Abstract
Successful human interaction is based on correct recognition, interpretation and appropriate reaction to facial affect. In depression, social skill deficits are among the most restraining symptoms leading to social withdrawal, thereby aggravating social isolation and depressive affect. Dysfunctional approach and withdrawal tendencies towards emotional stimuli have been documented but the investigation of their neural underpinnings has received limited attention. We performed an fMRI study including 15 depressive patients and 15 matched healthy controls. All subjects performed two tasks, an implicit joystick task as well as an explicit rating task both using happy, neutral, and angry facial expressions.
Behavioral data analysis indicated a significant group effect, with depressed patients showing more withdrawal than controls. Analysis of the functional data revealed significant group effects for both tasks. Amongst other regions, we observed significant group differences in amygdala activation with patients showing less response particularly during approach of happy faces. Additionally, significant correlations of amygdala activation with psychopathology emerged, suggesting that more pronounced symptoms are accompanied by stronger decreases of amygdala activation.
Hence, our results demonstrate that depressed patients show dysfunctional social approach and withdrawal behavior, which in turn may aggravate the disorder by negative social interactions contributing to isolation and reinforcing cognitive biases.
Keywords: depression, approach, withdrawal, emotion, amygdala, fMRI
Introduction
Emotional facial expressions are salient cues in social life and accurately recognizing, interpreting and responding to them is essential for successful social interaction. While evidence has accumulated on impaired facial emotion recognition and its dysfunctional neural underpinnings in patients suffering from depression (e.g., Dannlowski et al., 2007; Douglas & Porter, 2010; Suslow et al., 2010; for review see Leppänen, 2006), deficits in behavioral tendencies prompted by an emotional expression and the corresponding dysfunctional neural correlates have received limited attention in this clinical population. Regarding behavioral tendencies, Gray’s theory (Gray, 1982) of a behavioral approach (BAS) and a behavioral inhibition system (BIS) has been examined most extensively supposing two antipodal motivational systems: one appetitive (approach) and one aversive (withdrawal), both forming the basis of human behavior (cf. Elliot & Covington, 2001; Puca, Rinkenauer & Breidenstein, 2006). Though several aspects of this theory are still under debate (for overview see Corr, 2008), in its original version Gray (1972) postulated that the BIS is associated with punishment and frustrative non-reward and thus consequently with avoidance behavior. Contrary, the BAS is assumed to be related to reward and relief from punishment regulating appetitive motivation and approach behavior. It is widely acknowledged that the two systems do not function independently but instead influence and consequently inhibit each other (Gray, 1990; Gray & McNaughton, 2000). Due to this interaction, Gray (1994) speculated that depression is based on a combination of an elevated BIS and a decreased BAS sensitivity, with the BIS inhibiting the BAS, prompting a reduction in positive affect and appetitive motivation (Davidson, 1992), cognitive misinterpretations of social signals and disturbances in social interaction (for review see Segrin, 2000), eventually leading to social withdrawal and isolation. In this regard, several studies demonstrated a direct association between severity of depressive symptoms and self-reported BAS sensitivity (Campbell-Sills et al., 2004; Kasch et al., 2002). Moreover, supportive results stem from Tse and Bond (2004) who demonstrated that depressed patients tend to interpret social information in a negative way, feeling rejected by others and thus, avoid social interaction. Additionally, results from two prospective studies showed that self-reported BAS sensitivity was able to predict the clinical course of depression (Kasch et al., 2002; McFarland et al., 2006).
In a recent study from our lab (Seidel et al., 2010a) we investigated approach and withdrawal tendencies in depressed patients applying two different tasks: 1) an implicit joystick task, where participants were asked to pull or push the lever towards pictures of facial emotional expressions depending on the color of the frame encircling the expression, 2) an explicit rating task where subjects were instructed to indicate whether they would move towards or away from a person showing the presented emotional expression. Direct comparison of implicit and explicit results enabled analysis of more automatic vs. more conscious responses towards facial expressions of emotion. Although patients correctly recognized the emotional expressions they reacted differently to these social cues: especially female patients displayed stronger withdrawal tendencies in the explicit condition which was less pronounced in the implicit condition. Thus, we speculated that while automatic behavioral responses are still intact, conscious ratings seem to be negatively influenced by cognitive biases fitting Beck’s cognitive negativity model of depression (Beck, 2008).
Regarding the neural correlates, Gray (1982) assumed that a network comprising the septo-hippocampal system constitutes the BIS. Using fMRI, Reuter and colleagues (2004) demonstrated elevated activation of the amygdala, insula and temporo-frontal regions during viewing of disgust eliciting stimuli (BIS condition) in healthy controls. Thus, they reported activation of several regions know to be involved in various emotional competencies, such as emotion recognition (e.g., Derntl et al., 2009a,b,c; Fitzgerald et al., 2006; Habel et al., 2007; Moser et al., 2007, for review see Fusar-Poli et al., 2009) or empathy (e.g., Derntl et al., 2010; Lamm et al., 2007; Schulte-Rüther et al., 2008, for review see Lamm & Singer, 2009).
The neural network of the BAS is assumed to comprise the basal ganglia and the prefrontal cortex (Pickering & Gray, 1999) which is supported by results from electrophysiological studies demonstrating a relationship between asymmetrical frontal activity and self-reported BAS sensitivity (e.g., Coan & Allen, 2003; Harmon-Jones & Allen, 1997). Thus, these data support Davidson’s hypothesis that activity of the left prefrontal cortex is associated with the BAS whereas activity of the right prefrontal cortex is related to the BIS. Moreover, Davidson (1998) assumed that decreased left frontal activity reflects a reduced BAS constituting a vulnerability marker for depression. However, neuroimaging data explicitly prompting the BIS/BAS on behavioral tendencies in social situations in depressed patients are missing.
Since social withdrawal is one of the core symptoms in depression that affects multiple psychosocial domains, it is mandatory to further elucidate the neural underpinnings of behavioral approach and withdrawal tendencies towards facial emotional expressions. Therefore, the aim of the study is to investigate the neural correlates of social approach and withdrawal in depressed patients.
Based on our previous results (Seidel et al., 2010a) we hypothesized stronger withdrawal behavior in depressed patients as measured with self-report questionnaire data. Regarding the neural correlates, we expected stronger activation of patients during avoidance irrespective of emotion (in both tasks) particularly in brain regions involved in the BIS network, i.e. right prefrontal cortex and temporo-frontal regions. For the explicit task we hypothesized less amygdala activation for positive stimuli as shown in previous studies (e.g., Suslow et al., 2010).
Methods
Sample
Fifteen depressed patients (9 females, mean age = 34.1 years, SD = 12.0) fulfilling DSM-IV criteria for major depression and 15 healthy controls (9 females, mean age = 32.9 years, SD = 10.9) matched for gender, age and education participated. All subjects were native German speakers and right-handed as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971). The study was approved by the local Institutional Review Board and conducted according to the Declaration of Helsinki (1964). Written informed consent was obtained and all subjects were paid for participation (€30).
Depressed patients were recruited from the inpatient units of the Department of Psychiatry and Psychotherapy, University Hospital Aachen. They had no substance abuse for the last six months and no co-morbid psychiatric (axis I or axis II) or neurological disorder, as assessed by the German version of the Structured Clinical Interview for DSM IV (SCID, Wittchen, Zaudig & Fydrich, 1997). None of these patients experienced psychotic symptoms during the current or previous episodes. Severity of affective symptoms was assessed with the German versions of the Beck Depression Inventory (mean=25.6, SD=6.1; BDI, Beck et al., 1961) and the 17-item version of the Hamilton Depression Rating Scale (mean=19.9, SD=7.3; HAMD, Hamilton, 1960). The mean age of onset was 29.47 years (SD=10.8), with mean illness duration of 4.67 years (SD=7.1). All but two of the depressed patients were taking antidepressant medication at the time of testing (SSRI [n=2], SNRI [n=5], SSRI+SNRI [n=4], SNRI+quetiapine [n=2]).
The non-psychiatric control group consisted of 15 healthy adults (9 females) with no history of psychiatric (including substance abuse) or neurological illness. Subjects with such disorders or their first degree relatives were also excluded.
All participants additionally completed a neuropsychological test battery assessing crystallized verbal intelligence (Mehrfachwortwahltest – Version B [MWT-B], Lehrl, 1996), executive functions (Trail making test -A/-B [TMT-A/-B], Reitan, 1956), and working memory (digit span, Wechsler Adult Intelligence Scale [WAIS] III, Von Aster et al., 2006). Moreover, questionnaire data from the German version of the BIS/BAS scale by Carver and White (1994; Action Regulating Emotion Systems [ARES] scales, Hartig & Moosbrugger, 2003) were obtained.
Patients and controls differed significantly in their crystallized intelligence (MWT-B: t=3.293, p=.003), with patients showing lower scores, whereas performance in the other neurocognitive tasks did not differ significantly (TMT-A: t=1.274, p=.213; TMT-B: t=−0.013, p=.989; digit span forward: t=.555, p=.584; digit span backward: t=−.425, p=.675). Regarding group comparison of BIS/BAS scores, we observed significantly higher BAS scores in controls (t=5.713, p<.001) and significantly higher BIS scores in patients (t=10.241, p<.001). Demographic and neuropsychological characteristics are shown in Table 1.
Table 1.
Overview on demographic characteristics, neuropsychological performance (raw scores) and self-report questionnaire data of patients and controls. Moreover, for patients, mean values of the clinical rating scales are listed.
| Patients (n=15) | Controls (n=15) | t-values | p-values | |
|---|---|---|---|---|
| Gender (M:F) | 6:9 | 6:9 | - | - |
| Age (range) | 34.1 (11.95) | 32.9 (10.93) | 0.303 | 0.764 |
| Years of education | 16.1 (3.72) | 17.0 (4.00) | 0.614 | 0.544 |
| Verbal IQ | 106.9 (9.58) | 119.9 (12.84) | 3.293 | 0.003 |
| TMT-A (seconds) | 19.2 (3.76) | 20.6 (6.54) | 1.274 | 0.213 |
| TMT-B (seconds) | 40.1 (13.54) | 38.3 (11.28) | −0.013 | 0.989 |
| Digit span (raw score) | 15.6 (4.24) | 15.6 (4.52) | 0.009 | 0.993 |
| BIS | 33.4 (4.38) | 19.3 (2.94) | 10.24 | < 0.001 |
| BAS | 26.0 (4.47) | 33.8 (2.73) | 5.713 | < 0.001 |
| BDI | 25.6 (6.05) | |||
| HAMD | 19.9 (7.30) | |||
| GAF | 49.0 (9.49) | |||
| Mean age of onset | 29.5 (10.81) | |||
| Mean illness duration | 4.7 (7.12) | |||
Note. Standard deviations appear in parentheses. TMT = Trail Making Test, BIS = Behavioral inhibition scale, BAS = Behavioral activation scale, BDI = Beck Depression Inventory, HAMD = Hamilton Depression Rating Scale.
