa aerial extract on biphasic contractions of rat ileum induced by electrical field stimulation. Results are given as mean and vertical bars indicate SEM (n=6). Stars show statistically significant differences between extract and the initial controls. *P<0.05, **P,0.01, ***P<0.001 (Student's t-test).
DISCUSSION
The enteric nervous system is a collection of neurons located in the wall of the gastrointestinal system and their activity is responsible for spontaneous contractile activity in the ileum suspended in the organ bath. The enteric nervous system receives inputs from both sympathetic and parasympathetic systems(17). In addition to acetylcholine and noradrenaline, many other transmitter and neuromodulator substances have been iden-tified in enteric nervous system including serotonin, dopamine, ATP, nitric oxide, substance P, neuropeptide Y, vasoactive intestinal peptide, gastrin releasing peptide, calcitonin gene related peptide, cholecys-tokinin, enkephalin and other related opioid peptides(18). These mediators that often refer to as non-adrenergic non-cholinergic neuro-transmitters have major contribution for induction of biphasic responses have been seen with EFS in rat ileum. The biphasic contractile responses have already been reported in several papers that studied electrical field stimulation technique on rat ileum(19–24) and we have reproduced similar response with our locally designed stimulator. The local anes-thetic lidocaine, inhibited both EFS responses, whereas, in the presence of lidocaine, the tissue response to KCl remained intact. This evidence supports suggestion that the contractile response seen with EFS is mainly due to the nerve stimulation within the enteric nervous system.
Atropine is an antagonist which blocks effect of acetylcholine on muscarinic receptors on ileum smooth muscle. Atropine partially inhibited the contractile response to EFS indicating cholinergic activity during neuronal stimulations. However, even at concentrations higher than those which blocks acetylcholine response(25–29) full inhibitory effect was not achieved. This result is consistence with literature reports that gastrointestinal motility inhibition by atropine requires larger concentrations and it is not complete. This is because excitatory transmitters other than acetylcholine are important in normal function of myenteric plexus(30). Comparison of relative inhibition of initial and secondary EFS contractions with atropine reveals that acetylcholine release is more involved in primary response induced by ESF than the secondary contraction.
Nifedipine is an L-type calcium channel antagonist, which blocks Ca2+ influx during smooth muscle cells depolarization. Ca2+ has vital role in smooth muscle contraction and extracellular Ca2+ is a major source which enters the cell following activation of voltage gated calcium channels (including the L-type calcium channels)(31). Decrease in Ca2+ entry, reduces the magnitude of contraction(32). Nifedipine blocked both responses to EFS, although its effect was more pronounced on the secondary contractile response to EFS. This may suggest that extra-cellular Ca2+ source is more important in the secondary contraction induced by EFS.
P. spinosa root extract concentration dependently reduced magnitude of both initial and secondary contractile response to EFS in rat ileum. The inhibitory effect of root extract on EFS biphasic contractile responses is very similar to the inhibitory action seen with nifedipine; this may be an indication that there are substances in the extract that directly or indirectly are affecting Ca2+ ion channels. Inhibition of KCl response by the extract is also consistent with above mentioned suggestion. However, because of presence of several active substances in the extract, comment on more accurate mechanism of action is only possible when effect of each active component is compared with standard drugs. Qualitative and quantitative comparison of root and aerial extract of P. spinosa on rat ileum contraction induced by KCl or EFS does not show any significant differences. Therefore, to be green, we could use the active substances from aerial parts of P. spinosa without uprooting the plant because the anti-spasmodic effect of aerial extract is as good as the root extract. Furthermore, previous studies on aerial extract of P. spinosa shows that ileum relaxation is achieved with lower concentration than relaxant effect on uterus and bladder smooth muscle(12–13). In addition, anti-diarrhoeal action of aerial extract is equipotent to the standard drug loperamide(11). The yield and inhibitory effect of aerial extract on KCl is consistent with the previous reports(3–5,12,13).
CONCLUSION
Both root and aerial extract of P. spinosa are potent relaxant of rat ileum contractions. Although as folk medicine it is the root of the plant which are being used, but the aerial extract is as good as the root extract and therefore we recommend the use of aerial extract as anti-spasmodic and anti-diarrhoeal herbal medicine to be in line with plant conservation.
