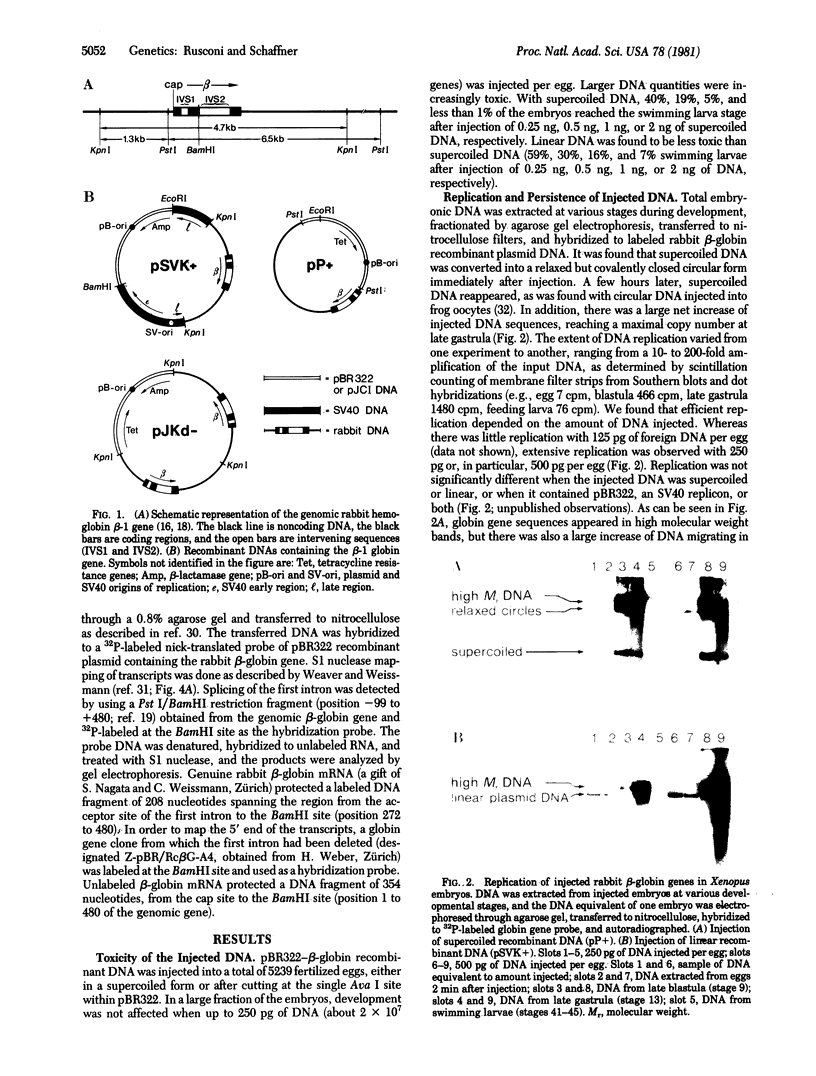
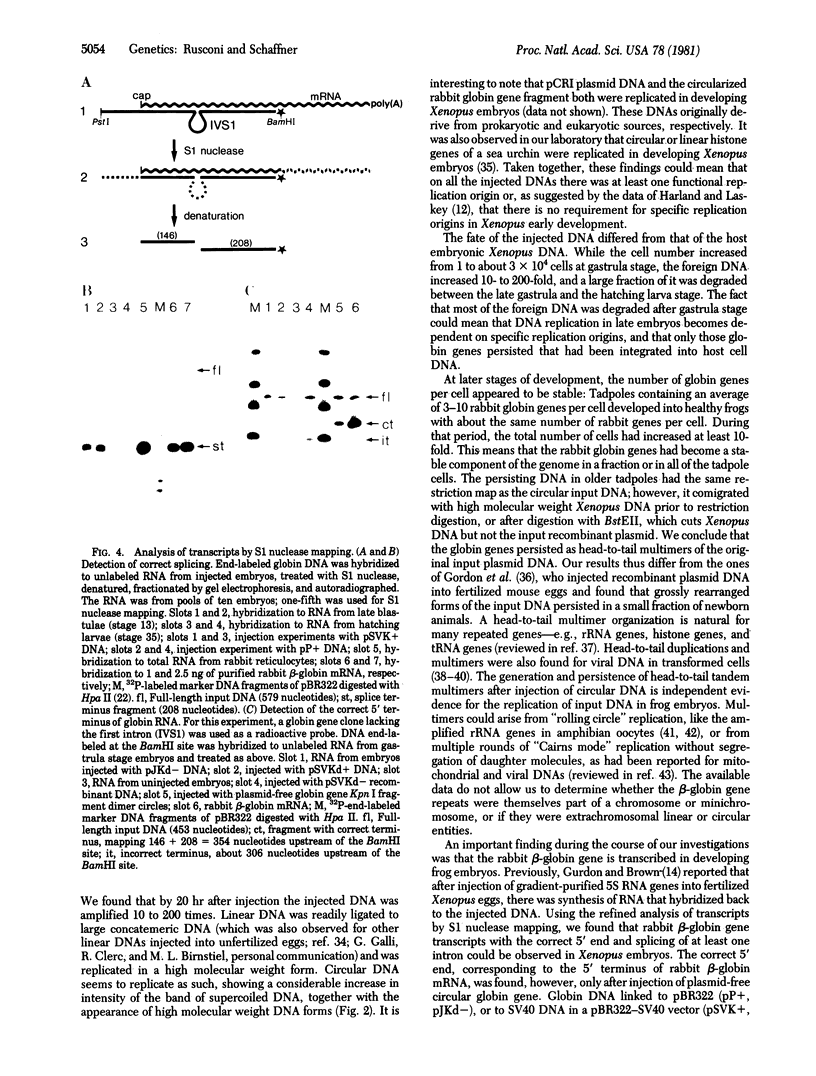
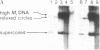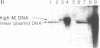Abstract
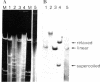
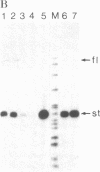
In order to study the fate and possible expression of foreign DNA during embryogenesis of the frog Xenopus laevis, we have injected a rabbit beta-globin gene into fertilized Xenopus eggs. Frog embryo DNA was extracted at various stages of development, fractionated by agarose gel electrophoresis, transferred to nitrocellulose filters, and hybridized to labeled beta-globin recombinant plasmid DNA. It was found that the injected DNA replicated extrachromosomally, reaching, at gastrula stage, a level equivalent to a 10- to 200-fold amplification of input DNA. At later stages, a majority of the foreign DNA was degraded, but a small fraction was maintained. Six-week-old tadpoles as well as six-month-old frogs contained an average of 3-10 copies of the rabbit globin gene per cell. Most of these persisting globin genes were present as long tandem repeats and comigrated in agarose gel electrophoresis with high molecular weight Xenopus DNA. Analysis of globin gene expression by S1 nuclease mapping showed that the rabbit beta-globin promoter was recognized in the frog embryo and that the transcripts were correctly spliced.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldi M. I., Benedetti P., Mattoccia E., Tocchini-Valentini G. P. In vitro catenation and decatenation of DNA and a novel eucaryotic ATP-dependent topoisomerase. Cell. 1980 Jun;20(2):461–467. doi: 10.1016/0092-8674(80)90632-7. [DOI] [PubMed] [Google Scholar]
- Benbow R. M., Krauss M. R., Reeder R. H. DNA synthesis in a multi-enzyme system from Xenopus laevis eggs. Cell. 1978 Feb;13(2):307–318. doi: 10.1016/0092-8674(78)90199-x. [DOI] [PubMed] [Google Scholar]
- Bendig M. M. Persistence and expression of histone genes injected into Xenopus eggs in early development. Nature. 1981 Jul 2;292(5818):65–67. doi: 10.1038/292065a0. [DOI] [PubMed] [Google Scholar]
- Birg F., Dulbecco R., Fried M., Kamen R. State and organization of polyoma virus DNA sequences in transformed rat cell lines. J Virol. 1979 Feb;29(2):633–648. doi: 10.1128/jvi.29.2.633-648.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. Studies on simian virus 40 excision from cellular chromosomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):709–719. doi: 10.1101/sqb.1979.043.01.079. [DOI] [PubMed] [Google Scholar]
- Cortese R., Melton D., Tranquilla T., Smith J. D. Cloning of nematode tRNA genes and their expression in the frog oocyte. Nucleic Acids Res. 1978 Dec;5(12):4593–4611. [PMC free article] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Mantei N., Weissmann C. DNA sequences preceding the rabbit beta-globin gene are required for formation in mouse L cells of beta-globin RNA with the correct 5' terminus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1411–1415. doi: 10.1073/pnas.78.3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. W., Scangos G. A., Plotkin D. J., Barbosa J. A., Ruddle F. H. