Abstract
Please cite this paper as: Romagosa et al. (2011) Sensitivity of oral fluids for detecting influenza A virus in populations of vaccinated and non‐vaccinated pigs. Influenza and Other Respiratory Viruses.
Background/objective We evaluated the sensitivity of PCR on oral fluids in detecting influenza virus in vaccinated and non‐vaccinated pigs.
Methods Three‐week‐old influenza‐free pigs were divided into three groups: (i) control, non‐vaccinated, (ii) vaccinated with a commercial, heterologous vaccine, and (iii) vaccinated with an experimental, homologous vaccine. After vaccination, an influenza‐infected pig was placed in contact with each of the groups. Individual nasal swabs and pen oral fluids were collected daily. Viral RNA was tested for the presence of influenza by RRT‐PCR and virus isolation attempted from oral fluids. A pen was considered positive if at least one nasal swab was positive.
Results Based on nasal swab results, 43·8% of pens were detected positive but only 35% based on oral fluids. Overall sensitivity of oral fluids was 80%, and virus was isolated from 51% of RRT‐PCR‐positive oral fluids. The kappa coefficient for agreement (ĸ) between oral fluids and nasal swabs was 0·82. Among groups, ĸ was 1 (95% CI, 1–1), 0·74 (95% CI, 0·55–0·92), and 0·76 (95% CI, 0·5–1) for control, heterologous, and homologous‐vaccinated groups, respectively. There was less agreement when within pen prevalence was 10% or less. Probability of detecting influenza virus in oral fluids was 99% when within pen prevalence was higher than 18% and decreased to 69% when prevalence was 9%.
Conclusions Results indicated that pen‐based collection of oral fluids is a sensitive method to detect influenza even when within pen prevalence is low and when pigs have been vaccinated and highlight the potential use of oral fluids for influenza surveillance.
Keywords: Diagnostic, influenza virus, oral fluids, surveillance, swine
Introduction
Monitoring diseases in populations using individual samples limits the ability to conduct surveillance because the number of samples required to detect infection in low prevalence populations is high. In addition, sample collection can be difficult and cumbersome, resulting in costly diagnostics. To overcome some of these limitations, oral fluids have become a popular method to detect pathogens in pigs. 1 Collection of oral fluids is non‐invasive, and samples can be collected by personnel with limited training and without special equipment. 1 , 2
Oral fluids are composed of saliva and other components from non‐salivary origin, such as oral mucosal transudation, inflammatory components, bronchial and nasal secretions, bacteria and viruses, plasma transudation, and epithelial cells. 3 The presence of pathogens and antibodies in saliva from infected humans and animals has been widely studied for diagnostic purposes. 4 In humans, whole saliva or oral fluid specimens have been used to detect antibodies against Helicobacter pylori, Shigella dysenteriae, Taenia Solium, and viral hepatitis, or to detect viral pathogens such as rubella virus and rotavirus in newborn infants. 5 , 6 , 7 , 8 , 9 , 10 Detection of human immunodeficiency virus antibodies in oral fluids is currently being applied for clinical use and epidemiological surveillance. 11 In pigs, foot‐and‐mouth disease virus, Erysipelothrix species, influenza virus, porcine reproductive and respiratory syndrome virus (PRRSV), and porcine circovirus type 2 have been detected in oral fluids from naturally or experimentally infected pigs. 1 , 2 , 12 , 13
Nowadays, oral fluid samples are routinely used to detect PRRSV in samples from pigs in the United States. 1 , 14 Porcine reproductive and respiratory syndrome virus can be detected in oral fluids for up to 4 weeks after exposure, 14 and as early as 1 day post‐infection. 15 Diagnostic agreement for detection of PRRSV in individual oral fluids and serum by quantitative RRT‐PCR has been estimated at 77%. Overall, these results indicate that testing oral fluids can be a sensitive method to monitor populations for viral pathogens. 1 , 15
More recently, Detmer et al. 2 showed that influenza virus can also be detected in oral fluids from experimentally and naturally infected pigs. Using the same USDA‐validated real‐time RRT‐PCR assay than the one used in the present study, the minimum influenza virus detection in spiked oral fluids was estimated at 316 TCID50/ml for the A/Sw/IA/00239/2004 H1N1 influenza virus and at 17 TCID50/ml for MN/07 H1N2 and KS/08 H3N2 influenza virus mixture, suggesting that testing oral fluids can be a sensitive method for influenza surveillance in pigs. 2
The above notwithstanding, there is no information on oral fluids and the predicted probability of detecting influenza virus at the population level, nor is there information regarding the sensitivities of testing pen‐level oral fluids compared to testing samples from individual animals nor whether oral fluids are a suitable sample for virus isolation. Furthermore, there is no information on how vaccination status may affect the ability to detect influenza infection in pig populations. Because influenza virus is endemic in pigs and vaccination is commonly carried out on swine farms in the United States, 16 it is important to understand the sensitivities of the different surveillance methods under the different conditions of infection and immunity.
