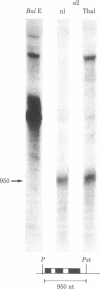
Abstract
The alpha 2-globin gene of an individual with alpha-thalassemia associated with the absence of alpha 2 mRNA was cloned in bacteriophage. This mutant globin gene was normally active in transcription in vitro. The DNA sequence of the gene, however, revealed a pentanucleotide deletion within the 5' splice junction of the first intervening sequence. Following the G of the invariant G-T dinucleotide normally located within such junctions, a deletion of T-G-A-G-G was found. No other sequence abnormalities within the mutant gene were present. We speculate therefore that this deletion within the splice junction is the primary genetic defect in this individual with thalassemia and that loss of a functional splice junction results in failure of stable mRNA formation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Sharp P. A. A gene chimaera of SV40 and mouse beta-globin is transcribed and properly spliced. Nature. 1981 Jan 29;289(5796):378–382. doi: 10.1038/289378a0. [DOI] [PubMed] [Google Scholar]
- Colby D., Leboy P. S., Guthrie C. Yeast tRNA precursor mutated at a splice junction is correctly processed in vivo. Proc Natl Acad Sci U S A. 1981 Jan;78(1):415–419. doi: 10.1073/pnas.78.1.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Grosveld G. C., Koster A., Flavell R. A. A transcription map for the rabbit beta-globin gene. Cell. 1981 Feb;23(2):573–584. doi: 10.1016/0092-8674(81)90153-7. [DOI] [PubMed] [Google Scholar]
- Gruss P., Lai C. J., Dhar R., Khoury G. Splicing as a requirement for biogenesis of functional 16S mRNA of simian virus 40. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4317–4321. doi: 10.1073/pnas.76.9.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer D. H., Leder P. Splicing and the formation of stable RNA. Cell. 1979 Dec;18(4):1299–1302. doi: 10.1016/0092-8674(79)90240-x. [DOI] [PubMed] [Google Scholar]
- Housman D., Forget B. G., Skoultchi A., Benz E. J., Jr Quantitative deficiency of chain-specific globin messenger ribonucleic acids in the thalassemia syndromes. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1809–1813. doi: 10.1073/pnas.70.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Gambino R., Dow L. W., Grossbard E., Natta C., Ramirez F., Spiegelman S., Marks P. A., Bank A. Decreased globin messenger RNA in thalassemia detected by molecular hybridization. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1886–1890. doi: 10.1073/pnas.70.6.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M., Trecartin R., Todd D. Identification of a nondeletion defect in alpha-thalassemia. N Engl J Med. 1977 Nov 17;297(20):1081–1084. doi: 10.1056/NEJM197711172972002. [DOI] [PubMed] [Google Scholar]
- Kantor J. A., Turner P. H., Nienhuis A. W. Beta Thalassemia: mutations which affect processing of the beta-Globin mRNA precursor. Cell. 1980 Aug;21(1):149–157. doi: 10.1016/0092-8674(80)90122-1. [DOI] [PubMed] [Google Scholar]
- Kinniburgh A. J., Ross J. Processing of the mouse beta-globin mRNA precursor: at least two cleavage-ligation reactions are necessary to excise the larger intervening sequence. Cell. 1979 Aug;17(4):915–921. doi: 10.1016/0092-8674(79)90331-3. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Khoury G. Deletion mutants of simian virus 40 defective in biosynthesis of late viral mRNA. Proc Natl Acad Sci U S A. 1979 Jan;76(1):71–75. doi: 10.1073/pnas.76.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer J., Shen C. K., Maniatis T. The chromosomal arrangement of human alpha-like globin genes: sequence homology and alpha-globin gene deletions. Cell. 1980 May;20(1):119–130. doi: 10.1016/0092-8674(80)90240-8. [DOI] [PubMed] [Google Scholar]
- Leder A., Miller H. I., Hamer D. H., Seidman J. G., Norman B., Sullivan M., Leder P. Comparison of cloned mouse alpha- and beta-globin genes: conservation of intervening sequence locations and extragenic homology. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6187–6191. doi: 10.1073/pnas.75.12.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Lewin B. Alternatives for splicing: recognizing the ends of introns. Cell. 1980 Nov;22(2 Pt 2):324–326. doi: 10.1016/0092-8674(80)90340-2. [DOI] [PubMed] [Google Scholar]