ACKNOWLEDGEMENT
We thank the botanist Dr. Mehregan* for identification of the plant. We also would like to thank the research department of Isfahan University of Medical Sciences, Isfahan, Iran, for financially supporting this project.
Footnotes
*Dr Mehregan has recently moved to Research Centre in Islamic Azad University, Tehran.
REFERENCES
- 1.Mozaffarian V. Tehran: Farhang Moaser; 1996. A dictionary of Iranian plant names; pp. 443–444. [Google Scholar]
- 2.Syder JH, Merson MH. The magnitude of the global problem of acute diarrhoeal disease: a review of active surveillance data. Bull WHO. 1982;60:605–613. [PMC free article] [PubMed] [Google Scholar]
- 3.Sadraei H, Asghari G, Naddafi A. Relaxant effect of essential oil and hydroalcoholic extract of Pycnocycla spinosa Decne. exBoiss on ileum contractions. Phytother Res. 2003;17:645–649. doi: 10.1002/ptr.1217. [DOI] [PubMed] [Google Scholar]
- 4.Sadraei H, Asghari G, Hekmatti AA. Anti-spasmodic effect of three fractions of hydro-alcoholic extract of Pycnocycla spinosa. J Ethnopharmacol. 2003;86:187–190. doi: 10.1016/s0378-8741(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 5.Sadraei H, Asghari G, Khazael M. Relaxant effect of four fractions separated from alkaloid extract of Pycnocycla spinosa on rat isolated ileum. Res Pharm Sci. 2008;3:79–86. [Google Scholar]
- 6.Seifollahkhani A. The th seminar of Iranian Pharmacy Students. Isfahan: 2001. Pharmacognostic studies of Pycnocycla nodiflora Decne. exBoiss The 7th seminar of Iranian Pharmacy Students; p. 184. [Google Scholar]
- 7.Ekdandi S. Medicinal herbs of Chhattisgarh, India having less known traditional uses (Pycnocyla glauca, family Apiceae). A letter to American Botanical. Available from: URL: http://www.botanical.com/site/column_poudhia/articles/878html.2007 .
- 8.Ahmadi L, Mirza M. Volatile constituents of the essential oil of Pycnocyla spinosa Decne ex. Boiss from Iran. J Essent Oil Res. 1988;10:197–198. [Google Scholar]
- 9.Asgahri G, Hoshfar G, Mahmoudi Z. Seasonal variation of mono- and sesquiterpenes in the essential oil of Pycnocyla spinosa Decne. exBoiss. Iranian J Pharm Res. 2002;1:61–63. [Google Scholar]
- 10.Jalili A, Jamzad Z. Tehran: Research Institute of Forests and Rangelands; 1999. Red data book of Iran, a preliminary survey of endemic, rare and endan-gered plant species in Iran; pp. 689–690. [Google Scholar]
- 11.Sadraei H, Asghari G, Shams M. Anti-diarrhoeal action of hydroalcoholic extract of Pycnocycla spinosa in comparison with loperamide and dicyclomine. Iranian J Pharm Res. In press. [PMC free article] [PubMed] [Google Scholar]
- 12.Sadraei H, Asghari G, Andisha M. Anti-spasmodic effect of Pycnocycla spinosa seed and aerial part extracts on rat ileum and uterus smooth muscle contractions. Daru. 2008;13:160–163. [Google Scholar]
- 13.Sadraei H, Asghari G, Arabzadah A. Effect of hydroalcoholic extract of Pycnocycla spinosa on rat isolated bladder contraction. Iranian J Pharm Res. 2004;4:237–241. [Google Scholar]
- 14.Sadraei H, Asghari G, Hajhashemi V, Nezami M. Evalutaion of cardiovascular effect of Pycnocycla spinosa Decne. exBoiss., var. spinosa extracts in anaensthetized rat. Daru. 2008;14:11–14. [Google Scholar]
- 15.Samuelsson G. 4th ed. Stockholm: Swedish Pharmaceutical Press; 1999. Drugs of natural origin; pp. 48–49. [Google Scholar]