The present study evaluated the collection of oral fluids to detect a triple reassortant H1N1 swine influenza virus by RRT‐PCR from populations of immunized pigs with different vaccines, estimated the diagnostic agreement and determined the sensitivity of oral fluids collected at the pen level to estimate the probability to detect influenza virus in pig populations. Virus isolation from oral fluid samples was also evaluated. This study provides new information on the use of oral fluids and offers a new approach to conduct surveillance in pig populations infected with influenza virus.
Materials and methods
Animals and animal housing
Eighty‐eight 3‐week‐old pigs from a high health farm were purchased. Pigs were free of infection with influenza virus, PRRSV and Mycoplasma hyopneumoniae, and born from influenza unvaccinated sows. All piglets were tested for influenza at the herd of origin prior to the start of the study using hemagglutination‐inhibition (HI) tests against four distinct influenza strains, and enzyme linked immunoabsorbent assay (ELISA) as described below.
Eight groups of eleven pigs each were randomly placed in individual isolated rooms located at the University of Minnesota Animal Research Facility (Saint Paul, MN, USA) and assigned to three different treatment groups as follows: (i) non‐vaccinated control, (ii) vaccinated with a commercially licensed, heterologous vaccine, and (iii) vaccinated with an experimental, homologous vaccine. There were two replicates for the control group and three replicates for the groups vaccinated with the heterologous and the homologous vaccine. Pigs were distributed in eight different rooms (one replicate/room). Each room contained a pen, and each pen 11 pigs. Space allowance was 6·3 square feet per pig (0·58 m2), and the pigs were fed on the floor ad libitum and with free access to water. Pigs were cared for according to University of Minnesota IACUC protocol number 0908A71965.
Experimental design
On arrival to the research facility, nasal swabs and blood samples were collected from all pigs and tested for influenza A virus antigen by RRT‐PCR, and for antibodies by HI and ELISA as described below. Pigs were also injected once with an antibiotic to reduce bacterial contaminants prior to the start of the study (ceftiofur crystalline‐free acid, 5·0 mg/kg body weight, Excede®; Pfizer Animal Health, New York, NY, USA).
Twenty‐four hours post‐arrival, pigs vaccinated with the heterologous vaccine received intramuscularly (IM) in the neck the heterologous influenza virus vaccine (2 ml, FluSure XP®; Pfizer Animal Health). Pigs vaccinated with the homologous vaccine were similarly vaccinated with an experimental, homologous, inactivated vaccine containing the same viral isolate as the challenge virus. Each vaccination was repeated 2 weeks later. The control pigs were injected with 2 ml of sterile saline solution IM in the neck at 2‐week intervals. In each room, one of the 11 pigs was left unvaccinated to be intratracheally and intranasally challenged with influenza A virus and serve as a source of infection (or “seeder” pig) for the other pigs in the group.
Thirteen days after the second vaccination, nasal swabs and blood samples were collected from all pigs, and the seeder pig from each room was moved to a separate room for challenge. At 48 hours post‐challenge, the eight seeder pigs were placed back to each room in contact with their original 10 pen mates (one seeder pig/room) until the termination of the study. Between 0 and 14 days post‐contact (dpc), pen‐based oral fluids were collected daily placing one rope in each room and allowing the pigs to chew the rope. Individual nasal swabs were also collected daily from all pigs just before oral fluids were collected. At 14 dpc, pigs were euthanized by injecting intravenously a lethal dose of pentobarbital (100 mg/kg, Fatal‐Plus Solution®; Vortech Pharmaceuticals, Dearborn, MI, USA).
Challenge virus and vaccine preparation
A triple reassortant H1N1 strain A/Sw/IA/00239/04 (IA04) belonging to the β H1 cluster and obtained from the University of Minnesota Veterinary Diagnostic Laboratory was used for challenge. For preparation of the homologous oil‐based adjuvanted vaccine, the same virus IA04 was grown in Madin‐Darby Canine Kidney (MDCK) cells using standard methods 17 and adjusted to an hemagglutination (HA) titer of 1:128/0·1 ml at the time of inactivation by the addition of formalin at a final concentration of 0·1%. The formalized virus was mixed with an adjuvant mixture of mineral oil (nine parts) and emulsifier (one part; equal volumes of Span 85 and Tween 85) in a 1:1 ratio and sonicated at 25 W for 2–3 minutes. The challenge virus was grown in MDCK cells. 17
The federally licensed, commercial, heterologous, swine influenza virus vaccine (FluSure XP®; Pfizer Animal Health) contained three distinct inactivated influenza isolates: A/Swine/North Carolina/031/05 (H1N1), A/Swine/Missouri/069/05 (H3N2), and A/Swine/Iowa/110600/00 (H1N1). The H1N1 vaccine strains A/Swine/North Carolina/031/05 and A/Swine/North Carolina/031/05 belonged to the γ and δ groups, respectively, and were genetically distinct from the challenge strain sharing 92·2% and 66·8% HA nucleotide similarity, respectively, with IA04. 18
Virus inoculation
Seeder pigs were inoculated intratracheally and intranasally with a total of 2 ml of IA04 H1N1 challenge virus at a titer of 106 TCID50/ml. Before the inoculation, all pigs were sedated by an intramuscular injection of a dissociative anesthetic (6·6 mg/kg, Telazol®; Fort Dodge Animal Health, Fort Dodge, IA, USA). Following satisfactory sedation, pigs were manually restrained while the mouth was opened to expose the larynx and the trachea, and a catheter was inserted and 1 ml of the virus was delivered using a syringe attached to the catheter. For intranasal inoculation, 0·5 ml of the same inoculum was delivered in each nostril by deep intranasal route to the same pig. Success of inoculation in the seeder pig was confirmed by positive influenza A virus RRT‐PCR from nasal swabs at 24 and 48 hour post‐inoculation. Viral isolation and titration was conducted from nasal swabs at 48 hour post‐inoculation.