Moreover, we explored the ability to recognize facial expressions of emotions in patients and controls by applying an emotion identification task (“Vienna Emotion Recognition Tasks”, VERT-K; Derntl et al., 2008). It consists of 36 colored photographs of facial expressions of five basic emotions (happiness, sadness, anger, fear and disgust) as well as neutral expressions out of the same stimulus set (Gur et al., 2002). The stimulus material is balanced for valence and gender. Only evoked expressions were shown in randomized order. The instruction was to recognize the emotion depicted as soon and as accurately as possible and to choose one out of six possible emotion categories. The faces remained on the screen until the participant selected a label.
Functional tasks
Similar versions of the functional tasks have been validated in a recent behavioral study in patients with major depression and are described in more detail there (Seidel et al., 2010a). Briefly, we applied two tasks tapping the implicit and explicit behavioral tendencies separately.
Implicit joystick task
Experimental paradigms of studies investigating behavioral tendencies, i.e. approach and avoidance, are based on findings of Cacioppo, Priester, and Berntson (1993). It has been shown that pushing a lever is faster than pulling in response to aversive stimuli and pulling is faster than pushing in response to appetitive stimuli (Chen & Bargh, 1999; Duckworth, Bargh, Garcia, & Chaiken, 2002; Neumann & Strack, 2000). Based on these findings, implicit tendencies were measured using an MR-compatible joystick with Y-axis limitation (Mag Design and Engineering, Sunnyvale, CA). Sixty pictures of facial expressions for each condition (happy, angry, neutral) displayed by sixty different Caucasian actors (balanced for gender) were presented. These pictures were taken from the same standardized stimulus set that has been frequently applied in behavioral and neuroimaging studies (for development see Gur et al., 2002). All stimuli depicted evoked facial expressions. The faces were presented twice, once within a blue and once within a yellow frame. Subjects were instructed to pull the lever when the stimulus appeared with a blue frame yielding approaching behavior or to push the lever when the stimulus was framed with a yellow line yielding avoidance behavior, irrespective of facial expression. The randomly presented faces remained on the screen for a maximum of three seconds (or until a response is given) followed by a randomized, variable interstimulus interval (ISI) ranging from 1900ms to 5100ms in steps of 400ms (during which subjects viewed a fixation cross). Six runs, containing sixty pictures each, were separated by a short break where the scan has not been interrupted but the participant could take a break for 23s indicated by the word “break” on the screen and then for 4s the word “attention” reminded the subjects to prepare for the task again. To keep data comparable to previous studies (e.g. Marsh, Ambady & Kleck, 2005; Seidel et al., 2010a,b), we defined reaction time (RT) as the time from stimulus onset to when the lever reached its maximal point. The difference in RTs for pushing vs. pulling revealed the dominant behavioral tendency, i.e. approach or avoidance. To familiarize participants with the task, a short practice run was conducted inside the scanner using an asterisk stimulus within blue and yellow frames. See Figure 1a for illustration of the implicit task.
Figure 1.
Illustration of the implicit (a) and the explicit task (b). In the implicit task participants had to push the joystick lever when the face was surrounded by a yellow frame, whereas a blue frame indicated to pull the lever. In the explicit task, participants were instructed to decide whether they want to move towards or away from the person displayed by pressing the corresponding button.
Explicit rating task
In the explicit rating task, we presented 90 evoked expressions (30 per anger, happiness, and neutral, balanced for gender and only Caucasian actors) of 30 posers randomly taken from the joystick paradigm stimulus set. Here, participants were asked to imagine standing face to face with the person and to indicate whether they would approach, avoid or show no tendency at all by pressing the corresponding button out of the three possibilities (“+” standing for approach, ”−” indicating avoidance, and “=“ meaning no tendency). Stimuli were presented maximally for 5s with a randomized, variable ISI ranging from 1900ms to 5100ms in steps of 400ms (during which subjects viewed a fixation cross). Manual responses triggered immediate progression to the next ISI.
Participants were told not to refer their rating to the attractiveness or trustworthiness of the person but only to the emotional expression. The aim of this rating scale was to measure the conscious behavioral tendency compared to the automatic motor reaction time (joystick task). We have already validated this task in a sample of depressed patients (Seidel et al., 2010a) and healthy controls (Seidel et al., 2010b). See Figure 1b for an illustration of the explicit rating task.
All stimuli were presented using goggles (VisuaStimDigital, Resonance Technology Inc., Los Angeles, CA). The presentation of images, recording of responses and acquisition of scanner triggers was achieved using the Presentation© software package (Neurobehavioral Systems, Inc., Albany, CA).
Behavioral data analysis
Statistical analyses of behavioral data were performed using SPSS (Statistical Packages for the Social Sciences, Version 15.0, SPSS Inc., USA). Due to the significant difference in verbal IQ we included MWT-B scores as a covariate in the behavioral data analysis.
For the analysis of RTs (in milliseconds) of the motor responses in the implicit joystick task we computed a repeated measures ANOVA with expression (anger, happiness, neutral) and direction (approach, avoid) as within subject factors and diagnosis as between subject factor.
The analysis of group differences in the explicit rating task was similarly performed using a 2 (diagnosis) × 3 (expression) repeated measures ANOVA on the average of participants’ ratings. Correlations (partial correlations controlling for verbal IQ) were computed between clinical characteristics (BDI, HAM-D, GAF), questionnaire data (BIS, BAS) and reactions in the implicit or explicit task.
We also applied a 2 (diagnosis) × 6 (facial expression) repeated measures ANOVA on the performance in the emotion recognition task (percent correct) and added MWT-B scores as a covariate.
FMRI acquisition parameters and data processing
Data acquisition
Functional MR images were acquired on a 3T Siemens MRI whole body scanner (SIEMENS Trio) at the Department of Psychiatry and Psychotherapy, RWTH Aachen University. We used a standard head coil and foam paddings to reduce head motion. Functional imaging was performed using a gradient echo EPI sequence with the following BOLD imaging parameters: TR = 2200 ms, TE = 30 ms, FoV = 200 mm, 36 slices, slice thickness = 3.1 mm, in-plane resolution = 3.1×3.1 mm, flip angle = 90°, and distance factor = 15%.
Measurement time of the joystick task was about 30 minutes, the rating task took about 10 minutes. Additionally, a high-resolution structural image (3-D Magnetization Prepared Rapid Gradient Echo [MP-RAGE]) was acquired at the end of the measurement with the following parameters: TR = 1900 ms; TE = 2.52 ms; TI = 900 ms; flip angle = 9°; 256 matrix; FoV = 250 mm; 176 slices per slab, which took four minutes.
Data preprocessing
Five dummy scans before the beginning of the experiment were discarded to allow for magnetic saturation. Functional data processing was performed using the Statistical Parametric Mapping software (SPM5; Wellcome Department of Imaging Neuroscience, London, UK) implemented in Matlab (Mathworks Inc., Sherborn, MA, USA). Functional images were realigned to correct for head movement between scans by an affine registration (Ashburner & Friston, 2003). Each subject’s T1-scans were coregistered to the mean image of the realigned functional images. The mean functional image was subsequently normalized to the Montreal Neurological Institute (MNI) single-subject template (Evans et al., 1992; Collins, Neelin, Peters & Evans, 1994) using linear proportions and a nonlinear sampling as derived from a segmentation algorithm (Ashburner & Friston, 2005). Normalization parameters were then applied to the functional images and coregistered to the T1-image. Images were resampled at a 1.5 × 1.5 × 1.5 mm voxel size and spatially smoothed using an 8mm full-width-at-half-maximum Gaussian kernel.
For this event-related design, each of the six experimental conditions in the implicit task (anger approach, anger avoidance, happy approach, happy avoidance, neutral approach, neutral avoidance) and three conditions in the explicit task (angry, happy, and neutral faces) were modeled with a separate regressor convolved with the canonical hemodynamic response function and its first-order temporal derivative.
Statistical analysis was performed at the individual and group level. Since we were specifically interested in group differences in behavioral tendencies and their neural correlates, we explored neural activation with specific t-contrasts highlighting the significant group differences. Concerning the implicit task, we applied several t-contrasts directly comparing approach and avoidance behavior as well as specific interesting behavioral tendencies which were hypothesis driven, such as approaching happy and avoiding angry faces. For the explicit task we directly compared neural activation during processing of happy and angry faces (versus neutral faces) of patients and controls by applying independent sample t-tests.
ROI analysis
We performed a ROI analysis for the amygdala region with the aim of maximizing the sensitivity to group as well as hemispheric lateralization differences in the amygdala. Furthermore, we aimed to determine its exact role in approach and avoidance behavior. The amygdala has been chosen due to several reasons: it plays a major role in emotion processing and several studies have revealed dysfunctional amygdala activation in depressed patients during processing of facial expressions of emotions (e.g., Dannlowski et al., 2007; Suslow et al., 2010). Values for amygdala ROIs were extracted using the probabilistic cytoarchitectonic maps (Amunts et al., 2005), as available in the Anatomy toolbox in SPM5 (Eickhoff et al., 2005, 2006). Mean parameter estimates were extracted for left and right amygdala ROI in each condition and Levene tests for homogeneity of variances indicated homoscedasticity for all parameter estimates of all tasks (explicit: happy left: p = 0.623, happy right: p=.779; anger left: p=.990, anger right: p=.934, neutral left: p=.920, neutral right: p=.911; implicit: happy approach left: p=.939, happy approach right: p=.1.000, anger approach left: p=.702, anger approach right: p=.960, neutral approach left: p=.912, neutral approach right: p=.961, happy avoid left: p=.216, happy avoid right: p=.955, anger avoid left: p=.805, anger avoid right: p=.986, neutral avoid left: p=.901, neutral avoid right: p=.689).
A three-way ANOVA was applied with diagnosis as between-subject factor as well as condition and laterality as repeated factors. In order to control for the significant group difference in verbal IQ we included MWT-B scores as a covariate in the analysis. Greenhouse-Geisser corrected p-values are presented.
Corollary analyses: Correlation analyses were performed for each task between performance (reaction time [implicit task] and scores for approach/avoidance [explicit task]) and amygdala activation (mean parameter estimates taken from the ROI analysis) for the whole group. Moreover, amygdala activation was also correlated with performance in the self-report BIS/BAS scores. Additionally, for depressed patients we also analyzed any association between clinical characteristics (BDI, HAM-D, GAF) and amygdala parameter estimates.
In order to account for multiple comparisons we applied a combined height and extent threshold technique based on Monte-Carlo simulations using AlphaSim (Cox, 1996). According to 1000 simulations based on a height threshold of p<.001 (uncorrected) and the spatial properties of the residual image an extent threshold of 55 contiguous voxels suffices to comply with a family wise error of p<.05. This correction for thresholding will be referred to as "height and extent corrected threshold" (HET) and group results as well as direct comparisons between patients and controls are depicted at this threshold.
Results
Behavioral data
Implicit task
Applying the repeated measures ANOVA on the RT data revealed no significant emotion effect (F(2,56)=0.603, p=.551), no significant diagnosis effect (F(1,28)=1.390, p=.248), and no significant emotion-by-diagnosis interaction (F(2,56)=0.025, p=.976). Moreover, we observed neither a significant direction effect (F(1,28)=1.001, p=.327), nor a significant effect of VIQ (F(1,28)=0.013, p=.909, and no other significant interaction emerged (emotion-by-VIQ: F(2,56)=0.693, p=.505; direction-by-VIQ: F(1,28)=0.541, p=.469; direction-by-diagnosis: F(1,28)=0.002, p=.962; emotion-by-direction: F(2,56)=0.393, p=.677; emotion-by-direction-by-VIQ: F(2,56)=1.901, p=.162; emotion-by-direction-by-diagnosis: F(2,56)=0.833, p=.440).
