Abstract
Objective
The objective of this study was to determine the pharmacokinetics of lidocaine in a 48-hour infusion in patients undergoing cardiac surgery with cardiopulmonary bypass.
Design
A retrospective substudy of a clinical trial assessing the efficacy of intravenous lidocaine for postoperative cognitive decline.
Setting
University hospital.
Participants
99 patients undergoing cardiac surgery with cardiopulmonary bypass.
Interventions
After induction of anesthesia, lidocaine was administered as bolus of 1 mg/kg and followed by a continuous infusion at 4 mg/min for the first hour, 2 mg/min for the second hour, and 1 mg/min for the next 46 hours.
Measurements and Main Results
Blood samples were taken at baseline, end of cardiopulmonary bypass, and 24 and 48 hours after cardiopulmonary bypass for measurement of plasma concentration of lidocaine. Lidocaine levels increased significantly over time despite a constant rate of infusion (p < 0.05). The pharmacokinetics of lidocaine was best described by a two-compartment model and body weight was found to be a significant factor for the volume of the central compartment and clearance. The final pharmacokinetic parameters were V1(L) = 0.0619*weight, V2(L) = 187, CL1(L/min) =0.00419*weight, CL2(L/min) = 8.92.
Conclusions
A two-compartment pharmacokinetic model best describes the plasma concentrations of 48-hour lidocaine infusion in patients undergoing heart surgery with cardiopulmonary bypass. The inclusion of body weight as a covariate on clearance and central compartment improves the model. Lidocaine infusions should be dosed by body weight and decreased after 24 hours to avoid potential toxicity in long-term infusions.
Keywords: lidocaine, cardiopulmonary bypass, pharmacokinetic
Introduction
Population pharmacokinetic models have become increasingly popular for intravenous anesthetics. Assessing the variability in plasma concentrations between and within individuals through such modeling can identify factors that must be adjusted for in order to minimize risks of drug toxicity. To date, the pharmacokinetics of lidocaine has been defined only after bolus injections or short-term infusions.1-5 Much of the data also suggests that patients with congestive heart failure or hepatic disease have pronounced changes in the clearance of lidocaine.4,6 This reduction in lidocaine metabolic clearance can lead to increased accumulation during long-term infusion and enhance the likelihood of toxicity, particularly in patients with cardiovascular disease. Using data from a previously published clinical trial assessing the effect of lidocaine upon postoperative cognition,7 we therefore sought to develop a population pharmacokinetic model of lidocaine that may improve the accuracy and safety of longer-term lidocaine infusion during cardiac surgery with cardiopulmonary bypass (CPB).
Methods
After we received institutional review board approval and written informed consent from participants, patients scheduled to undergo coronary artery bypass grafting or an open chamber procedure with cardiopulmonary bypass (CPB) were enrolled into the primary clinical trial. Excluded from the study were patients with a history of symptomatic cerebrovascular disease, psychiatric illness, renal failure (serum creatinine > 2 mg/dL), liver disease (liver function tests > 1.5 times the upper limit of normal), higher alcohol consumption (> 2 drinks/day), those who were unable to read or had less than a seventh grade education, and patients undergoing circulatory arrest. Only the patients treated with lidocaine were assessed in the current substudy.
Lidocaine 1 mg/kg was administered as a bolus dose over 2 minutes after induction of anesthesia and followed immediately by a continuous infusion at 4 mg/min for the first hour, 2 mg/min for the second hour, and 1 mg/min for the next 46 hours. Anesthesia was induced and maintained with midazolam, fentanyl, and isoflurane while muscle relaxation was provided with pancuronium and/or vecuronium. All patients underwent nonpulsatile hypothermic (30° to 32°C) CPB with a membrane oxygenator and an arterial line filter. The pump was primed with crystalloid and prime volume was standard during the study period at 1500 ml. Serial hematocrit levels were kept at ≧0.21. Perfusion was maintained at pump flow rates of 2 to 2.4 L · min-1 · m2 throughout CPB to maintain mean arterial pressure at 50 to 80 mm Hg. Arterial blood gases were measured every 15 to 30 minutes to maintain arterial carbon dioxide partial pressures of 35 to 40 mm Hg, unadjusted for temperature (α-stat), and oxygen partial pressures of 150 to 250 mm Hg.