Sample collection
Nasal swabs, oral fluids, and blood samples
Nasal swabs were collected from individual pigs using rayon‐tipped swab applicators with Stuart’s medium (BBL CultureSwab™ liquid, Stuart single plastic applicator; Becton, Dickinson and Com, Sparks, MD, USA). Oral fluids were collected as described by Prickett et al. 1 Briefly, three‐strand twisted unbleached 100% cotton ropes with 5/8″ diameter (WebRiggingSupply.com, Barrington, IL, USA) were placed in each pen at approximately 40 cm above from the floor for 20–30 minutes for the pigs to chew on the ropes. Oral fluids were extracted from the rope immediately after collection by wringing the wet portion into a plastic bag [Ziploc® bags (3·79 lt); S.C. Johnson & Son, Inc., Racine, WI, USA]. A bottom corner of the bag was cut to drain the fluid into a 5‐ml plastic sterile tube, and samples were refrigerated at 4°C overnight to allow debris to deposit at the bottom of the tube. Supernatant from each oral fluid sample was processed by RRT‐PCR as described below. Blood samples were collected using venipuncture. After collection, serum was separated and stored at −20°C.
Sample processing and diagnostic tests
Nasal swabs were suspended in 2 ml of MEM supplemented with 4% BSA prior to processing for RRT‐PCR. Samples from the supernatant of each oral fluid specimen were also processed by RRT‐PCR. The viral RNA was extracted using the magnetic particle processor procedure (Ambion® MagMAX™ AM1835, Viral RNA Isolation Kit; Applied Biosystems, Foster City, CA, USA) and subsequently tested using the procedure provided by the USDA‐NVSL 19 for detection of influenza A virus Matrix gene by RRT‐PCR 20 A positive result is defined by a cycle threshold (C t) value of <35, suspect range includes C t values >35 but <40, and negative results are defined as C t values >40 (the C t value is determined by the number of cycles needed to exceed the background signal).
Virus isolation from oral fluids was performed following standard procedures as described before. 17 Briefly, 300 μl of oral fluids was inoculated in 96‐well plates containing monolayers of MDCK cells and incubated for up to 4 days at what time plates were assessed for CPEs. Only RRT‐PCR‐positive oral fluids were submitted for virus isolation.
Sera were analyzed by HI test and ELISA assay. HI tests were performed following standard procedures. 21 Samples were tested by HI against the challenge strain (IA04), the commercial heterologous vaccine isolates (A/Sw/IA/110600/00 and A/Sw/NC/031/2005), and H3N2 (A/Sw/MO/069/2005 H3N2) at the farm of origin, at arrival, thirteen days after the second vaccine, and at necropsy. Additionally, all sera were tested using the Influenza A Multiscreen Elisa (IDEXX FlockChek™ AI Multi‐Screen Ab Test Kit; IDEXX Lab, Westbrook, ME, USA) for detecting anti‐influenza A nucleoprotein antibodies following manufacturer’s protocols.
Statistical methods
To perform the statistical analysis, each pen collection in a given time point was considered a unit. A pen was considered positive if influenza virus was detected from nasal swabs of at least one pig within the pen. The research facilities had one pen per room.