Explicit task
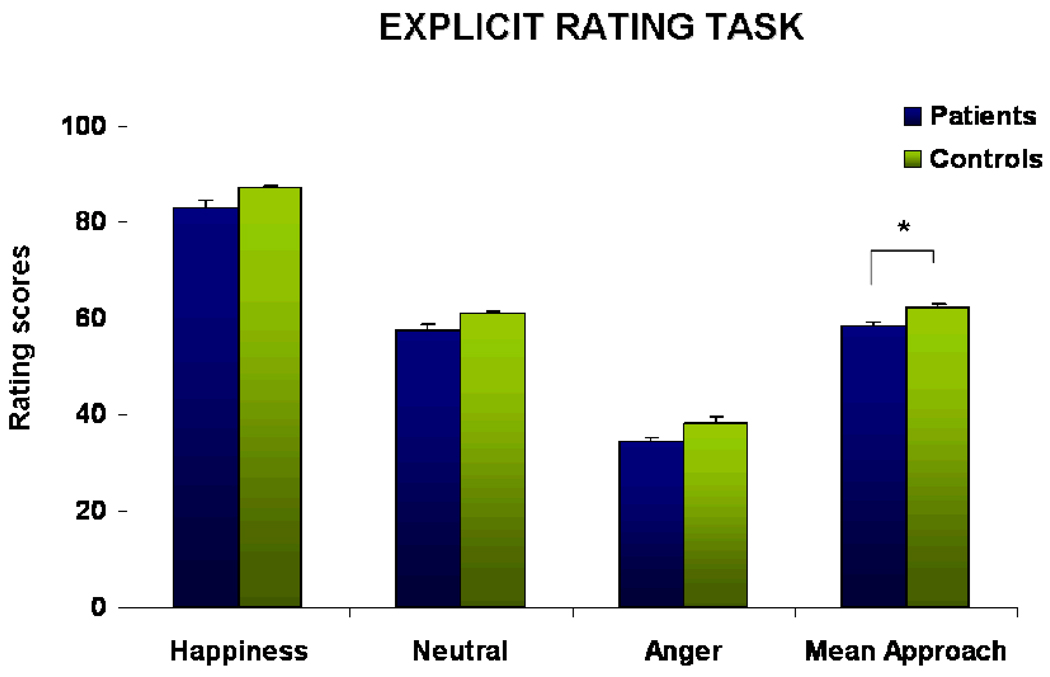
Analysis of the rating data including VIQ as a covariate showed a significant emotion effect (F(2,56)=11.495, p<.001, partial eta-sq=.315) with highest values and thus strongest approach towards happy faces followed by neutral expressions. Angry faces elicited least approach but strongest avoidance. Furthermore, we observed a significant diagnosis effect (F(1,28)=8.895, p=.006, partial eta-sq=.262), with stronger avoidance in patients, and no significant emotion-by-diagnosis interaction (F(2,56)=1.443, p=.246). VIQ showed no significant impact (F(1,28)=0.081, p=.779) and no significant emotion-by-VIQ interaction emerged (F(2,56)=3.514, p=.073). For illustration of the behavioral performance in the explicit task please see Figure 2.
Figure 2.
Results of the explicit rating task demonstrating approaching behavior towards happy, neutral, and angry facial expressions and a mean approach score. Data analysis revealed a significant group difference (p=.005), indicating stronger withdrawal in the patient group across all emotions.
Corollary analysis
Using partial correlations to control for VIQ differences, we analyzed whether behavioral performance showed any association with BIS/BAS scores and observed a significant positive correlation between BAS scores and happy ratings (r=0.586, p=.002) as well as a significant negative correlation between BIS scores and happy ratings (r=−0.502, p=.009) across the whole group. Correlations with neutral and angry ratings or with the implicit task did not reach significance (all ps>.09).
Further analysis between symptom severity (BDI and HAMD scores) and reaction times in the implicit task revealed no significant association (all ps>.12). However, correlation analysis for the explicit rating task and psychopathological parameters revealed significant associations between the HAMD scores and the rating results for happy faces (r=−0.602, p=.017) and for angry faces (r=0.725, p=.002). They indicate that patients with more severe symptoms show less approach towards happy faces and more avoidance towards faces expressing anger.
Emotion recognition
To clarify whether patients correctly classified the emotional facial expressions presented in the implicit and explicit task, serving as a necessary prerequisite to adequately fulfill the implicit and explicit task, we conducted a repeated measures ANOVA including VIQ as a covariate which revealed a significant emotion effect (F(5,140)=2.975, p=.030, partial eta-sq.=.115) with highest accuracy for happy and lowest for disgusted faces, a significant emotion-by-diagnosis interaction (F(5,140)=2.653, p=.042, partial eta-sq.=.103) but no significant effect of diagnosis (F(1,28)=1.764, p=.197), nor VIQ (F(1,28)=2.365, p=.138) or emotion-by-VIQ interaction (F(5,140)=2.245, p=.080). Post-hoc analysis disentangling the significant emotion-by-diagnosis interaction revealed only a significant difference for fear recognition (p=.001) with better accuracy in the patient group. All other comparisons remained not significant (all p>.298).
Functional data
Implicit task
The t-contrast comparing patients vs. controls for approach reactions showed more activation in the left calcarine gyrus (BA 17) and the left postcentral gyrus (BA 1). The reverse t-contrast showed that approach in controls (compared to patients) is associated more strongly with neural activation in the cerebellum bilaterally (vermis), the right calcarine gyrus (BA 17), the left middle temporal gyrus, the left superior frontal gyrus, the right temporo-parietal junction, the hippocampus bilaterally, and the left precuneus. Directly comparing patients vs. controls for avoidance reactions revealed stronger neural activation in the left calcarine gyrus, left postcentral gyrus and left paracentral lobule in the patient group. In controls (compared to patients) avoidance reactions yielded stronger neural responses of the cerebellum bilaterally, the right calcarine gyrus, the right middle frontal gyrus, right fusiform gyrus, the right lingual gyrus and the left superior frontal gyrus (for a detailed list see Table 2).
Table 2.
Significant group effects of approach and avoidance (threshold: t>3.14, p<.05 HET corr.) are given and regions are listed with MNI coordinates, cluster size (k), and t-values.
| Contrast | Cluster | MNI | t-value | K | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Approach | ||||||
| Patients > Controls | L. calcarine Gyrus | 0 | −89 | −6 | 6.01 | 520 |
| L. postcentral Gyrus | −44 | −33 | 63 | 4.16 | 226 | |
| Controls > Patients | R. Cerebellum (Vermis) | 3 | −59 | −12 | 5.31 | 1264 |
| R. calcarine Gyrus (BA 17) | 12 | −89 | 8 | 4.16 | 264 | |
| L. middle temporal Gyrus | −50 | −56 | 2 | 3.98 | 262 | |
| L. superior frontal Gyrus | −29 | −8 | 65 | 5.25 | 250 | |
| R. temporo-parietal Junction | 30 | −39 | 39 | 4.71 | 233 | |
| L. Hippocampus | −24 | −27 | −2 | 4.13 | 143 | |
| R. middle frontal Gyrus | 36 | 2 | 59 | 4.09 | 138 | |
| R. fusiform Gyrus | 24 | −42 | −20 | 4.05 | 119 | |
| L. Cerebellum | −23 | −77 | −26 | 4.10 | 106 | |
| R. superior parietal Lobe | 5 | −83 | 47 | 4.40 | 101 | |
| L. supramarginal Gyrus | −57 | −29 | 41 | 4.09 | 97 | |
| L. Precuneus | −9 | −75 | 56 | 3.62 | 68 | |
| R. Hippocampus | 30 | −8 | −38 | 3.71 | 65 | |
| R. lingual Gyrus | 27 | −89 | −15 | 4.04 | 58 | |
| R. precentral Gyrus (BA 44) | 62 | 11 | 21 | 4.49 | 57 | |
| Avoidance | ||||||
| Patients > Controls | L. calcarine Gyrus (BA 17) | 0 | −87 | −5 | 6.39 | 587 |
| L. postcentral Gyrus (BA 1) | −44 | −32 | 62 | 4.68 | 353 | |
| L. paracentral Lobule (BA 4) | −5 | −26 | 54 | 4.72 | 167 | |
| Controls > Patients | R. Cerebellum (Vermis) | 2 | −60 | −14 | 4.66 | 631 |
| R. calcarine Gyrus (BA 17) | 12 | −89 | 8 | 4.68 | 337 | |
| L. Cerebellum | −23 | −77 | −26 | 4.02 | 103 | |
| R. middle frontal Gyrus | 38 | 2 | 59 | 4.17 | 97 | |
| R. fusifom Gyrus | 36 | −45 | −9 | 3.87 | 90 | |
| R. lingual Gyrus (BA 18) | 27 | −89 | −15 | 4.42 | 87 | |
| L. superior frontal Gyrus | −29 | −8 | 65 | 4.10 | 66 | |
Since one major interest of our study was to analyze group differences in approaching happy and avoiding angry faces (i.e. the behavioral tendencies typically prompted by these emotions), we applied the respective t-contrasts. For approaching happy faces the t-contrast comparing patients vs. controls demonstrated that patients exhibited stronger activation of the left calcarine gyrus (BA 17), the left postcentral gyrus (BA 1), and the left inferior occipital gyrus. The reverse contrast showed that controls recruited the right cerebellum, the left superior frontal gyrus (BA 6), the right fusiform gyrus, and the right superior parietal lobule more strongly (for detailed information see Table 3).
Table 3.