Blood samples were drawn from an indwelling arterial catheter at baseline and end of CPB and by venipuncture at 24 and 48 hours after CPB for measurement of lidocaine levels. A 10cc sample was first extracted from the arterial catheter to ensure that there was no contamination from dead space volume. The whole blood was centrifuged immediately and the plasma was stored at -70°Celsius until the point of analyses. The concentrations of monoethylglycinexylidide (MEGX), an active metabolite of lidocaine, were also measured at 48 hours after CPB. Primary study enrollment ended in April 2003 and lidocaine and MEGX levels were assayed in August 2008. Lidocaine and MEGX quantitative analyses were performed at NMS Labs (Willow Grove, PA) by gas chromatography with a nitrogen selective detector. In summary, internal standard (8-methoxyloxapine) was added to a 0.5-ml aliquot that was made strongly basic with ammonium hydroxide and extracted with 5% isopropanol in methylene chloride. The extracted samples were analyzed on a 15 meters × 0.32 mm I.D. capillary column with 0.15 micron DB-17 film (Agilent Technologies) using an Agilent 6890 gas chromatograph with nitrogen selective detection. Calibrators were run at concentrations of 0.05, 0.20, 0.50, 1.0, 2.5, 5.0 mcg/mL of each analyte in human serum, and were linear over that range. The inter-assay imprecision for lidocaine was 10.1 and 7.4 % at 1.0 and 3.5 mcg/mL, respectively. The inter-assay imprecision for MEGX was 6.8 and 5.6 % at 0.4 and 3.5 mcg/mL, respectively. Elution times were approximately 4.6, 5.7 and 8.4 minutes for lidocaine, MEGX and the internal standard, respectively.
Pharmacokinetic parameters were estimated using a nonlinear mixed effect model regression program, NONMEM version VI (Globomax LLC, Hanover, MD). The parameters of two or three compartment mammillary models, with input into and elimination from the central compartment, were fitted to the data. NONMEM not only estimates the structural pharmacokinetic parameters that describe the data set, but also the interindividual and intraindividual variability of the pharmacokinetic parameters. The interindividual variability was estimated using a log-normal distribution model. For the intra-individual variability, a constant coefficient of variation model was used to describe the residual errors resulting from assay errors, time-recording inaccuracy, and model misspecification. A global measure of fitness is the objective function based on the final parameter estimates in performing nonlinear regression analysis, which, in the case of NONMEM, is minus twice the log likelihood of the data. A model with a smaller objective function offers an improvement in the fitness; thus, during model building, a fall in objective function value of 3.84 (p < 0.05) when a single new parameter is introduced indicates that the new model has substantially improved the overall fitness.
To investigate possible relationships between parameter values and the potential covariates, the residuals were plotted against the potential covariates. Examination of these plots can provide us a visual basis for specifying the possible covariates that appear to affect the pharmacokinetic parameters. The following covariates were analyzed: age, gender, weight, and diabetes mellitus (DM) status. The covariates showing a correlation with the residuals would be further examined to determine which parameter should include the covariate. To assess the performances of the models with and without inclusion of the different covariates, bias and accuracy were examined. The weighted residual (WR) was calculated for each blood sample as:
| (Equation 1) |
where Cp is the measured concentration of lidocaine, and Cpred is the corresponding predicted concentration. The median weighted residual (MDWR) was used as an estimate of model bias:
| (Equation 2) |
where n = the total number of observations in the study. The median absolute weighted residual (MDAWR) was used to evaluate the precision of the models. The performance of the models with covariates was also assessed graphically using residual error plots to determine whether the overall accuracy of the model could be improved with the addition of one or more of these covariates.
NONMEM calculates the mean pharmacokinetic parameters of the population, interindividual and intraindividual variability, and the standard errors for all parameters in each run. If the covariance step was unsuccessful, then the 95% confidence intervals (CI) of the pharmacokinetic parameters were obtained by bootstrapping (sampling with replacement) method using PLT tools version 2.5 software (PLT tools, San Francisco, CA). This method consisted of repeatedly fitting the model to 1000 bootstrap replicates of the data. Then, the confidence intervals were determined by the range of parameter estimates that cover 95% of the values from these 1000 runs. A post-hoc simulation with the final pharmacokinetic parameters to determine whether the protocol of lidocaine administration should be adjusted to avoid lidocaine toxicity (i.e. concentration >5 μg/ml) was also performed using NONMEM.8
Comparisons of lidocaine levels between the 4 sampling times were performed using analysis of variance, followed by Tukey's post hoc test. P < 0.05 was considered significant.