Suspect RRT‐PCR results from nasal swabs and oral fluids were considered negative for statistical analysis. Results from nasal swabs and oral fluids were compared by group of treatment and all the groups combined. Fisher’s exact test and simple kappa coefficient (κ) were used to test agreement between results. Values of ĸ below 0·4 are considered low agreement, values between 0·4 and 0·6 moderate agreement, values between 0·6 and 0·8 good agreement, and values higher than 0·8 are considered excellent. Results from virus isolation and RRT‐PCR C t values were compared using paired Student’s t‐test. RRT‐PCR‐positive C t value means from oral fluids and nasal swabs were also compared using the Student’s t‐test. P‐values <0·05 were considered statistically significant. Logistic regression was used to calculate the estimated probability to detect influenza in oral fluids. Statistical analyses were performed using sas ® version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Pigs were both influenza virus antibody and antigen negative at the start of the study. Table 1 summarizes the HI antibody results after vaccination and prior to contact. Two weeks after the second vaccination, the homologous vaccine induced robust titers against IA/04 H1N1 with titers more than 1:320. However, mean titers against IA/04 in the group vaccinated with the heterologous vaccine were below the positive cutoff (<1:40). As expected, the pigs vaccinated with the heterologous vaccine had positive titers against the H1N1 and the H3N2 strains contained in the heterologous, commercially licensed vaccine, while pigs in the group vaccinated with the homologous vaccine were negative to the strains in the licensed vaccine. Titers in the non‐vaccinated control group were <1:10. Virus titers from nasal swabs in the seeder pigs at the time of contact ranged from 3 × 102 to 1 × 105 TCID50 per ml. Seeder pigs showed limited signs of influenza infection with fever and mild cough and nasal secretions. Clinical signs in the vaccinated pigs were almost non‐existent compared with the unvaccinated pigs, but overall clinical signs were mild.
Table 1.
Hemagglutination‐inhibition titers (reciprocal geometric means) against four flu strains prior to infection by group of treatment. The flu strains 2, 3, and 4 were contained in the commercial vaccine
| Group | Strain | |||
|---|---|---|---|---|
| IA04* | 012XP** | 31XP*** | 69XP† | |
| Control | <10 | <10 | <10 | 13 |
| Heterologous | 14 | 305 | 232 | 485 |
| Homologous | 297 | 36 | <10 | <10 |
*Challenge strain A/Sw/0239/IA/04 H1N1 (IA04).
**A/Swine/North Carolina/031/05 H1N1 (012XP).
***A/Swine/Iowa/110600/00 H1N1 (31XP).
†A/Swine/Missouri/069/05 H3N2 (69XP).
Prior to placing the seeder pig in contact with the contact pigs, all oral fluids and nasal swabs from the contact pigs in all groups were negative. Influenza virus RRT‐PCR results from nasal swabs and oral fluids are shown in Table 2. Results from nasal swabs of the seeder pigs and contact pigs are shown separately. Individual nasal PCR results indicated that influenza virus was detected in all contact pigs from the control group at an average of 4 (±1) dpc. In contrast, influenza could not be detected in any of the contact pigs in the group vaccinated with the homologous vaccine throughout the duration of the study. Results from the heterologous commercial vaccine group were more variable. In that group, the cumulative percentage of positive pigs at the end of the study was 10%, 50%, and 20% for the three replicates. These differences were most likely due to the varying levels of immunity present among the pigs.
Table 2.
Summary of PCR results of oral fluids (OF) and nasal swabs (NS) from contact and seeder pigs and virus isolation of oral fluids ( cells) by collection day, replica, and treatment (each room had one seeder pig and 10 contacts)
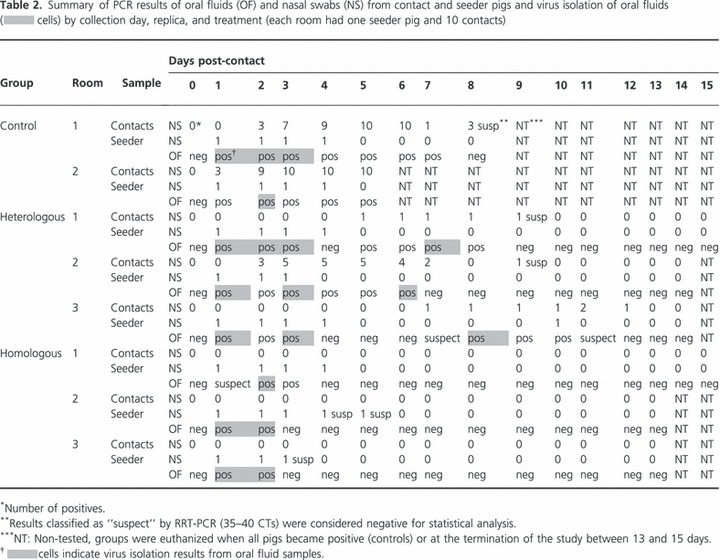
A total of 1155 nasal swabs were collected throughout the study, and 13·2% were positive which corresponded to 46 positive (43%) pen collections (those pens had at least one positive animal in a given collection day). From the 105 oral fluid samples collected, 37 samples or 35·2% pens were positive.
In the control group, 12 of the 15 pen collections had at least one positive nasal swab. All 12 collections were positive by oral fluids. In the group vaccinated with the homologous vaccine, positive results from nasal swabs were detected only from the seeder pigs, and they shed influenza for an average of 3 dpc. Oral fluid samples were only positive when the seeder pig was detected positive. As a whole in the group vaccinated with the homologous vaccine, nine of the 44 pen collections were positive, and only six were positive according to oral fluid samples. In the heterologous commercial vaccine group, 25 of the 46 pen collections were positive, but only 19 were detected positive by oral fluid samples. Overall, RRT‐PCR results from nasal swabs and oral fluids were strongly associated (Fisher’s exact test, P < 0·001), and differences in overall RRT‐PCR C t values between oral fluids and nasal swabs were also statistically significant, P‐value 0·001 (results not shown).