- Liebhaber S. A., Goossens M. J., Kan Y. W. Cloning and complete nucleotide sequence of human 5'-alpha-globin gene. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7054–7058. doi: 10.1073/pnas.77.12.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luse D. S., Roeder R. G. Accurate transcription initiation on a purified mouse beta-globin DNA fragment in a cell-free system. Cell. 1980 Jul;20(3):691–699. doi: 10.1016/0092-8674(80)90315-3. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquat L. E., Kinniburgh A. J., Beach L. R., Honig G. R., Lazerson J., Ershler W. B., Ross J. Processing of human beta-globin mRNA precursor to mRNA is defective in three patients with beta+-thalassemia. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4287–4291. doi: 10.1073/pnas.77.7.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Orkin S. H. The 3' untranslated regions of the duplicated human alpha-globin genes are unexpectedly divergent. Cell. 1980 Nov;22(2 Pt 2):371–377. doi: 10.1016/0092-8674(80)90347-5. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Alter B. P., Altay C., Mahoney M. J., Lazarus H., Hobbins J. C., Nathan D. G. Application of endonuclease mapping to the analysis and prenatal diagnosis of thalassemias caused by globin-gene deletion. N Engl J Med. 1978 Jul 27;299(4):166–172. doi: 10.1056/NEJM197807272990403. [DOI] [PubMed] [Google Scholar]
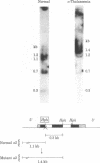
- Orkin S. H., Goff S. C. The duplicated human alpha-globin genes: their relative expression as measured by RNA analysis. Cell. 1981 May;24(2):345–351. doi: 10.1016/0092-8674(81)90324-x. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Michelson A. Partial deletion of the alpha-globin structural gene in human alpha-thalassaemia. Nature. 1980 Jul 31;286(5772):538–540. doi: 10.1038/286538a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Old J., Lazarus H., Altay C., Gurgey A., Weatherall D. J., Nathan D. G. The molecular basis of alpha-thalassemias: frequent occurrence of dysfunctional alpha loci among non-Asians with Hb H disease. Cell. 1979 May;17(1):33–42. doi: 10.1016/0092-8674(79)90292-7. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. The duplicated human alpha globin genes lie close together in cellular DNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5950–5954. doi: 10.1073/pnas.75.12.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Shander M. H., Manley J. L., Gefter M. L., Maniatis T. Structure and in vitro transcription of human globin genes. Science. 1980 Sep 19;209(4463):1329–1336. doi: 10.1126/science.6158093. [DOI] [PubMed] [Google Scholar]
- Rimm D. L., Horness D., Kucera J., Blattner F. R. Construction of coliphage lambda Charon vectors with BamHI cloning sites. Gene. 1980 Dec;12(3-4):301–309. doi: 10.1016/0378-1119(80)90113-4. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Directory of restriction endonucleases. Methods Enzymol. 1980;65(1):1–15. doi: 10.1016/s0076-6879(80)65003-4. [DOI] [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman J. G., Leder P. A mutant immunoglobulin light chain is formed by aberrant DNA- and RNA-splicing events. Nature. 1980 Aug 21;286(5775):779–783. doi: 10.1038/286779a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spritz R. A., Jagadeeswaran P., Choudary P. V., Biro P. A., Elder J. T., deRiel J. K., Manley J. L., Gefter M. L., Forget B. G., Weissman S. M. Base substitution in an intervening sequence of a beta+-thalassemic human globin gene. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2455–2459. doi: 10.1073/pnas.78.4.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkington C. A., Nishioka Y., Leder P. In vitro transcription of normal, mutant, and truncated mouse alpha-globin genes. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7132–7136. doi: 10.1073/pnas.77.12.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Curtis P. J., Tiemeier D. C., Leder P., Weissmann C. The intervening sequence of a mouse beta-globin gene is transcribed within the 15S beta-globin mRNA precursor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1309–1313. doi: 10.1073/pnas.75.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Tiemeier D. C., Seidman J. G., Peterlin B. M., Sullivan M., Maizel J. V., Leder P. Intervening sequence of DNA identified in the structural portion of a mouse beta-globin gene. Proc Natl Acad Sci U S A. 1978 Feb;75(2):725–729. doi: 10.1073/pnas.75.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Derbyshire R., Guy A., Molko D., Roget A., Téoule R., Chambon P. Specific in vitro transcription of conalbumin gene is drastically decreased by single-point mutation in T-A-T-A box homology sequence. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7024–7028. doi: 10.1073/pnas.77.12.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Kédinger C., Corden J., Brison O., Chambon P. Specific in vitro initiation of transcription on conalbumin and ovalbumin genes and comparison with adenovirus-2 early and late genes. Nature. 1980 Jun 5;285(5764):367–373. doi: 10.1038/285367a0. [DOI] [PubMed] [Google Scholar]
- Weatherall D. J., Clegg J. B. Recent developments in the molecular genetics of human hemoglobin. Cell. 1979 Mar;16(3):467–479. doi: 10.1016/0092-8674(79)90022-9. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]