Results
Of the 114 subjects allocated to the lidocaine arm of the primary trial, 106 had their blood samples assayed for lidocaine levels. Seven subjects with apparent outlier data points, likely resulting from sampling in the same vein as the infusion were excluded leaving samples from 99 patients in the final pharmacokinetic analyses using NONMEM. Demographic characteristics of these 99 subjects are listed in Table 1. Isoflurane was administered at a dose of 0.5-1.0%, fentanyl at 14.4 ± 5.7 mcg/kg, midazolam at 0.09 ± 0.05 mg/kg, and pancuronium at 0.17 ± 0.07 mg/kg or vecuronium at 0.21 ± 0.13 mg/kg. Figure 1 shows the plasma concentration-time profile of lidocaine. The mean plasma concentrations of lidocaine at baseline, end of CPB, 24 hours after CPB, and 48 hours after CPB were 0, 2.32±0.63, 2.56±0.86, and 3.34±0.91 μg/ml, respectively. Plasma concentrations at 48 hours after CPB were significantly higher than the other 3 timepoints (p < 0.05). In addition, the measured concentrations of MEGX on 48 hours after CPB were 0.25±0.15 μg/ml and the ratio of serum levels of MEGX to lidocaine was 8.9±5.4%.
Table 1. Demographic Characteristics.
| Variables | Mean (SD) |
|---|---|
| Age, y (SD) | 61.4 (12.0) |
| Gender, % female | 28.3 |
| Race, % white | 90.1 |
| Weight, kg (SD) | 86.1 (23.8) |
| History of hypertension, % | 60.4 |
| Diabetes, % | 22.8 |
| Previous MI, % | 25.7 |
| CHF (%) | 52.3 |
| Preop AFIB (%) | 20.8 |
| Ejection fraction (SD) | 50.3 (13.1) |
| Surgical Procedure, % | |
| CABG | 44.5 |
| CABG+Valve | 19.8 |
| Valve | 35.6 |
| Redo surgery, % | 12 |
| No. grafts (SD) | 1.9 (1.7) |
| Cross-clamp time, min (SD) | 98.7 (49.3) |
| CPB time, min (SD) | 167.7 (72.9) |
CPB=cardiopulmonary bypass, MI=myocardial infarction, CABG= coronary artery bypass grafting, CHF=congestive heart failure, SD=standard deviation, AFIB=atrial fibrillation.
Figure 1.
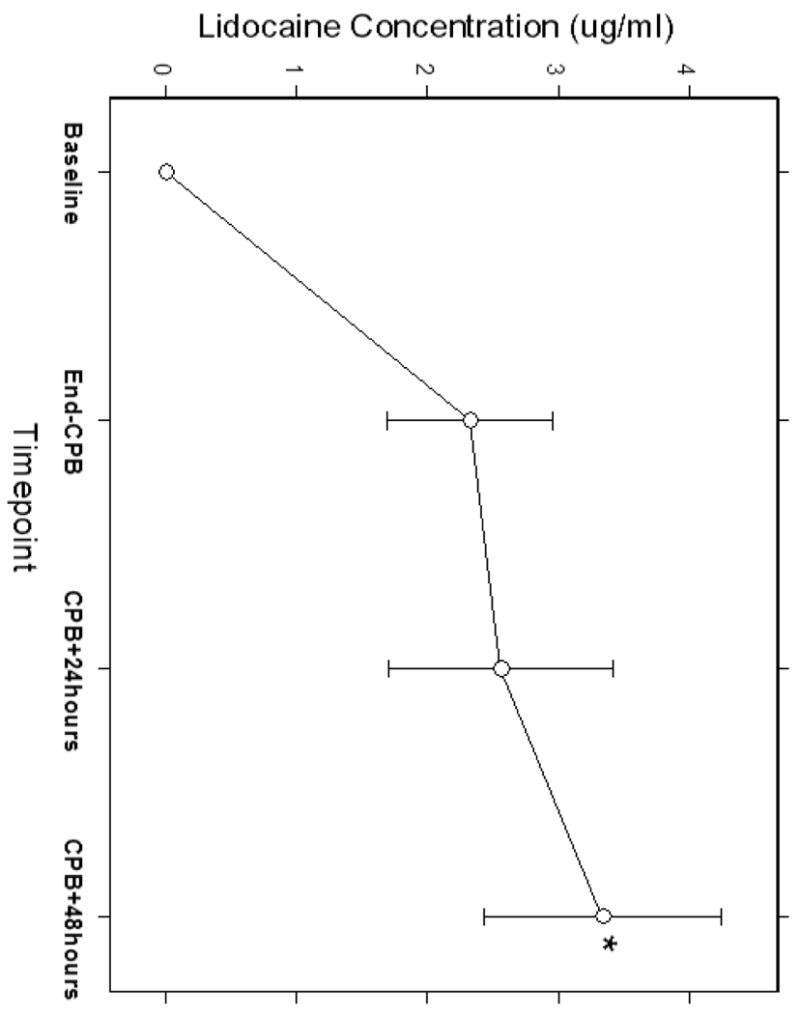