The simple kappa coefficient of agreement between results in the control group was 1 (95% CI, 1–1). In the group vaccinated with the heterologous commercial vaccine, ĸ was 0·74 (95% CI, 0·55–0·92) and in the group vaccinated with the homologous vaccine, it was 0·76 (95% CI, 0·5–1). When results from all treatment groups were combined, the simple kappa coefficient was very high (ĸ = 0·82, 95% CI, 0·71–0·93). Results can be seen in Table 3.
Table 3.
Detection of the challenge virus (IA04) using RRT‐PCR in oral fluids (OF) from the control, heterologous, homologous, and all groups combined. Information is presented in 2 by 2 tables. Results from the seeder pigs are included
| Pen status* | Control OF | Heterologous OF | Homologous** OF | Overall OF | ||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | |
| Positive | 12 | 0 | 19 | 6 | 6 | 3 | 37 | 9 |
| Negative | 0 | 3 | 0 | 21 | 0 | 35 | 0 | 59 |
| Total | 12 | 3 | 19 | 27 | 6 | 38 | 37 | 68 |
| ĸ coefficient (95% CI) | 1 | 0·74 (0·55–0·92) | 0·76 (0·5–1) | 0·82 (0·71–0·93) | ||||
*Number of positive pens, with pens defined as positive if ≥1 nasal swab tested positive by RRT‐PCR.
**Positive results from the homologous group originated only from the seeder pigs.
Fifty‐one percent (19/37) of RRT‐PCR‐positive oral fluids were also positive by virus isolation. Virus isolates were recovered from all treatment groups mostly during the first 3 days post‐contact with the seeder pigs (Table 2). In the heterologous group, virus isolates could also be recovered at 6, 7, and 8 days post‐contact. There were no statistically significant differences between virus isolation from oral fluids and RRT‐PCR C t values from oral fluids (P‐value, 0·06) or nasal swabs (P‐value, 0·56) (results not shown.)
The sensitivity of detecting influenza virus at the pen level by testing oral fluids compared with individual nasal swabs was estimated at 80·4% and the specificity at 100%. Most of the negative oral fluid results that occurred in positive pens corresponded to the heterologous commercial vaccine group. In that group, the frequency of samplings with only one PCR‐positive pig within the pen was higher than in the control group (P > 0·05). On the other hand, the frequency of samplings with more of the nasal swabs testing positive (>8 swabs) was significantly higher in the control group than in neither of the vaccinated groups (Figure 1).
Figure 1.
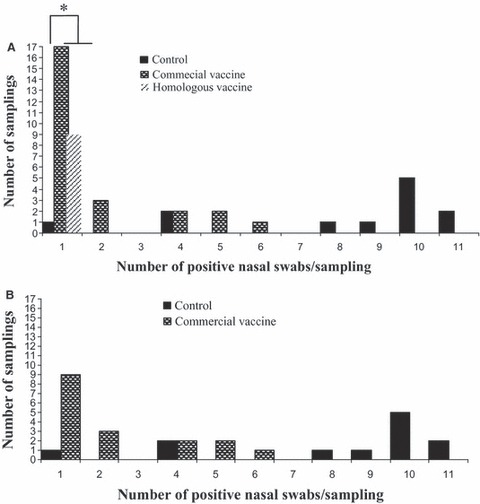
Number of positive nasal swabs per pen collection by group of treatment. (A) Includes results from the seeder and contact pigs. (B) Includes results only from the contact infected pigs by group of treatment. *In vaccinated groups, the frequency of pen collections with only one positive pig within the pen was higher than in the control group (P < 0.05).
Figure 2 shows the predictive probability of detecting influenza‐positive pigs in a pen using oral fluids based on the percentage of infected pigs in the pen. The predictive probability was 69% when the prevalence of infected pigs was 9%, increasing to 99% when the prevalence was 18% or higher.
Figure 2.
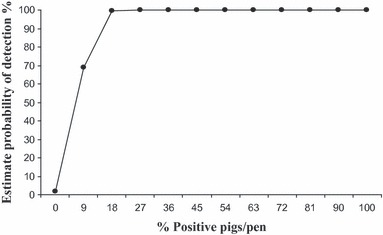
Predicted probability of detecting influenza virus‐positive pigs using oral fluids based on the number of positive pigs in a room. The predicted probability was 69% when the prevalence of positive pigs was 9%, increasing to 99% when the prevalence was 18% or higher.
Discussion
The collection of oral fluid facilitates monitoring, surveillance, and detection of viruses in populations. 1 , 2 , 4 Population‐based sampling using oral fluids may result in lower test costs, while increasing the number of pigs monitored and pathogens that can be detected. 1 , 14 Collection of oral fluids using ropes takes advantage of the natural curious behavior of pigs to interact and play with the rope while depositing the oral fluids in the rope.