Significant group effects of approaching happy and avoiding angry faces (threshold: t>3.14, p<.05 HET corr.) are given and regions are listed with MNI coordinates, cluster size (k), and t-values.
| Contrast | Cluster | MNI | t-value | k | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Approach HAPPY | ||||||
| Patients > Controls | L. calcarine Gyrus | 0 | −89 | −6 | 5.51 | 437 |
| L. postcentral Gyrus (BA 1) | −45 | −32 | 62 | 4.55 | 409 | |
| L. inferior occipital Gyrus | −51 | −77 | −9 | 4.29 | 73 | |
| Controls > Patients | R. Cerebellum | 3 | −59 | −14 | 4.74 | 326 |
| L. superior frontal Gyrus | −29 | −8 | 65 | 4.31 | 111 | |
| R. fusiform Gyrus | 32 | −80 | −3 | 4.02 | 61 | |
| R. superior parietal Lobe | 29 | −39 | 38 | 3.99 | 56 | |
| Avoid ANGER | ||||||
| Patients > Controls | L. calcarine Gyrus | 0 | −87 | −5 | 5.38 | 364 |
| L. postcentral Gyrus (BA 1) | −44 | −32 | 60 | 3.96 | 163 | |
| Controls > Patients | R. Cerebellum (Vermis) | 3 | −60 | −14 | 5.09 | 1126 |
| R. calcarine Gyrus (BA 17) | 12 | −89 | 8 | 4.87 | 436 | |
| L. Cerebellum | −24 | −77 | −26 | 3.81 | 178 | |
| L. SMA | 0 | 9 | 56 | 3.81 | 178 | |
| L. dorsal ACC | −8 | 17 | 33 | 3.72 | 153 | |
| R. fusiform Gyrus | 36 | −45 | −8 | 4.51 | 153 | |
| L. middle frontal Gyrus | 35 | 0 | 59 | 4.22 | 142 | |
| L. superior frontal Gyrus | −29 | −8 | 65 | 4.22 | 108 | |
| R. superior parietal lobe | 5 | −84 | 45 | 4.27 | 65 | |
| R. Insula | 38 | 0 | −5 | 3.77 | 61 | |
| Approach HAPPY vs. Avoid HAPPY | ||||||
| Patients > Controls | R orbitofrontal Gyrus | 30 | 39 | 3 | 4.28 | 66 |
| R supramarginal Gyrus | 50 | −48 | 30 | 3.77 | 57 | |
| Controls > Patients | L posterior cingulate Cortex | −21 | −51 | 18 | 4.46 | 238 |
| R posterior cingulate Cortex | 11 | −24 | 38 | 3.78 | 72 | |
| Avoid ANGER vs. Approach ANGER | ||||||
| Patients > Controls | L caudate Nucleus | −12 | 5 | 17 | 4.22 | 64 |
| R Cerebellum | 6 | −45 | −18 | 3.67 | 62 | |
| Controls > Patients | R orbitofrontal Gyrus | 14 | 26 | −17 | 4.18 | 67 |
| L inferior frontal Gyrus | −36 | −5 | 27 | 4.10 | 87 | |
| R inferior frontal Gyrus | 38 | −6 | 23 | 4.09 | 95 | |
For avoiding angry faces patients (compared to controls) showed stronger activation of the left calcarine gyrus (BA 17) and the left postcentral gyrus (BA 1). Controls (compared to patients) showed elevated activation in the cerebellum bilaterally, the right calcarine gyrus (BA 17), the left supplementary motor area, the left dorsal anterior cingulate, the right fusiform gyrus, the right middle and the left superior frontal gyrus, the right superior parietal lobule, and the right insula (for more details see Table 3).
Directly comparing approach vs. avoidance towards happy faces in patients vs. controls revealed significantly stronger activation of the right orbitofrontal gyrus and the right supramarginal gyrus in patients, while controls demonstrated elevated activation of the posterior cingulate bilaterally only (please see Table 3 for detailed information).
Additionally, we analyzed avoidance vs. approach of angry faces again directly comparing patients vs. controls. While patients recruited the left caudate and the right cerebellum, controls showed stronger activation of the inferior frontal gyrus bilaterally and the right orbitofrontal gyrus (please see Table 3 for detailed information).
Explicit task
Analysis of functional data of the explicit task by means of t-contrasts directly comparing patients and controls for each condition revealed significant group differences for angry and happy expressions (versus neutral, t=3.19, p<.05 HET corr.). During perception of angry faces and imagination of approaching or avoiding these faces, patients (compared to controls) showed stronger activation in the left precuneus, the right calcarine gyrus (BA 17) and the left posterior cingulate cortex. Controls (compared to patients) demonstrated elevated responses of a whole network of regions including the cerebellum bilaterally, the fusiform gyrus bilaterally, the left superior occipital gyrus, the right middle frontal gyrus, and the right cuneus.
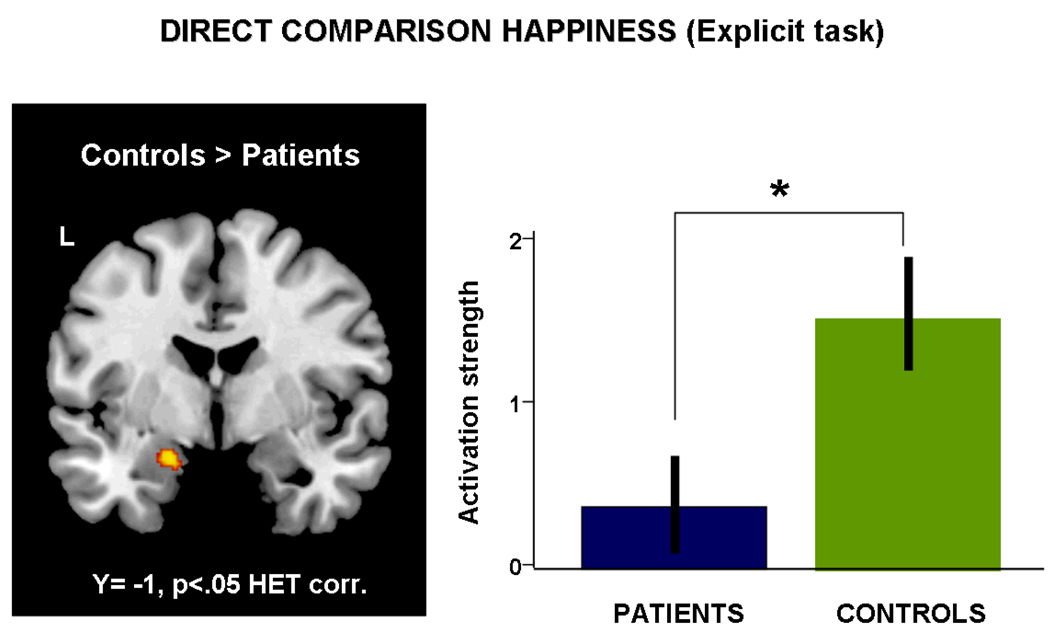
Comparing patients vs. controls for the happy condition, i.e. processing of happy faces and imaging moving towards or away from the face, we observed stronger activation of the right calcarine gyrus and the left inferior occipital gyrus in patients. Controls (compared to patients) showed a much more widespread network including the left lingual gyrus, the cerebellum bilaterally, and the left amygdala. Figure 4 depicts this significant difference in amygdala activation. For a detailed list of activated regions see Table 4.
Figure 4.
Results from the t-contrast directly comparing neural activation during processing of happy faces of patients and controls. Controls showed significantly stronger activation of the left amygdala (x,y,z: −18, −2, −2, k=69, t=4.06, p<.05 HET corr.) which is also apparent in the parameter estimates.
Table 4.
Significant group effects for the explicit task are given (t>3.19, p<.05 HET corr.) and regions are listed with MNI coordinates, cluster size (k), and t-values.
| Contrast | Cluster | MNI | t-value | k | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| HAPPY | ||||||
| Patients > Controls | R. calcarine Gyrus (BA 17) | 14 | −98 | −6 | 3.98 | 132 |
| L. inferior occipital Gyrus (BA 19) | −50 | −80 | −9 | 3.97 | 60 | |
| Controls > Patients | L. lingual Gyrus (BA 18) | −3 | −65 | 0 | 4.30 | 130 |
| L. Cerebellum | −5 | −63 | −20 | 3.80 | 119 | |
| L. Cerebellum | −8 | −47 | −11 | 4.14 | 85 | |
| R. Cerebellum | 18 | −44 | −48 | 4.04 | 71 | |
| L. Amygdala | −18 | −2 | −20 | 4.06 | 69 | |
| R. Cerebellum | 47 | −48 | −29 | 3.96 | 68 | |
| ANGER | ||||||
| Patients > Controls | - | - | - | - | - | - |
| Controls > Patients | L. Cerebellum | −8 | −77 | −32 | 4.92 | 868 |
| L. fusiform Gyrus | −39 | −57 | −21 | 4.53 | 239 | |
| L. Cerebellum | −2 | −65 | −2 | 5.56 | 206 | |
| R. fusiform Gyrus | 45 | −50 | −29 | 4.30 | 176 | |
| L. superior occipital Gyrus | −15 | −93 | 35 | 4.55 | 130 | |
| L. Cerebellum | −20 | −69 | −47 | 3.99 | 96 | |
| R. inferior frontal Gyrus | 50 | 30 | 14 | 3.71 | 95 | |
| R. Cerebellum | 38 | −75 | −24 | 4.21 | 88 | |
| L. Cerebellum | −3 | −60 | −21 | 4.03 | 68 | |
| L. Cerebellum | −8 | −47 | −11 | 3.97 | 56 | |
ROI analysis
Implicit task
Applying a repeated measures ANOVA including VIQ as a covariate revealed only a trend for a diagnosis effect (F(1,28)=3.735, p=.065, partial eta-sq.=.130) indicating stronger activation in the control group, while no other significant main effect (emotion: F(2,56)=1.555, p=.221; laterality: F(1,28)=0.210, p=.651; direction: F(1,28)=0.290, p=.595; VIQ: F(1,28)=1.495, p=.233) or interaction (all ps>.271) emerged.
Explicit task
The repeated measures ANOVA including VIQ as a covariate revealed a significant effect of diagnosis (F(1,28)=4.312, p=.043, partial eta-sq.=.122), with stronger amygdala activation in controls, but neither a significant emotion effect (F(2,56)=0.435, p=.650), nor laterality effect (F(1,28)=0.641, p=.431) or VIQ effect (F(1,28)=0.422, p=.522). Moreover, no interaction reached significance (all ps>. 224).
Corollary analyses
Implicit task
Since we observed no significant laterality effect we used the mean amygdala parameter estimates to investigate an association between BIS scores and avoidance of angry expressions and between BAS scores and approach of happy and neutral faces. We observed a significant positive association between BAS scores and amygdala activation during approach of happy faces (r=0.392, p=.024), however no other correlation reached significance (all ps>.170). Exploring the association of psychopathology (BDI, HAMD, GAF) with amygdala activation in the implicit task also revealed no significant correlation (all ps>.268).
Explicit task
Correlating mean amygdala parameter estimates with BIS/BAS values revealed a significant correlation between amygdala activation during perception of angry faces and BIS scores (r=−0.533, p=.050). However, BAS values did not show any significant association with processing of happy or neutral faces in the amygdala (all ps>.287).