The plasma concentration-time profile of lidocaine demonstrating increasing concentrations with increased duration of lidocaine infusion. Plasma concentrations at 48 hours after CPB were significantly higher than the other 3 timepoints. (p < 0.05)
Pharmacokinetic analysis revealed that the 3-compartment linear PK model was unsuccessful because the model was unstable and exhibited convergence difficulties. The two-compartment model was therefore used for subsequent analysis. The pharmacokinetic parameters estimated included central compartment (V1), clearance (CL1), peripheral compartment (V2), and intercompartmental clearance (CL2). A two-compartment linear model best described the time course of plasma lidocaine concentration during and after CPB. Covariate analysis suggested body weight was positively correlated with clearance (CL1) and central compartment (V1). The inclusion of body weight as a covariate on CL1 and V1 resulted in improvements in goodness of fit. No influence of age, gender, and DM status could be found for the analyzed subjects. Figure 2 shows the correlation between body weight and weighted residuals suggesting that body weight is a potential covariate. The pharmacokinetic parameters of the models with and without body weight as a covariate are shown at Table 2. The precision (MDAWR) was 20.2% and the bias (MDWR) was -1.4% of the final model. Figure 3 and 4 are model performance and diagnostic plots. Figure 3 shows the predicted lidocaine concentration calculated with the parameters derived from the final model plotted against the measured lidocaine concentrations. The weighted residual was calculated for each sample and plotted against the corresponding predicted concentration (Figure 4).
Figure 2.
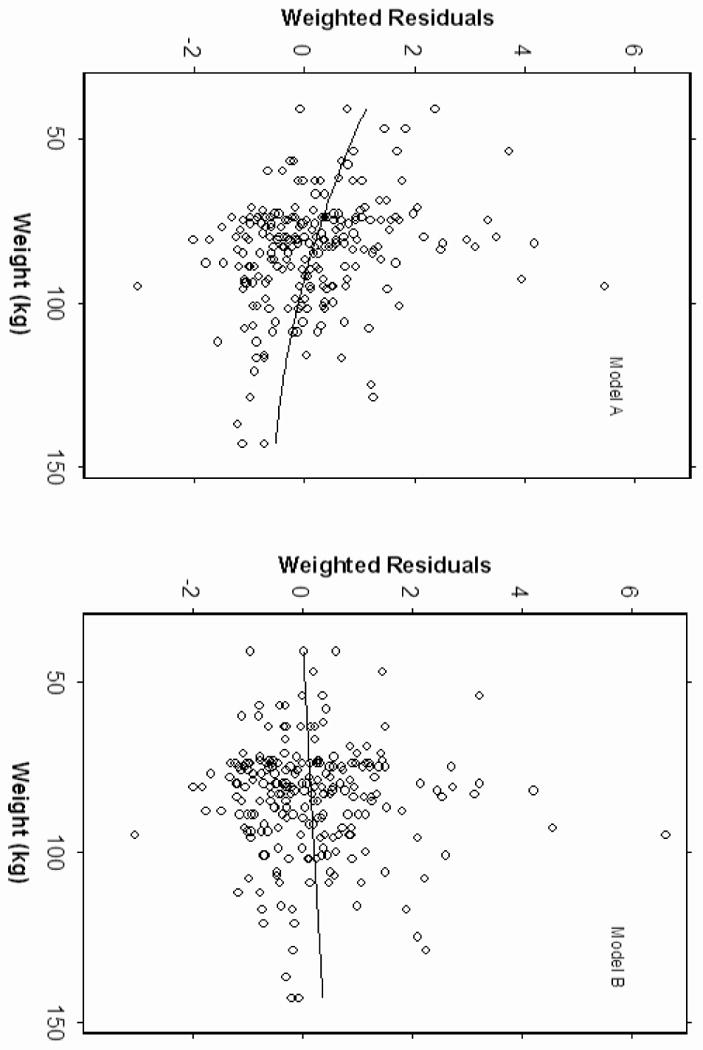
Diagnostic plots of weighted residuals versus body weight. The plot of model A indicates the weighted residuals are correlated with body weight. Model B includes body weight as a covariate.