The purpose of this study was to examine the use of oral fluid specimens to detect the presence of influenza A virus in populations. This study compared results from oral fluid samples collected from groups of pigs with nasal swab samples collected from individual pigs. Nasal swabs are one of the most used methods for collecting field samples for surveillance of influenza virus in pigs, but collection can be difficult and negative results may occur if not enough samples are collected.
Samples collected for this study originated from an experimental setting that included vaccinated and non‐vaccinated pigs. The advantage of including vaccinated pigs was our ability to replicate conditions of low infection prevalence also found in populations in the field using influenza vaccines. Influenza vaccination in pigs is common and vaccination can decrease clinical signs, transmission, and the probability to detect infection. 22 , 23 , 24 Therefore, there is a need to evaluate detection of influenza virus in both vaccinated and non‐vaccinated populations.
In this study, all pigs interacted quickly with the ropes placed in the pens. Oral fluids and nasal swabs were collected daily at approximately the same time to assess how each collection method correlated with each other. In addition, this study allowed the evaluation of the dynamics of influenza virus detection throughout the acute infection period in a seeder pig transmission model to mimic the ability to detect transmission under field conditions. Detection of influenza virus in oral fluids from experimentally infected animals has been reported before and offers promising results for influenza surveillance. 2 However, its use to monitor populations needs to be properly evaluated prior to its widespread use.
In this study, the experimental unit was the pen at each collection which was classified as positive or negative according to the PCR results from nasal swabs collected from the individual pigs. In a given collection point, a pen was considered positive if at least one pig was detected as positive for influenza A virus by nasal swab RRT‐PCR. None of the contact pigs vaccinated with the homologous vaccine tested positive by PCR on individual nasal swabs. Therefore, any positive results in this group originated most likely from the seeder pig, but not from the contact pigs.
Influenza infection could not be established in the group vaccinated with the homologous virus suggesting that the vaccine was protective which can be explained by the high level of antibody titers against the challenge strain. Therefore, it appears that vaccination prevented transmission within this group, and therefore, virus detection was not possible. However, influenza virus was detected in samples from the group vaccinated with the commercial heterologous vaccine and in samples from the non‐vaccinated (immunologically naïve) control pigs. In both groups, influenza was detected by RRT‐PCR in oral fluids and individual nasal swabs even after the seeder pig had stopped shedding. Interestingly, most of collections with just one or few positive nasal swabs originated from the heterologously vaccinated group and in most of these cases oral fluids also tested positive. There were no positive oral fluid samples in collections where all pigs tested negative by nasal PCR.
In our study, collection of nasal swabs from the entire population on a daily basis was a powerful tool to estimate the probability of detecting influenza virus in oral fluids based on the prevalence of known positive pigs in a pen. The probability was similar for prevalence ranging from 18% to 100% with an estimate of detection of 99%. Probability of detecting influenza virus decreased to 69% when the within pen prevalence was 9%. One limitation of this study is that we were not able to evaluate the probability of detecting influenza when the prevalence was below 9% because of the limited group size. The overall sensitivity of oral fluids related to nasal swabs was above 80% and that included scenarios with low within pen prevalence. However, results from our study must be validated in the field where the number of individual pigs that can be sampled in a given pen by nasal swabs is limited, and where the number of pigs per pen and per rope can differ. In addition, sensitivity of PCR on oral fluids to identify infected pens was not as high as when pen status was measured by the individual results because all the animals within the pen were tested. Unfortunately, sampling of all animals in large populations is almost impossible, and under those conditions, we speculate that the use of oral fluids would have superior results compared to individual animal testing.
Overall agreement between RRT‐PCR results from oral fluids and pen status was excellent (ĸ = 0·82). Although a few pens could not be properly identified, the differences were mostly due to low prevalence situations from pigs vaccinated with the heterologous vaccine, or in situations where the total amount of virus was not sufficient to test positive. In a previous study conducted in experimentally infected pigs, influenza virus could be detected in 92% of the pen‐based oral fluid samples collected at days 3, 4, 5, and 6 post‐infection. 2 However, this study only included few collections and all from acutely infected naïve pigs at the time of infection. Furthermore, other studies focused on detection of PRRSV in experimentally infected pigs and in individual boars by comparing PCR results from oral fluid and blood samples showed similar results to the ones reported here where the agreement between pen‐based oral fluids and serum PRRSV status was 77% in finishing barns, and 100% in paired samples from individual boars at 7 days post‐infection with PRRSV. 1 , 15
Among the different treatments, the highest agreement occurred in the unvaccinated control group (ĸ = 1). In the homologous vaccine group, the agreement was very good (ĸ = 0·76), and the lowest agreement was observed in the heterologous commercial vaccine group (ĸ = 0·74) although the differences between these last two groups were not significant.