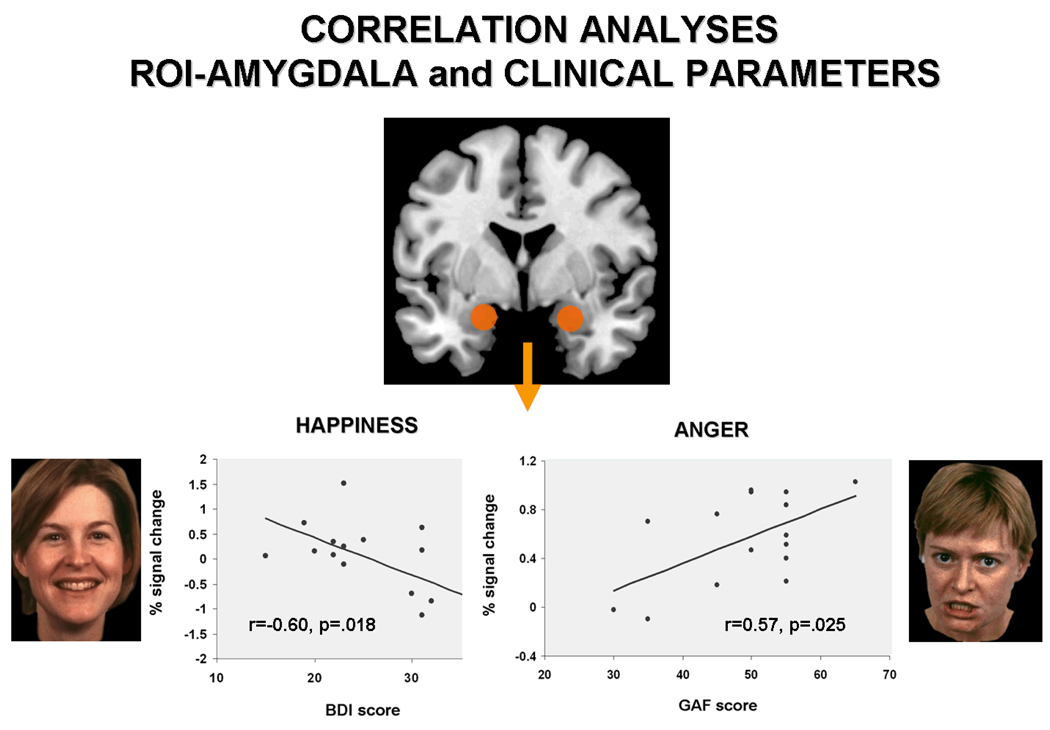
Analysis of associations between psychopathological rating scales and amygdala activation revealed significant negative correlations between BDI values and amygdala activation for happy faces (r=−0.600, p=.018). Stronger depressive symptomatology was associated with less amygdala involvement. Amygdala activation during perception of angry faces showed a significant positive association with global assessment of functioning scores (r=0.574, p=.025), hence a higher level of functioning indicated a stronger amygdala response to angry faces. HAMD scores did not show any significant association with amygdala response (all ps>.396). Figure 5 illustrates the significant correlations between amygdala activation and clinical parameters.
Figure 5.
Results from correlation analyses showing a significant negative association between amygdala activation during processing of happy faces and BDI scores (left), revealing that the more severe the symptoms (higher BDI scores) the less amygdala activation was observable. Moreover, a significant positive correlation between amygdala activation during processing of angry faces and global assessment of functioning (GAF) scores (right) emerged, indicating that the higher the GAF scores (the better patients cope with everyday living) the higher the amygdala activation.
Discussion
The aim of the present fMRI study was to examine the neural underpinnings of social approach and withdrawal in patients suffering from depression. Therefore, we explored behavioral approach and avoidance tendencies in response to evoked expressions of happy, neutral, and angry facial expressions. Moreover, in contrast to previous studies (e.g., Hasler et al., 2010; Kasch et al., 2002; McFarland et al., 2006), we directly compared rather automatic with more conscious behavioral reactions to these salient emotional cues by relying on an implicit as well as an explicit task.
In comparison to age-, gender-, and education-matched controls and despite unimpaired emotion recognition ability, we observed significant avoidance behavior in the patient sample that was accompanied by dysfunctional neural activation including the amygdala region.
Amygdala and social withdrawal
In the present study, we observed significantly less amygdala activation in patients compared to controls during approach and withdrawal in the explicit task, whereas in the implicit task only a trend in this direction emerged. Across all participants, correlation analysis revealed significant negative associations of the self-report BIS data with amygdala activation during processing of angry faces, indicating lower amygdala activation with higher BIS scores. In patients this finding was further extended to symptom severity and global functioning. BDI scores correlated negatively with amygdala activation during processing of happy expressions indicating that the more depressed patients feel the less amygdala participation they reveal during expected explicit approach towards positive faces. This specific problem of depressed patients is also reflected in significant correlations between amygdala activation and BDI scores, indicating that the more depressed patients feel the more they tend to avoid which will also be reflected in less amygdala activation. Correspondingly, the higher their level of functioning the more amygdala activation was demonstrated during explicit avoidance and approach of angry faces, thus the better patients cope with their life and everyday needs the more amygdala activation was demonstrated.
Dysfunctional activation of the amygdala, a central structure in the limbic emotion network, has been observed in several neuroimaging studies addressing emotion processing in patients with major depression. Mostly, previous studies reported a hyperactivation of the amygdala when patients were confronted with mood-congruent facial expressions, i.e. facial expressions depicting sadness (e.g., Phillips et al., 2003; Whalen et al., 2002). Recently, Suslow and colleagues (2010) applied a backward-masking design using happy and sad faces and neutral expressions as masks and observed this mood-congruency effect, i.e. elevated amygdala activation during processing of sad faces in patients, but also a decreased activation during processing of happy faces. Similarly, for approaching happy faces in the explicit task we also observed decreased amygdala activation of the patient sample. Suslow et al. (2010) assume that the significantly lower amygdala response to positive emotional faces might implicate less engagement in the encoding of positively valenced stimuli eventually leading to disturbed relationships in the sense of less attunement and mutual involvement (Bouhuys, Geerts, & Mersch, 1996; Surguladze et al., 2004). Hence, in addition to a stronger avoidance response to angry faces, depressed patients also withdraw from happy faces. Thus, patients also avoid persons with a positive, smiling expression which in most cultures (at least the Western culture all patients stem from) is considered the nonverbal sign of an invitation to join someone, to take a step closer, thus, a prompt which is followed by most people in everyday life. Hence, our results demonstrate that patients show an inadequate and abnormal behavioral tendency in a positive socio-emotional context.
Neural correlates of social withdrawal
Patients and controls performed equally well in the implicit task, however, group differences were detected for the neural correlates of approach and avoidance behavior.
Direct comparison of implicit avoidance vs. approach of angry faces in patients vs. controls revealed stronger activation of the left caudate and the right cerebellum in patients.
The left caudate nucleus a region known to be involved in reward processing (Balleine, Delgado & Hikosako, 2007) as well as feedback processing (Graybiel, 2005; Packard & Knowlton, 2002), and emotion regulation (e.g., Beer & Lombardo, 2007). Concerning emotion processing and depression, our results support findings from Scheuerecker et al. (2010) who reported stronger response of the left caudate nucleus in depressed patients vs. controls during an emotion matching task. Interestingly, increased activation of the caudate region has also been shown during emotional stress situations in cocaine-dependent patients (Li & Sinha, 2008). Probably, our task instructing patients to avoid and approach other people or at least simulate it with the joystick put them in a strong emotional stress situation reflected in the increased caudate activation. However, as mentioned by Beer and Lombard (2007) the putative role of the caudate in regulating emotions and stress responses is still speculative and needs further examination.
Explicit evaluation of angry faces elicited stronger activation of the left precuneus, the right calcarine gyrus, and the posterior cingulate. In their meta-analysis, Cavanna and Trimble (2006) demonstrate that activation of the anterior precuneus has repeatedly been observed during mental and motor imagery but evidence has accumulated that this region also plays a particular role in social cognition, self-agency, and self-processing (e.g., Kjaer et al., 2002; Lou et al., 2004; Koenigsberg et al., 2009; Vogeley & Fink, 2003). Moreover, den Ouden and colleagues (2005) speculate, that the precuneus together with the posterior cingulate is specifically involved in processing of intentions related to the self. Thus, patients seem to be specifically aware of the intention to withdraw from angry faces which was the major tendency in patients (and most controls).
Regarding social withdrawal in controls (compared to patients), we observed significantly stronger activation of the right orbitofrontal gyrus and the inferior frontal gyrus bilaterally.
Activity in the inferior frontal gyrus has been repeatedly observed during various emotional processes (e.g., passive viewing of faces, Dapretto et al., 2006; emotion recognition and evaluation, Carr et al., 2003; Seitz et al., 2008) and only recently has been reported to play a major role in emotional perspective taking (Schulte-Rüther et al., 2007, 2008; Derntl et al., 2010). Schulte-Rüther and colleagues (2007) assume that this activation might mirror the degree of interpersonal emotional involvement, suggesting that controls are able to stronger participate and respond emotionally when confronted with a salient stimulus communicating the request to go away (cf. Horstmann, 2003) thereby facilitating avoidance behavior (cf. Marsh, Ambady & Kleck, 2005). Thus,
Neural correlates of social approach
Analyzing approach vs. avoidance of happy faces revealed that patients (compared to controls) relied on activation of the right orbitofrontal gyrus and the right supramarginal gyrus. Only recently, Hsu et al. (2010) observed significant positive correlations between self-report scores on recent negative life stressors and activation of the orbitofrontal gyrus bilaterally during processing of negative words in depressed patients. Furthermore, Surguladze and colleagues (2010) observed stronger activation of this region in depressed patients during processing of facial expressions of strong disgust. According to findings from Kringelbach and Rolls (2004), the orbitofrontal gyri are specifically engaged in representing the emotional impact of anticipated negative outcomes. Moreover, this region has also been found to be ineffective in down-regulating amygdala activation during effortful reappraisal of negative stimuli (Johnstone et al., 2007). Hence, approaching someone, even when the person is smiling, seems to be associated with anticipated negative outcome and inefficient emotion regulation in patients suffering from depression.
Moreover, patients (compared to controls) recruited the right supramarginal gyrus more strongly during approach of happy faces. Activation of this region has consistently been observed during memory retrieval (e.g., Naghavi & Nyberg, 2005; Wagner, Shannon, Kahn & Buckner, 2005), and has been shown to mediate attention towards stimuli that are potentially important for the individual (e.g., Downar, Crawley, Mikulis & Davis, 2002). Consequently, Ciarimelli, Grady, and Moscovitch (2008) demonstrated elevated activation of the inferior parietal lobe, including the supramarginal gyrus, when individuals subjectively felt as if they are reliving their memories, were confident about their memories, and when these memory were strong. Depressed patients have to endure many negative experiences in social interaction, particularly regarding approach behavior, thus they may anticipate exclusion and memorize prior experiences of social rejection which might be reflected in the neural activation.
In controls (compared to patients), the posterior cingulate bilaterally was associated with approach towards happy faces. Notably, patients recruited this region more strongly when they processed angry faces in the explicit task, thereby mostly imagined to avoid these faces. The posterior cingulate cortex is an important node in the processing of social-affective stimuli (e.g., Amodio & Frith, 2006; Kross et al., 2007; Northoff & Bermpohl, 2004), is involved in emotional experience (Britton et al., 2006; Koenigsberg et al., 2009), emotion regulation, particularly distancing oneself from aversive images (Koenigsberg et al., 2010), and has recently been shown to be essential when forming social preferences (Chen et al., 2010).