Table 2. Pharmacokinetic Parameters of the Models with and without Weight as Covariates.
| Model A | Model B | |
|---|---|---|
| PK parameters | CL1=THETA(1)*EXP(ETA(1)) | CL1=THETA(1)*WT *EXP(ETA(1)) |
| V1=THETA(2)*EXP(ETA(2)) | V1=THETA(2)*WT*EXP(ETA(2)) | |
| CL2=THETA(3)*EXP(ETA(3)) | CL2=THETA(3)*EXP(ETA(3)) | |
| V2=THETA(4)*EXP(ETA(4)) | V2=THETA(4)*EXP(ETA(4)) | |
| CL1 | 0.352 | 0.00419*WT |
| CL2 | 7.5 | 8.92 |
| V1 | 4.85 | 0.0619*WT |
| V2 | 185 | 187 |
| Variance of Epsilon | 22.7% | 22.8% |
| OBJ | 126 | 117 |
| MDWR(%) | 1.9 | -1.4 |
| MDAWR(%) | 22.0 | 20.2 |
V1 = volume of the central compartment, V2 = volume of the peripheral compartment, CL1 = elimination clearance, CL2 = intercompartmental clearance, WT=weight, OBJ=objective function, MDWR=median weighted residual, MDAWR= median absolute weighted residual
Figure 3.
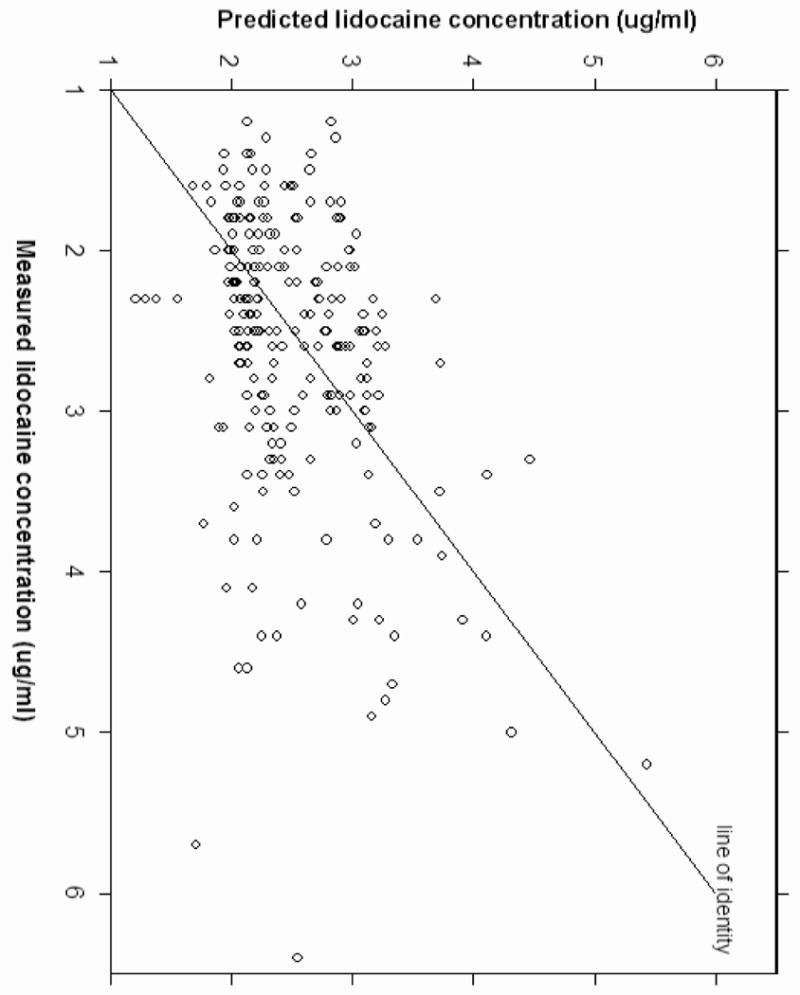
Model performance plot indicates good correlation between the measured lidocaine concentrations and the predicted lidocaine concentration calculated with the final parameters.