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7380–7384. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Birnstiel M. L. Identification of regulatory sequences in the prelude sequences of an H2A histone gene by the study of specific deletion mutants in vivo. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1432–1436. doi: 10.1073/pnas.77.3.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Birnstiel M. L. Spacer DNA sequences upstream of the T-A-T-A-A-A-T-A sequence are essential for promotion of H2A histone gene transcription in vivo. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7102–7106. doi: 10.1073/pnas.77.12.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B., Birnstiel M. L., Speight V. A. The replication of purified DNA introduced into living egg cytoplasm. Biochim Biophys Acta. 1969 Feb 18;174(2):614–628. doi: 10.1016/0005-2787(69)90291-3. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Molecular biology in a living cell. Nature. 1974 Apr 26;248(5451):772–776. doi: 10.1038/248772a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Woodland H. R., Lingrel J. B. The translation of mammalian globin mRNA injected into fertilized eggs of Xenopus laevis I. Message stability in development. Dev Biol. 1974 Jul;39(1):125–133. doi: 10.1016/s0012-1606(74)80014-x. [DOI] [PubMed] [Google Scholar]
- Hardison R. C., Butler E. T., 3rd, Lacy E., Maniatis T., Rosenthal N., Efstratiadis A. The structure and transcription of four linked rabbit beta-like globin genes. Cell. 1979 Dec;18(4):1285–1297. doi: 10.1016/0092-8674(79)90239-3. [DOI] [PubMed] [Google Scholar]
- Harland R. M., Laskey R. A. Regulated replication of DNA microinjected into eggs of Xenopus laevis. Cell. 1980 Oct;21(3):761–771. doi: 10.1016/0092-8674(80)90439-0. [DOI] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourcade D., Dressler D., Wolfson J. The amplification of ribosomal RNA genes involves a rolling circle intermediate. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2926–2930. doi: 10.1073/pnas.70.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu H., Vinograd J. Replication of circular DNA in eukaryotic cells. Annu Rev Biochem. 1974;43(0):695–719. doi: 10.1146/annurev.bi.43.070174.003403. [DOI] [PubMed] [Google Scholar]
- Kressmann A., Clarkson S. G., Telford J. L., Birnstiel M. L. Transcription of xenopus tDNAmet1 and sea urchin histone DNA injected into the Xenopus oocyte nucleus. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1077–1082. doi: 10.1101/sqb.1978.042.01.108. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Gurdon J. B. Induction of polyoma DNA synthesis by injection into frog-egg cytoplasm. Eur J Biochem. 1973 Sep 3;37(3):467–471. doi: 10.1111/j.1432-1033.1973.tb03007.x. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Honda B. M., Mills A. D., Morris N. R., Wyllie A. H., Mertz J. E., De Roberts E. M., Gurdon J. B. Chromatin assembly and transcription in eggs and oocytes of Xenopus laevis. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):171–178. doi: 10.1101/sqb.1978.042.01.019. [DOI] [PubMed] [Google Scholar]