Regions which show activation irrespective of behavioral tendency & emotional expression
Irrespective of behavioral tendency and emotional expression, patients showed stronger reactivity of the primary visual cortex (calcarine gyrus, BA 17) compared to controls. Interestingly, greater activation of this region to negative facial expressions has been reported to be associated with a good clinical outcome in depression (Keedwell et al., 2010). Additionally, patients exhibited stronger activation of the postcentral gyrus (BA 4) which has been repeatedly observed in studies investigating neural dysfunctions during processing of emotional faces in depression (e.g., Beevers et al., 2010; Fu et al., 2004). Concerning emotion processing, Adolphs (2002) indicated that the somatosensory cortex is essential for emotional contagion. Hence, this elevated activation of primary visual and primary somatosensory cortices might reflect intensified neural processing at a very basic level. Probably when confronted with emotional expressions and instructed to approach or avoid these faces, depressed patients not only show perceptual biases yielding stronger visual responses but are also affected more strongly emotionally.
Besides its association with various motor functions and even speech perception and production (e.g., Ackermann et al., 2007 for review), the cerebellum is also known to be involved in emotion processing (Schmahmann, 2000), emotional modulation of cognitive processing (Simpson et al., 2000), and emotional experience (Derntl et al., 2010; Hofer et al., 2006, 2007). We observed cerebellar activation during approach and avoidance in the implicit task in both groups, however, controls showed significantly stronger recruitment of this region irrespective of behavioral tendency. Together with further functional abnormalities (Naismith et al., 2010; Liu et al., 2010) one can speculate that this region not only plays a neglected role in emotion but also in the pathophysiology of depression.
Behavioral performance and self-report data
The behavioral performance partly corroborates previous results from our lab (Seidel et al., 2010) where we also observed that patients showed stronger avoidance during the explicit task, i.e. conscious focusing on approach or avoidance of the presented face. However, during the implicit task which rather prompts automatic behavioral tendencies no group difference was apparent. As hypothesized, we observed significantly higher BIS and lower BAS scores in patients, thereby supporting previous findings (e.g., McFarland et al., 2006; Kasch et al., 2002; Seidel et al., 2010a).
Hence, we assume that the self-reported behavioral tendencies and the performance in the explicit task are prone to perceptual and interpretative biases of depressed patients, who tend to consciously draw back from positive social context and hence putatively positive social experiences (Gable & Shean, 2000). Beevers (2005) postulated a dual process model of information processing in depression, proposing an associative mode, which acts automatically, and a reflective mode which is effortful and consumes cognitive resources. This model suggests that depressive cognition is characterized by a negatively biased automatic processing not corrected by the reflective mode. Our data indicate that with respect to the aberrant approach and avoidance tendencies in depression, and thus social withdrawal, the reflective mode (explicit task) is disturbed. These disturbances in consciously controlled aspects of social interaction might therefore be accessible to cognitive behavioral therapy (CBT) and dysfunctional behavioral tendencies to behavioral activation therapy (BAT). Hence our data have a clinical implication which should be addressed in greater detail in future studies.
Regarding emotion recognition we observed no significant group difference but a significant group-by-emotion interaction indicating that patients showed higher accuracy for fearful faces. We did not expect this finding, however, looking at the published studies reporting emotion recognition deficits in depression there are several differences in task design and sample characteristics that might explain the diversity in findings (for overview see Bourke, Douglas & Porter, 2010). While we used an explicit emotion recognition task showing 36 stimuli (6 per emotion and 6 neutral expression) with a forced-choice answering format, several other studies only showed a limited range of emotions including happiness, sadness and neutral expressions (e.g., Gur et al., 1992; Mikhailova et al., 1996), some relied on the face in the crowd task (e.g., Suslow et al., 2001, 2004) which does not directly assess recognition accuracy or presented schematic faces in an emotion discrimination task (e.g., Bouhuys et al., 1996). Moreover, we investigated depressed patients without any comorbidity which is in contrast to most other studies who included depressed patients with a comorbid anxiety disorder (e.g., Bouhuys et al., 1997; Gilboa-Schechtman et al., 2002) or even merged depressed and bipolar patients (e.g., Gur et al., 1992; Rubinow & Post, 1992). In the latter we reported emotion recognition deficits (Derntl et al., 2009d). Moreover, recent results from Anderson and colleagues (2011) indicate no significant difference in recognition accuracy between controls and currently depressed patients but showed that remitted patients performed significantly better than the two other groups. Thus, these results support our data showing no significant difference in emotion recognition performance between currently depressed patients and matched controls.
Limitations
Due to the small sample size, analysis of gender differences was not possible. However, depression in men and women differs in prevalence (e.g., Kessler et al., 2005), etiology (e.g., Piccinelli & Wilkinson, 2000), severity and symptom presentation (e.g., Smith et al., 2008). Moreover, in a previous study from our lab applying the implicit and explicit task to measure approach and avoidance behavior in depression (Seidel et al., 2010a) we observed significantly stronger social withdrawal in female patients. Future studies should explore whether these behavioral differences are accompanied by distinct neural responses further characterizing female and male depression. Moreover, due to the small sample size and the exploratory nature of most corollary analyses we did not apply an alpha correction for multiple correlations.
Considering the impact of medication on neural processing, it has recently been proposed that antidepressants rather modulate affective processing than directly affect mood (Harmer, 2010; Harmer, Goodwin & Cowen, 2009). Consequently, previous studies have repeatedly shown that antidepressant medication affects amygdala responses to emotional stimuli: while some observed reduced amygdala activation to negative stimuli (e.g., Fu et al., 2004; Harmer et al., 2006; Norbury et al., 2007; Sheline et al., 2001), others observed enhanced amygdala activation to positive faces (e.g., Fu et al., 2007; Norbury et al., 2009; Schaefer et al., 2006). We observed significantly less amygdala activation to happy and angry faces in the explicit task and a trend towards stronger amygdala activation irrespective of emotion in controls in the implicit joystick task. Thus, our data from medicated patients only partly support pervious findings. In light of the small number of fMRI studies addressing antidepressant treatment effects on the neural substrates of emotion processing in depressed patients and considering our small sample size with mixed medication it is hard to infer how medication influenced the current results. Therefore, future neuroimaging studies should highlight how and where antidepressants influence the neural correlates of emotional competencies, such as social approach and withdrawal.
The color of the frames (blue = pull, yellow = push) was not counterbalanced across subjects, thus we cannot rule out that color may represent a potential confound. Future studies should used counterbalanced designs to control for this issue.
Conclusion
This study aimed at investigating the behavioral and neural correlates of implicit and explicit social approach and withdrawal in patients suffering from major depression. We found stronger social withdrawal in depressed patients which was also reflected in stronger neural activation of a widespread network during avoidance of angry faces. Moreover, during approach patients show stronger activation of orbitofrontal and supramarginal gyrus, regions that have been associated with anticipated negative outcome and memory retrieval. We also observed a significant decrease in amygdala activation, particularly during processing of happy faces in patients. Additionally, the significant correlations between psychopathology and amygdala activation as well as behavioral data support the notion that more pronounced depressive symptoms are accompanied by stronger neural dysfunctions and inadequate behavioral tendencies. This in turn may aggravate the disorder by negative social interactions contributing to isolation and reinforcing cognitive biases.
Figure 3.
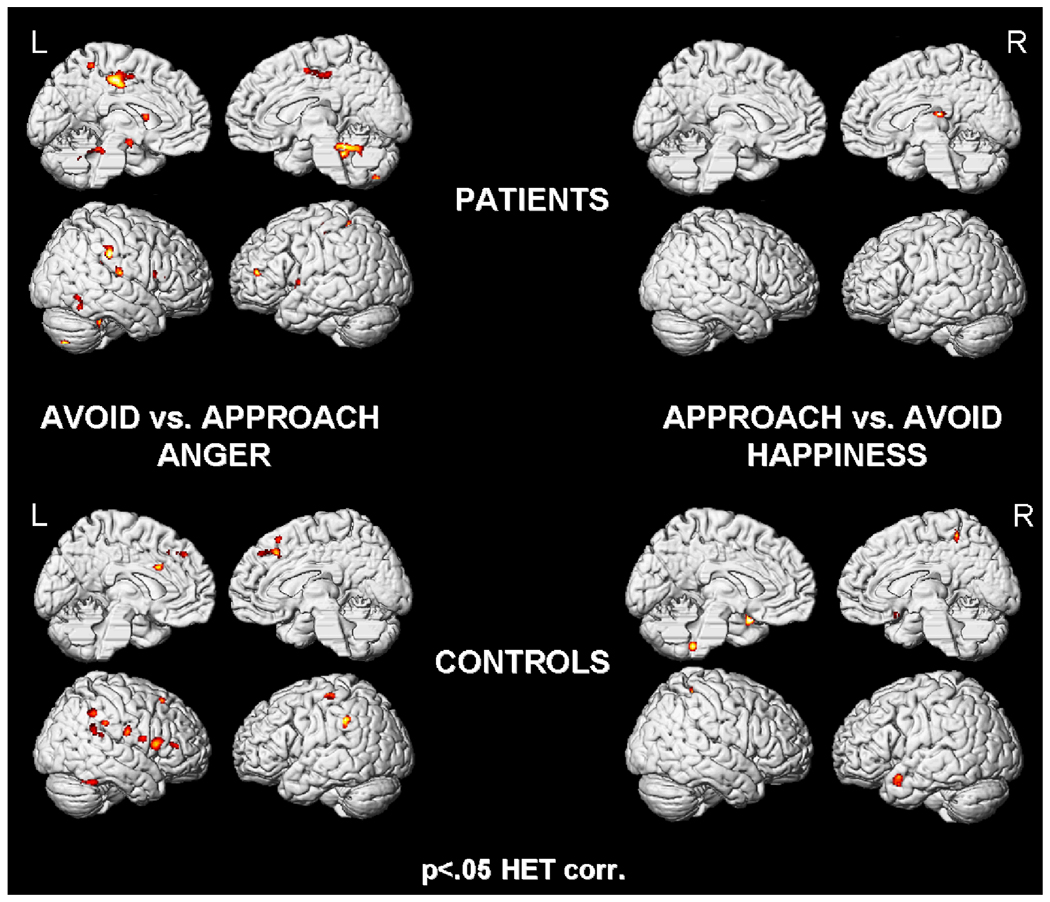
Results of the implicit task, revealing widespread activation in patients (top) and controls (bottom) for avoid vs. approach angry faces (left). On the right side, the direct comparison of approach vs. avoid happy faces is depicted with less activation in patients (top) than controls (bottom).
Acknowledgments
This study was supported by the Medical Faculty of the RWTH Aachen University (START 609811 to BD) and the DFG (IRTG 1328). SBE was supported by the Human Brain Project (R01-MH074457-01A1) and the Helmholtz-Initiative on Systems Biology. Work at the University of Pennsylvania was supported by Grant MH-60722 from the National Institutes of Health.