In addition, in this study, we were able to isolate influenza virus from oral fluids. Isolation of viruses from oral fluids in pigs is difficult 25 , and for influenza, it proved unsuccessful in a previous study. 2 In our study, about 50% of RRT‐PCR‐positive oral fluids yielded positive virus isolation suggesting the potential application of oral fluids for influenza virus surveillance in pig populations. However, we were not able to compare virus isolation from oral fluids and nasal swabs; therefore, a complete understanding of the sensitivity of virus isolation from oral fluids remains to be elucidated. Furthermore, RRT‐PCR C t values from neither oral fluids nor nasal swabs proved to be a good predictor of virus isolation from oral fluid samples. Interestingly, virus isolation was positive in 13 pen collections where only one pig was RRT‐PCR‐positive and in only five collections where multiple pigs were positive. Nevertheless, more studies are needed to evaluate the performance of virus isolation from oral fluids in pigs.
Differences in our ability to detect influenza virus by RRT‐PCR in oral fluids between groups may be due to several factors. Most of the negative oral fluids that occurred in positive pens happened in vaccinated pens when only one pig within the pen was detected positive. Decreased shedding has been reported in vaccinated pigs with pigs shedding less virus for shorter periods of time when they become infected. 23 Furthermore, viral titers in oral fluids could be affected by the time between collection and processing, and by the artifacts present in the samples. Nasal swabs were processed immediately after collection, but oral fluids were kept overnight at 4°C to allow separation of the particles by sedimentation. This procedure was used because centrifugation is not always available in the field and because in a recent study with the same influenza virus strain (IA04) spiked in swine oral fluids at different concentrations and assayed at different time points, it was shown that the RRT‐PCR threshold cycle values (C t) did not change after 5 hours at room temperature. 2 Therefore, the time from collection to sample processing is not likely to have influenced the results of this study. Additionally, the time that the pigs were interacting with the rope is also unlikely to have been a factor because all pigs in the groups were observed to interact with the rope constantly. After an acute infection, changes in behavior because of fever and/or lethargy can reduce the odds of interaction, but in this study, clinical signs were very mild, and we did not observe changes in motivation of the pigs to interact with the ropes. 26 In addition, differences in virus isolation rates from oral fluids between groups of pigs need to be further investigated because differences can be due to many factors beyond the scope of this study.
The research reported highlights the application of oral fluid samples for diagnosis of swine pathogens and provides insightful information on the ability to detect influenza virus in oral fluids from vaccinated and non‐vaccinated populations, scenarios commonly found in the field. The results of our study showed that pen‐based collection of oral fluids using ropes can be a sensitive method of detecting influenza virus in infected pig populations even when the prevalence within a pen is low, reducing the cost for diagnostics and the number of samples needed to detect influenza, and highlight the potential application of oral fluids for influenza virus surveillance in pigs.
Acknowledgements
The study was supported in whole or in part with the federal funds from the NIH, National Institute of Allergy and Infectious Diseases and Department of Health and Human Services under the contract No.HHSN266200700007C and funds from the Swine Disease Eradication Center (University of Minnesota).
Special thanks to Drs Matt Allerson, Seth Baker, Cesar Corzo, Susan Detmer, and Daniel Linhares from the University of Minnesota Veterinary Population Medicine Department, College of Veterinary Medicine.
References
- 1. Prickett JR, Kim W, Simer R, Yoon KJ, Zimmerman J. Oral‐fluid samples for surveillance of commercial growing pigs for porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 infections. J Swine Health Prod 2008; 16:86–91. [Google Scholar]
- 2. Detmer SE, Patnayak DP, Jiang Y, Gramer MR, Goyal SM. Detection of Influenza A virus in porcine oral fluid samples. J Vet Diagn Invest 2011; 23:241–247. [DOI] [PubMed] [Google Scholar]
- 3. Mandel ID, Wotman S. The salivary secretions in health and disease. Oral Sci Rev 1976; 8:25–47. [PubMed] [Google Scholar]
- 4. Robinson L, Bonita EL, Kothapalli S, Craig WR, Fox JD. Use of throat swab or saliva specimens for detection of respiratory viruses in children. Clin Infect Dis 2008; 46:61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feldman M, Plancarte A, Sandoval M, Wilson M, Flisser A. Comparison of two assays (EIA and EITB) and two samples (saliva and serum) for the diagnosis of neurocysticercosis. Trans R Soc Trop Med Hyg 1990; 84:559–562. [DOI] [PubMed] [Google Scholar]