- Long E. O., Dawid I. B. Repeated genes in eukaryotes. Annu Rev Biochem. 1980;49:727–764. doi: 10.1146/annurev.bi.49.070180.003455. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Perlman S., Rosbash M. Analysis of Xenopus laevis ovary and somatic cell polyadenylated RNA by molecular hybridization. Dev Biol. 1978 Mar;63(1):197–212. doi: 10.1016/0012-1606(78)90125-2. [DOI] [PubMed] [Google Scholar]
- Probst E., Kressmann A., Birnstiel M. L. Expression of sea urchin histone genes in the oocyte of Xenopus laevis. J Mol Biol. 1979 Dec 15;135(3):709–732. doi: 10.1016/0022-2836(79)90173-6. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D., Bird A., Barkken A. Ribosomal RNA gene amplification by rolling circles. J Mol Biol. 1974 Aug 15;87(3):473–487. doi: 10.1016/0022-2836(74)90098-9. [DOI] [PubMed] [Google Scholar]
- Sagata N., Shiokawa K., Yamana K. A study on the steady-state population of poly(A)+RNA during early development of Xenopus laevis. Dev Biol. 1980 Jun 15;77(2):431–448. doi: 10.1016/0012-1606(80)90486-8. [DOI] [PubMed] [Google Scholar]
- Schaffner W. Direct transfer of cloned genes from bacteria to mammalian cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2163–2167. doi: 10.1073/pnas.77.4.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Taylor J. H. Cloning of an origin of DNA replication of Xenopus laevis. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5292–5296. doi: 10.1073/pnas.77.9.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
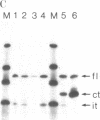
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold B., Wigler M., Lacy E., Maniatis T., Silverstein S., Axel R. Introduction and expression of a rabbit beta-globin gene in mouse fibroblasts. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5684–5688. doi: 10.1073/pnas.76.11.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H., Laskey R. A., Finch J., Gurdon J. B. Selective DNA conservation and chromatin assembly after injection of SV40 DNA into Xenopus oocytes. Dev Biol. 1978 May;64(1):178–188. doi: 10.1016/0012-1606(78)90069-6. [DOI] [PubMed] [Google Scholar]
- van Ooyen A., van den Berg J., Mantei N., Weissmann C. Comparison of total sequence of a cloned rabbit beta-globin gene and its flanking regions with a homologous mouse sequence. Science. 1979 Oct 19;206(4416):337–344. doi: 10.1126/science.482942. [DOI] [PubMed] [Google Scholar]
- van den Berg J., van Ooyen A., Mantei N., Schamböck A., Grosveld G., Flavell R. A., Weissmann C. Comparison of cloned rabbit and mouse beta-globin genes showing strong evolutionary divergence of two homologous pairs of introns. Nature. 1978 Nov 2;276(5683):37–44. doi: 10.1038/276037a0. [DOI] [PubMed] [Google Scholar]