References
- Ackermann H, Mathiak K, Riecker A. The contribution of the cerebellum to speech production and speech perception: clinical and functional imaging data. Cerebellum. 2007;6(3):202–213. doi: 10.1080/14734220701266742. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Recognizing emotion from facial expressions: psychological and neurological mechanisms. Behavioral and Cognitive Neuroscience Reviews. 2002;1(1):21–62. doi: 10.1177/1534582302001001003. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and Embryology. 2005;210(5–6):343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Anderson IM, Shippen C, Juhasz G, Chase D, Thomas E, Downey D, Toth ZG, et al. State-dependent alteration in face emotion recognition in depression. British Journal of Psychiatry. 2011 doi: 10.1192/bjp.bp.110.078139. doi:10.1192/bjp.bp.110.078139. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Rigid body registration. In: Frackowiak RS, Friston KJ, Frith C, Dolan R, Price CJ, Zeki S, Ashburner J, Penny WD, editors. Human Brain function. London, UK: Academic Press; 2003. pp. 635–655. [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. Journal of Neuroscience. 2007;27(31):8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. American Journal of Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Beck AT, Erbaugh J, Ward CH, Mock J, Mendelsohn M. An Inventory for Measuring Depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beer JS, Lombardo MV. Insights into emotion regulation from neuropsychology. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford; 2007. pp. 69–86. [Google Scholar]
- Beevers CG, Clasen P, Stice E, Schnyer D. Depression symptoms and cognitive control of emotion cues: a functional magnetic resonance imaging study. Neuroscience. 2010;167(1):97–103. doi: 10.1016/j.neuroscience.2010.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG. Cognitive vulnerability to depression: a dual process model. Clinical Psychology Review. 2005;25(7):975–1002. doi: 10.1016/j.cpr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Bouhuys AL, Geerts E, Mersch PP. Relationship between perception of facial emotions and anxiety in clinical depression: does anxiety-related perception predict persistence of depression? Journal of Affective Disorders. 1997;43(3):213–223. doi: 10.1016/s0165-0327(97)01432-8. [DOI] [PubMed] [Google Scholar]
- Bouhuys AL, Geerts E, Mersch PP, Jenner JA. Nonverbal interpersonal sensitivity and persistence of depression: perception of emotions in schematic faces. Psychiatry Research. 1996;64(3):193–203. doi: 10.1016/s0165-1781(96)02930-7. [DOI] [PubMed] [Google Scholar]
- Bourke C, Douglas K, Porter R. Processing of facial emotion expression in major depression: a review. Australian and New Zealand Journal of Psychiatry. 2010;44(8):681–696. doi: 10.3109/00048674.2010.496359. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, Liberzon I. Neural correlates of social and nonsocial emotions: An fMRI study. NeuroImage. 2006;31(1):397–409. doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Priester JR, Berntson GG. Rudimentary determinants of attitudes .2. Arm flexion and extension have differential-effects on attitudes. Journal of Personality and Social Psychology. 1993;65(1):5–17. doi: 10.1037//0022-3514.65.1.5. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Liverant GI, Brown TA. Psychometric evaluation of the behavioral inhibition/behavioral activation scales in a large sample of outpatients with anxiety and mood disorders. Psychological Assessment. 2004;16(3):244–254. doi: 10.1037/1040-3590.16.3.244. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of Science U. S. A. 2003;100(9):5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TI. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain: A Journal of Neurology. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention to memory: a hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46(7):1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Chen AC, Welsh RC, Liberzon I, Taylor SF. 'Do I like this person?' A network analysis of midline cortex during a social preference task. NeuroImage. 2010;51(2):930–939. doi: 10.1016/j.neuroimage.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Bargh JA. Consequences of automatic evaluation: Immediate behavioral predispositions to approach or avoid the stimulus. Personality and Social Psychology Bulletin. 1999;25(2):215–224. [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology. 2003;40(1):106–114. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3d intersubject registration of mr volumetric data in standardized Talairach space. Journal of Computer Assisted Tomography. 1994;18(2):192–205. [PubMed] [Google Scholar]
- Corr PJ. The reinforcement sensitivity theory of personality. Cambridge University Press; 2008. [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computational and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W, et al. Amygdala reactivity to masked negative faces is associated with automatic judgmental bias in major depression: a 3 T fMRI study. Journal of Psychiatry & Neuroscience: JPN. 2007;32(6):423–429. [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9(1):28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition. 1992;20(1):125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Derntl B, Finkelmeyer A, Eickhoff S, Kellermann T, Falkenberg DI, Schneider F, Habel U. Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology. 2010;35(1):67–82. doi: 10.1016/j.psyneuen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Derntl B, Habel U, Windischberger C, Robinson S, Kryspin-Exner I, Gur RC, Moser E. General and specific responsiveness of the amygdala during explicit emotion recognition in females and males. BMC Neuroscience. 2009a;10:91. doi: 10.1186/1471-2202-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B, Habel U, Robinson S, Windischberger C, Kryspin-Exner I, Gur RC, Moser E. Amygdala activation during recognition of emotions in a foreign ethnic group is associated with duration of stay. Social Neuroscience. 2009b;4(4):294–307. doi: 10.1080/17470910802571633. [DOI] [PubMed] [Google Scholar]
- Derntl B, Windischberger C, Robinson S, Kryspin-Exner I, Gur RC, Moser E, Habel U. Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrinology. 2009c;34(5):687–693. doi: 10.1016/j.psyneuen.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Derntl B, Seidel E, Kryspin-Exner I, Hasmann A, Dobmeier M. Facial emotion recognition in patients with bipolar I and bipolar II disorder. British Journal of Clinical Psychology. 2009d;48(Pt 4):363–375. doi: 10.1348/014466509X404845. [DOI] [PubMed] [Google Scholar]
- Derntl B, Kryspin-Exner I, Fernbach E, Moser E, Habel U. Emotion recognition accuracy in healthy young females is associated with cycle phase. Hormones and Behavior. 2008;53(1):90–95. doi: 10.1016/j.yhbeh.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Douglas KM, Porter RJ. Recognition of disgusted facial expressions in severe depression. British Journal of Psychiatry. 2010;197:156–157. doi: 10.1192/bjp.bp.110.078113. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. Journal of Neurophysiology. 2002;87(1):615–620. doi: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- Duckworth KL, Bargh JA, Garcia M, Chaiken S. The automatic evaluation of novel stimuli. Psychological Science. 2002;13(6):513–519. doi: 10.1111/1467-9280.00490. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. NeuroImage. 2006;32(2):570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Evans AC, Marrett S, Neelin P, Collins L, Worsley K, Dai W, Milot S, Meyer E, et al. Anatomical mapping of functional activation in stereotactic coordinate space. Neuroimage. 1992;1(1):43–53. doi: 10.1016/1053-8119(92)90006-9. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: amygdala reactivity across multiple expressions of facial affect. NeuroImage. 2006;30(4):1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Williams SCR, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61(9):877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Williams SCR, Brammer MJ, Suckling J, Kim J, Cleare AJ, Walsh ND, et al. Neural responses to happy facial expressions in major depression following antidepressant treatment. The American Journal of Psychiatry. 2007;164(4):599–607. doi: 10.1176/ajp.2007.164.4.599. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- Gable SL, Shean GD. Perceived social competence and depression. Journal of Social and Personal Relationships. 2000;17:139–150. [Google Scholar]
- Gilboa-Schechtman E, Erhard-Weiss D, Jeczemien P. Interpersonal deficits meet cognitive biases: memory for facial expressions in depressed and anxious men and women. Psychiatry Research. 2002;113(3):279–293. doi: 10.1016/s0165-1781(02)00266-4. [DOI] [PubMed] [Google Scholar]
- Gray JA. Framework for a taxonomy of psychiatric disorder. In: van Goozen SHM, Van de Poll NE, editors. Emotions: Essays on emotion theory. Mahwah, NJ: Lawrence Erlbaum Associates, Inc.; 1994. pp. 29–59. [Google Scholar]
- Gray JA. Brain systems that mediate both emotion and cognition. Cognition & Emotion. 1990;4(3):269–288. [Google Scholar]
- Gray JA. The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system. Oxford: Oxford University Press; 1982. [Google Scholar]
- Gray JA. The psychophysiological basis of introversion-extraversion: A modification of Eysenck's theory. In: Nebylitsyn VD, Gray JA, editors. Biological bases of individual behavior. New York: Academic; 1972. pp. 182–205. [Google Scholar]
- Gray JA, McNaughton N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System. 2nd ed. USA: Oxford University Press; 2000. [Google Scholar]
- Graybiel AM. The basal ganglia: learning new tricks and loving it. Current Opinion in Neurobiology. 2005;15(6):638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. Journal of Neuroscience Methods. 2002;115(2):137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Gur RC, Erwin RJ, Gur RE. Neurobehavioral probes for physiologic neuroimaging studies. Archives of General Psychiatry. 1992;49(5):409–414. doi: 10.1001/archpsyc.1992.01820050073013. [DOI] [PubMed] [Google Scholar]
- Habel U, Windischberger C, Derntl B, Robinson S, Kryspin-Exner I, Gur RC, Moser E. Amygdala activation and facial expressions: explicit emotion discrimination versus implicit emotion processing. Neuropsychologia. 2007;45(10):2369–2377. doi: 10.1016/j.neuropsychologia.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A Rating Scale for Depression. Journal of Neurology Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ. Antidepressant drug action: a neuropsychological perspective. Depression and Anxiety. 2010;27:231–233. doi: 10.1002/da.20680. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. British Journal of Psychiatry. 2009;195:102–108. doi: 10.1192/bjp.bp.108.051193. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biological Psychiatry. 2006;59(9):816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJ. Behavioral activation sensitivity and resting frontal EEG asymmetry: covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology. 1997;106(1):159–163. doi: 10.1037//0021-843x.106.1.159. [DOI] [PubMed] [Google Scholar]
- Hartig J, Moosbrugger H. Die "ARES-Skalen" zur Erfassung der individuellen BIS- und BAS-Sensitivität: Entwicklung einer Lang- und einer Kurzfassung. Zeitschrift für Differentielle und Diagnostische Psychologie. 2003;24:291–308. [Google Scholar]
- Hasler BP, Allen JJB, Sbarra DA, Bootzin RR, Bernert RA. Morningness-eveningness and depression: preliminary evidence for the role of the behavioral activation system and positive affect. Psychiatry Research. 2010;176(2–3):166–173. doi: 10.1016/j.psychres.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer A, Siedentopf CM, Ischebeck A, Rettenbacher MA, Verius M, Felber S, Wolfgang Fleischhacker W. Sex differences in brain activation patterns during processing of positively and negatively valenced emotional words. Psychological Medicine. 2007;37(1):109–119. doi: 10.1017/S0033291706008919. [DOI] [PubMed] [Google Scholar]
- Hofer A, Siedentopf CM, Ischebeck A, Rettenbacher MA, Verius M, Felber S, Fleischhacker WW. Gender differences in regional cerebral activity during the perception of emotion: a functional MRI study. NeuroImage. 2006;32(2):854–862. doi: 10.1016/j.neuroimage.2006.03.053. [DOI] [PubMed] [Google Scholar]