Figure 4.
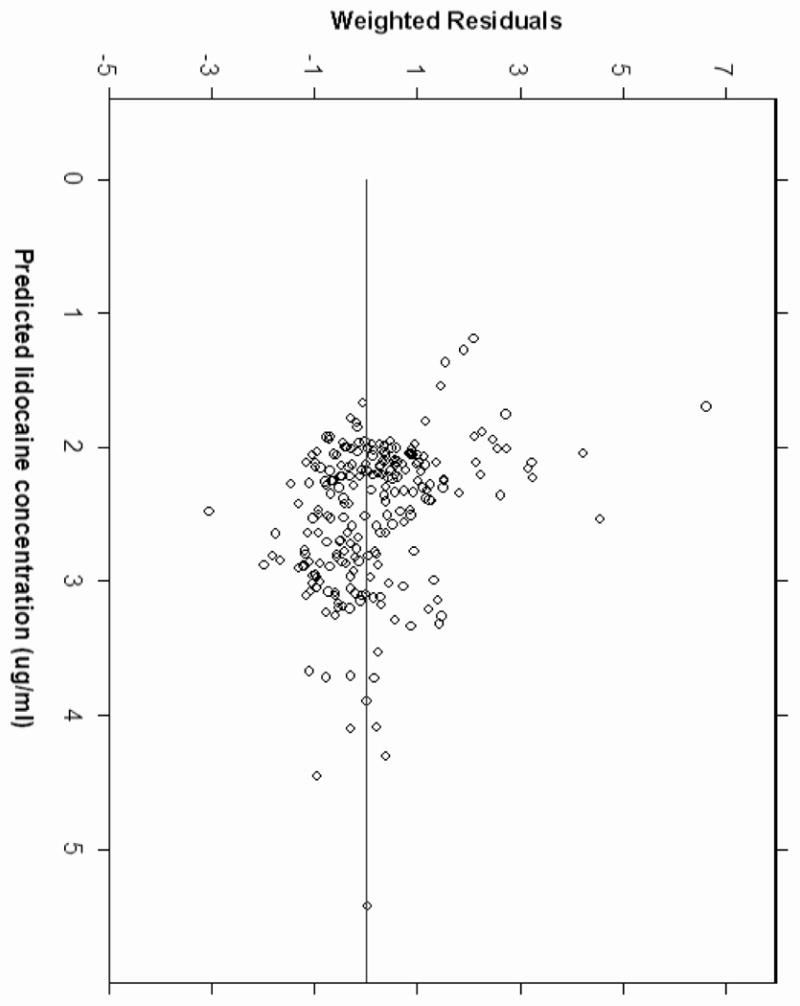
Plot of weighted residuals versus model-predicted concentrations.
During NONMEM runs, the covariance step was aborted due to numerical instability. Therefore, the 95% confidence intervals (CI) of the pharmacokinetic parameters were calculated using the bootstrap method. The parameter estimates of the final pharmacokinetic model for lidocaine and the 95% confidence intervals (CI) using the bootstrap resampling procedure are shown on Table 3. The population estimates obtained from the final pharmacokinetic models and the mean of 1000 bootstrap replicates were very similar.
Table 3. Pharmacokinetic Parameters of the Final Model and the 95% Confidence Interval Using the Bootstrap Resampling Procedure.
| Original Data Set | 1000 Bootstrap Replicates | |||
|---|---|---|---|---|
| Parameter | Estimate | % CV | Estimate | 95% Confidence Interval |
| V1 (l/kg) | 0.062 | 12% | 0.056 | 0.031 – 0.64 |
| V2 (l) | 187 | 24% | 187 | 137 – 207 |
| CL1 (l/kg/min) | 0.0042 | 26% | 0.0042 | 0.0038 – 0.0045 |
| CL2 (l/min) | 8.92 | 73% | 6.76 | 3.6 – 65.1 |
V1 = volume of the central compartment; V2 = volume of the peripheral compartment CL1 = elimination clearance; CL2 = intercompartmental clearance; CV = coefficient of variation.