- 16.Eglen RM, Whiting RL. Competitive and non-competitive antatgonism exhibited by selective antagonists at atrial and ileal muscarinic receptor subtypes. Br J Pharmacol. 1987;90:701–707. doi: 10.1111/j.1476-5381.1987.tb11223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Ann Rev Physiol. 1999;61:117–142. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- 18.Katzung BG. Introduction to autonomic pharmacology. In: Katzung BG, editor. Basic and clinical pharmacology. 9th ed. Norwalk, CN: Appleton and Lange; 2006. pp. 102–110. [Google Scholar]
- 19.Smith GJ, Lefebvre RA. Nonadrenergic non-cholinergic responses in the rat ileum. Eur J Pharmacol. 1996;303:79–87. doi: 10.1016/0014-2999(96)00089-1. [DOI] [PubMed] [Google Scholar]
- 20.Smith GJM, Lefebvre RA. ATP and nitric oxide: inhibitory NANC neurotransmitters in the longitudinal muscle-myenteric plexus preparation of rat ileum. Br J Pharmacol. 1996;118:695–703. doi: 10.1111/j.1476-5381.1996.tb15456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ekblad E, Sundler F. Motor responses in rat ileum evoked by nitric oxide donors vs.field stimulation: Modulation by pituitary adenylate cyclase activating peptide, forskolin and guanylate cyclase inhibitors. J Pharmacol Exp Ther. 1997;283:23–28. [PubMed] [Google Scholar]
- 22.Kurjak M, Sattler V, Schusdiarra V, Allwscher HD. Characterization of prejunctional and postjunctional muscarinic receptors of the ascending reflex contraction in rat ileum. J Pharmacol Exp Ther. 1999;290:893–900. [PubMed] [Google Scholar]
- 23.Storr M, Thammer J, Dunkel R, Schusdziarra V, Allescher HD. Modulatory effect of adenosine receptors on the ascending and descending neural reflex responses of rat ileum. BMC Neurosci. 2002;3:21–27. doi: 10.1186/1471-2202-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaji M, Ohta M, Yamazaki Y, Fujinami K, Fujita A, Takeuchi T, et al. A possible role of neurotensin in NANC relaxation of longitudinal muscle of the jejunum and ileum of Wistar rats. Br J Pharmacol. 2002;137:629–636. doi: 10.1038/sj.bjp.0704914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elorriaga M, Anselmi E, Hernandez JM, Docon P, Ivorra D. The source of Ca2+ for muscarinic receptor-induced contraction in rat ileum. J Pharm Pharmacol. 1996;48:817–819. doi: 10.1111/j.2042-7158.1996.tb03980.x. [DOI] [PubMed] [Google Scholar]
- 26.Goyal RK. Identification, localisation and classi-fication of muscarinic receptor subtypes in the gut. Life Sci. 1988;43:2209–2220. doi: 10.1016/0024-3205(88)90414-6. [DOI] [PubMed] [Google Scholar]
- 27.Levey AI. Immunological localization of M1-M5 muscarinic acetylcholine receptors in peripheral tissue and brain. Life Sci. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- 28.Caulfield MP. Muscarinic receptor characteri-zation, coupling and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 29.Eglen RM, Hege SS, Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- 30.Rang HP, Dale MM. Cholinergic transmission. In: Rang HP, Dale MM, Ritter JM, Flower RJ, editors. Rang & Dale's Pharmacology. 6th ed. London: Churchill Livingstone; 2007. p. 153. [Google Scholar]
- 31.Rang HP, Dale MM. Rang & Dale's Pharmacology. 6th ed. London: Churchill Livingstone; 2007. How drugs act: cellular aspects-excitation, contraction and secretion; pp. 54–71. [Google Scholar]
- 32.Reynolds IJ, Gould RJ, Snyder SH. Loperamide: blocked of calcium channels as a mechaninsm for anti-diarrheal effect. J Pharmacol Exp Ther. 1984;231:628–632. [PubMed] [Google Scholar]