- Horstmann G. What do facial expressions convey: feeling states, behavioral intentions, or action requests? Emotion. 2003;3(2):150–166. doi: 10.1037/1528-3542.3.2.150. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Langenecker SA, Kennedy SE, Zubieta J, Heitzeg MM. fMRI BOLD responses to negative stimuli in the prefrontal cortex are dependent on levels of recent negative life stress in major depressive disorder. Psychiatry Research. 2010;183(3):202–208. doi: 10.1016/j.pscychresns.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. Journal of Abnormal Psychology. 2002;111(4):589–597. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Drapier D, Surguladze S, Giampietro V, Brammer M, Phillips M. Subgenual cingulate and visual cortex responses to sad faces predict clinical outcome during antidepressant treatment for depression. Journal of Affective Disorders. 2010;120(1–3):120–125. doi: 10.1016/j.jad.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, Wang P, et al. Prevalence and treatment of mental disorders, 1990 to 2003. New England Journal of Medicine. 2005;352:2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. NeuroImage. 2002;17(2):1080–1086. [PubMed] [Google Scholar]
- Koenigsberg HW, Fan J, Ochsner KN, Liu X, Guise K, Pizzarello S, Dorantes C, et al. Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia. 2010;48(6):1813–1822. doi: 10.1016/j.neuropsychologia.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW, Fan J, Ochsner KN, Liu X, Guise KG, Pizzarello S, Dorantes C, et al. Neural correlates of the use of psychological distancing to regulate responses to negative social cues: a study of patients with borderline personality disorder. Biological Psychiatry. 2009;66(9):854–863. doi: 10.1016/j.biopsych.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kross E, Egner T, Ochsner K, Hirsch J, Downey G. Neural dynamics of rejection sensitivity. Journal of Cognitive Neuroscience. 2007;19(6):945–956. doi: 10.1162/jocn.2007.19.6.945. [DOI] [PubMed] [Google Scholar]
- Lamm C, Singer T. The social neuroscience of empathy. Annals of the New York Academy of Sciencies. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience. 2007;19(1):42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Lehrl S. Der MWT- ein Intelligenztest für die ärztliche Praxis. Praxis für Neurologie und Psychiatrie. 1996;7:488–491. [Google Scholar]
- Leppänen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Current Opinion in Psychiatry. 2006;19(1):34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- Li CR, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neuroscience and Biobehavioral Reviews. 2008;32(3):581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Xu C, Xu Y, Wang Y, Zhao B, Lv Y, Cao X, et al. Decreased regional homogeneity in insula and cerebellum: a resting-state fMRI study in patients with major depression and subjects at high risk for major depression. Psychiatry Research. 2010;182(3):211–215. doi: 10.1016/j.pscychresns.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, Sackeim HA, et al. Parietal cortex and representation of the mental Self. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(17):6827–6832. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Ambady N, Kleck RE. The effects of fear and anger facial expressions on approach- and avoidance-related behaviors. Emotion (Washington, D.C.) 2005;5(1):119–124. doi: 10.1037/1528-3542.5.1.119. [DOI] [PubMed] [Google Scholar]
- McFarland BR, Shankman SA, Tenke CE, Bruder GE, Klein DN. Behavioral activation system deficits predict the six-month course of depression. Journal of Affective Disorders. 2006;91(2–3):229–234. doi: 10.1016/j.jad.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Mikhailova ES, Vladimirova TV, Iznak AF, Tsusulkovskaya EJ, Sushko NV. Abnormal recognition of facial expression of emotions in depressed patients with major depression disorder and schizotypal personality disorder. Biological Psychiatry. 1996;40(8):697–705. doi: 10.1016/0006-3223(96)00032-7. [DOI] [PubMed] [Google Scholar]
- Moser E, Derntl B, Robinson S, Fink B, Gur RC, Grammer K. Amygdala activation at 3T in response to human and avatar facial expressions of emotions. Journal of Neuroscience Methods. 2007;161(1):126–133. doi: 10.1016/j.jneumeth.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Consciousness and Cognition. 2005;14(2):390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Naismith SL, Lagopoulos J, Ward PB, Davey CG, Little C, Hickie IB. Fronto-striatal correlates of impaired implicit sequence learning in major depression: an fMRI study. Journal of Affective Disorders. 2010;125(1–3):256–261. doi: 10.1016/j.jad.2010.02.114. [DOI] [PubMed] [Google Scholar]
- Neumann R, Strack F. Approach and avoidance: The influence of proprioceptive and exteroceptive cues on encoding of affective information. Journal of Personality and Social Psychology. 2000;79(1):39–48. doi: 10.1037//0022-3514.79.1.39. [DOI] [PubMed] [Google Scholar]
- Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ. Short-term antidepressant treatment and facial processing. Functional magnetic resonance imaging study. The British Journal of Psychiatry: The Journal of Mental Science. 2007;190:531–532. doi: 10.1192/bjp.bp.106.031393. [DOI] [PubMed] [Google Scholar]
- Norbury R, Taylor MJ, Selvaraj S, Murphy SE, Harmer CJ, Cowen PJ. Short-term antidepressant treatment modulates amygdala response to happy faces. Psychopharmacology. 2009;206(2):197–204. doi: 10.1007/s00213-009-1597-1. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8(3):102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- den Ouden HEM, Frith U, Frith C, Blakemore S. Thinking about intentions. NeuroImage. 2005;28(4):787–796. doi: 10.1016/j.neuroimage.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Piccinelli M, Wilkinson G. Gender differences in depression. Critical review. The British Journal of Psychiatry: The Journal of Mental Science. 2000;177:486–492. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- Pickering AD, Gray JA. Pervin L, John O. Handbook of Personality. 2nd Edition. New York: Guilford Press; 1999. The neuroscience of personality; pp. 277–299. [Google Scholar]
- Puca RM, Rinkenauer G, Breidenstein C. Individual differences in approach and avoidance movements: how the avoidance motive influences response force. Journal of Personality. 2006;74(4):979–1014. doi: 10.1111/j.1467-6494.2006.00400.x. [DOI] [PubMed] [Google Scholar]
- Rauch AV, Reker M, Ohrmann P, Pedersen A, Bauer J, Dannlowski U, et al. Increased amygdala activation during automatic processing of facial emotion in schizophrenia. Psychiatry Research. 2010;182(3):200–206. doi: 10.1016/j.pscychresns.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Trail Making Test: manual for administration, scoring and interpretation. Indianapolis: 1956. [Google Scholar]
- Reuter M, Stark R, Hennig J, Walter B, Kirsch P, Schienle A, Vaitl D. Personality and emotion: test of Gray's personality theory by means of an fMRI study. Behavioral Neuroscience. 2004;118(3):462–469. doi: 10.1037/0735-7044.118.3.462. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Post RM. Impaired recognition of affect in facial expression in depressed patients. Biological Psychiatry. 1992;31(9):947–953. doi: 10.1016/0006-3223(92)90120-o. [DOI] [PubMed] [Google Scholar]
- Schaefer HS, Putnam KM, Benca RM, Davidson RJ. Event-related functional magnetic resonance imaging measures of neural activity to positive social stimuli in pre- and post-treatment depression. Biological Psychiatry. 2006;60(9):974–986. doi: 10.1016/j.biopsych.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Schulte-Rüther M, Markowitsch HJ, Shah NJ, Fink GR, Piefke M. Gender differences in brain networks supporting empathy. NeuroImage. 2008;42(1):393–403. doi: 10.1016/j.neuroimage.2008.04.180. [DOI] [PubMed] [Google Scholar]
- Schulte-Rüther M, Markowitsch HJ, Fink GR, Piefke M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: a functional magnetic resonance imaging approach to empathy. Journal of Cognitive Neuroscience. 2007;19(8):1354–1372. doi: 10.1162/jocn.2007.19.8.1354. [DOI] [PubMed] [Google Scholar]
- Segrin C. Social skills deficits associated with depression. Clinical Psychology Review. 2000;20:379–403. doi: 10.1016/s0272-7358(98)00104-4. [DOI] [PubMed] [Google Scholar]
- Seidel E, Habel U, Finkelmeyer A, Schneider F, Gur RC, Derntl B. Implicit and explicit behavioral tendencies in male and female depression. Psychiatry Research. 2010a;177(1–2):124–130. doi: 10.1016/j.psychres.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Seidel E, Habel U, Kirschner M, Gur RC, Derntl B. The impact of facial emotional expressions on behavioral tendencies in women and men. Journal of Experimental Psychology. Human Perception and Performance. 2010b;36(2):500–507. doi: 10.1037/a0018169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz RJ, Schäger R, Scherfeld D, Friederichs S, Popp K, Wittsack HJ, et al. Valuating other people’s emotional face expression: a combined functional magnetic resonance imaging and electroencephalography study. Neuroscience. 2008;152(3):713–722. doi: 10.1016/j.neuroscience.2007.10.066. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry. 2001;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Ongür D, Akbudak E, Conturo TE, Ollinger JM, Snyder AZ, et al. The emotional modulation of cognitive processing: an fMRI study. Journal of Cognitive Neuroscience. 2000;12 Suppl 2:157–170. doi: 10.1162/089892900564019. [DOI] [PubMed] [Google Scholar]
- Singer T, Lamm C. The social neuroscience of empathy. Annals of the New York Academy of Sciences. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- Smith KLW, Matheson FI, Moineddin R, Glazier RH. Gender, income and immigration differences in depression in Canadian urban centres. Canadian Journal of Public Health. Revue Canadienne De Santé Publique. 2007;98(2):149–153. doi: 10.1007/BF03404328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze SA, El-Hage W, Dalgleish T, Radua J, Gohier B, Phillips ML. Depression is associated with increased sensitivity to signals of disgust: A functional magnetic resonance imaging study. Journal of Psychiatric Research. 2010 doi: 10.1016/j.jpsychires.2010.02.010. doi:10.1016/j.jpsychires.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze SA, Young AW, Senior C, Brébion G, Travis MJ, Phillips ML. Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology. 2004;18(2):212–218. doi: 10.1037/0894-4105.18.2.212. [DOI] [PubMed] [Google Scholar]
- Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schöning S, et al. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biological Psychiatry. 2010;67(2):155–160. doi: 10.1016/j.biopsych.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Suslow T, Dannlowski U, Lalee-Mentzel J, Donges U, Arolt V, Kersting A. Spatial processing of facial emotion in patients with unipolar depression: a longitudinal study. Journal of Affective Disorders. 2004;83(1):59–63. doi: 10.1016/j.jad.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Suslow T, Junghanns K, Arolt V. Detection of facial expressions of emotions in depression. Perceptual and Motor Skills. 2001;92(3 Pt 1):857–868. doi: 10.2466/pms.2001.92.3.857. [DOI] [PubMed] [Google Scholar]
- Tse WS, Bond AJ. The impact of depression on social skills. Journal of Nervous and Mental Disease. 2004;192(4):260–268. doi: 10.1097/01.nmd.0000120884.60002.2b. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Fink GR. Neural correlates of the first-person-perspective. Trends in Cognitive Sciences. 2003;7(1):38–42. doi: 10.1016/s1364-6613(02)00003-7. [DOI] [PubMed] [Google Scholar]
- Von Aster M, Neubauer A, Horn R. Hamburg-Wechsler-Intelligenz-Test für Erwachsene III. Harcourt: Frankfurt; 2006. [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, Somerville LH, McLean AA, Kim H. Functional neuroimaging studies of the amygdala in depression. Seminars in Clinical Neuropsychiatry. 2002;7(4):234–242. doi: 10.1053/scnp.2002.35219. [DOI] [PubMed] [Google Scholar]
- Wittchen H-U, Zaudig M, Fydrich T. Strukturiertes Klinisches Interview für DSM-IV. Hogrefe: Göttingen; 1997. [Google Scholar]