- 6. Jayashree S, Bhan MK, Kumar R, Raj P, Glass R, Bhandari N. Serum and salivary antibodies as indicators of rotavirus infection in neonates. J Infect Dis 1988; 158:1117–1120. [DOI] [PubMed] [Google Scholar]
- 7. Li C, Ha T, Ferguson DA, Chi DS, Zhao R, Patel NR. A newly develop PCR assay of H. pylori in gastric biopsy, saliva, and feces. Evidence of high prevalence of H. pylori in saliva supports oral transmission. Dig Dis Sci 1996; 41:2142–2149. [DOI] [PubMed] [Google Scholar]
- 8. Parry JV, Perry KR, Panday S, Mortimer PP. Diagnosis of hepatitis A and B by testing saliva. J Med Virol 1989; 28:255–260. [DOI] [PubMed] [Google Scholar]
- 9. Perry KR, Brown DW, Parry JV, Panday S, Pipkin C, Richards A. Detection of measles, mumps, and rubella antibodies in saliva using antibody captures radioimmunoassay. J Med Virol 1993; 40:235–240. [DOI] [PubMed] [Google Scholar]
- 10. Schultsz C, Qadri F, Hossain SA, Ahmed F, Ciznar I. Shigella‐specific IgA in saliva of children with bacillary dysentery. FEMS Microbiol Immunol 1992; 4:65–72. [DOI] [PubMed] [Google Scholar]
- 11. Holmstrom P, Syrjanen S, Laine P, Valle SL, Suni J. HIV antibodies in whole saliva detected by Elisa and Western blot assays. J Med Virol 1990; 30:245–248. [DOI] [PubMed] [Google Scholar]
- 12. Callahan JD, Brown F, Osorio F et al. Use of a portable real‐time reverse transcriptase polymerase chain reaction assay for rapid detection of foot‐and‐mouth disease virus. J Am Vet Med Assoc 2002; 220:1636–1642. [DOI] [PubMed] [Google Scholar]
- 13. Bender JS, Shen HG, Irwin C, Schwartz KJ, Opriessnig T. Characterization of Erysipelothrix species isolates from clinically affected pigs, environmental samples, and vaccine strains from six recent swine erysipelas outbreaks in the United States. Clin Vaccine Immunol 2010; 17:1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prickett J, Simer R, Christopher‐Hennings J, Yoon KJ, Evans RB, Zimmerman JJ. Detection of porcine reproductive and respiratory syndrome virus infection in porcine oral fluid samples: a longitudinal study under experimental conditions. J Vet Diagn Invest 2008; 20:156–163. [DOI] [PubMed] [Google Scholar]
- 15. Kittawornrat A, Prickett J, Chittick W et al. Porcine reproductive and respiratory syndrome virus (PRRSv) in serum and oral fluid samples from individual boars: will oral fluid replace serum for PRRSv surveillance? Virus Res 2010; 154:170–176. [DOI] [PubMed] [Google Scholar]
- 16. USDA . Part II: reference of swine health and health management in the United States, 2000, NAHMS swine 2006: National Animal Health Monitoring System, [on line] 2006. Available at http://www.aphis.usda.gov/vs/ceah/ncahs/nahms/swine/swine2000/Swine2000drPartII.pdf (Accessed 30 November 2010); 16–20.
- 17. Meguro H, Bryant JD, Torrence AE, Wright PF. Canine kidney cell line for isolation of respiratory virus. J Clin Microbiol 1979; 9:175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vincent LA, Larger KM, Ma W et al. Evaluation of hemagglutinin subtype 1 influenza viruses from the United States. Vet Microbiol 2006; 118:212–222. [DOI] [PubMed] [Google Scholar]
- 19. United States Department of Agriculture, National Animal Health Laboratory Network , Protocol BPA‐9034.01.
- 20. Spackman E, Senne DA, Myers TJ et al. Development of a real time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol 2002; 40:3256–32560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pedersen JC. Hemagglutination‐inhibition test for avian influenza virus subtype identification and the detection and quantization of serum antibodies to the avian influenza virus Methods in Molecular Biology, Vol 436. Avian Influenza Virus. Totowa, NJ: Erica Spackman © Humana Press, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Romagosa A, Gramer M, Joo HS, Deen J, Torremorell M. Influenza virus transmission rates in vaccinated and non‐vaccinated pig populations. Proceedings of the International Conference on Options for the control of Influenza VII, Hong Kong, China: International Society for Influenza and other Respiratory Diseases, 2010: 9.
- 23. Heinen PP, van Niewstadt AP, de Boer‐Luijtze EA, Bianchi ATJ. Analysis of the quality of protection induced by a porcine influenza A vaccine to challenger with H3N2 virus. Vet Immunol Immunopathol 2001; 82:39–56. [DOI] [PubMed] [Google Scholar]
- 24. Van Reeth K, Labarque G, De Clercq S, Pensaert M. Efficacy of vaccination of pigs with different H1N1 swine influenza viruses using a recent challenge strain and different parameters of protection. Vaccine 2001; 19:4479–4486. [DOI] [PubMed] [Google Scholar]
- 25. Wills RW, Zimmerman JJ, Yoon K‐J et al. Porcine reproductive and respiratory syndrome virus: routes of excretion. Vet Microbiol 1997; 57:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Millman ST, Brooks RM, Zimmerman J, Irwin C. Effects of acute influenza virus infection on swine behaviour associated with collection of oral fluid specimens for disease surveillance; in: McGill University, (ed.). Proceedings of the 9th North American Regional Meeting of the International Society for Applied Ethology. Montreal, Canada, 2009; 28.