Lidocaine levels above 5 μg/ml were found at 48 hours after CPB in 6.4% of patients with the currently recommended (non-weight-based) infusion protocol. Using the final weight-adjusted pharmacokinetic parameters, a new protocol for lidocaine administration was simulated to avoid lidocaine concentrations above 5 μg/ml after 24 hours of infusion. Results of the post-hoc simulations suggest the ideal lidocaine infusion protocol is a bolus of 1 mg/kg, followed by 50 μg/kg/min for the first hour, 25 μg/kg/min for the second hour, 12 μg/kg/min for the next 22 hours, and then 10 μg/kg/min for 24 to 48 hours.
Discussion
In a previously published clinical trial, intravenous lidocaine, administered for 48 hours without consideration of body weight or impairment of clearance with prolonged infusions, did not reduce the high rate of postoperative cognitive decline (POCD).7 Post hoc analyses of the trial results, however, revealed a detrimental effect of higher total dose of lidocaine, suggesting that standard dosing guidelines (mg/min) were not optimal for patients undergoing cardiac surgery.7 In the current study, which we believe is the first population pharmacokinetic model of lidocaine in cardiac surgery patients receiving a lidocaine infusion for 48 hours, we have shown that a two-compartment pharmacokinetic model including body weight as a covariate on the clearance and central compartment best describes the concentration data. Thus, weight-based dosing is recommended to reduce the risk of toxicity. Furthermore, our results confirm previous reports that prolonged infusion of lidocaine will result in decreasing clearance and increased elimination time after 24 hours. According to our pharmacokinetic simulation, the infusion rate should be reduced by 20% after 24 hours infusion to minimize the risk of lidocaine toxicity.
Intravenous infusions of lidocaine have been used not only in the prevention of POCD, but also in the treatment of ventricular arrhythmia and chronic pain. Optimal infusion therapy often requires the rapid achievement and maintenance of therapeutic plasma concentrations of lidocaine. On the other hand, the adverse effects of lidocaine are generally related to its high plasma concentration; an accepted therapeutic range is 2 to 5 μg/mL and side effects usually occur at levels above 6 to 10 μg/mL.9,10 Lidocaine is metabolized primarily by the liver with less than 10% of a dose excreted unchanged in the urine and the elimination half-life of lidocaine following an intravenous bolus injection is typically 1.5 to 2 hours. Through sequential oxidative N-dealkylation, the initial active metabolite is monoethylglycinexylidide (MEGX) and glycinexylidide (GX).11 However, the pharmacokinetics of lidocaine appear to change with prolonged infusions of lidocaine; a phenomenon that has been attributed to the inhibitory effect of MEGX on clearance of lidocaine. This inhibitory effect is possibly the result of competition between lidocaine and MEGX for the binding sites of hepatic enzymes.12 A pharmacokinetics and pharmacodynamics study of lidocaine and MEGX has shown that when administered in combination with MEGX, lidocaine clearance was significantly reduced from 58±18 to 48 ±13 L/hr.13 LeLorier et al.14 has also demonstrated that the half-life prolongs to 3 hours or longer following infusions of lidocaine greater than 24 hours. In our study, we found the terminal half-life was 6.9 hours when lidocaine was infused for 48 hours. Two factors may cause the significantly prolonged terminal half-life. First, as described above, the accumulation of MEGX may inhibit the biotransformation of lidocaine. In our study, the measured concentrations of MEGX at 48 hours after CPB was 0.25±0.15 μg/ml and the ratio of serum levels of MEGX to lidocaine was 8.9±5.4%. Although the ratio of MEGX to lidocaine in our study is lower than that reported by Drayer et al.15 in a study of only 33 subjects (36±26%), the presence of any MEGX impairs lidocaine clearance and can contribute to central nervous system toxicity. Another factor which may alter lidocaine disposition is congestive heart failure, which is common in cardiac surgical patients and has been found to decrease the clearance of lidocaine.16 In such patients, the volume of distribution of the central compartment is smaller so that the same dose will achieve a higher plasma concentration whereas a diminished cardiac index results in a decrease in clearance.
Limitations to our study include the fact that there is a lack of sampling data at the early time points immediately following the start of infusion. In addition, there are only 3 time-points for each subject. Due to sparse nature of the data, there was numerical instability for a standard three-compartment analysis in the NONMEM analysis and a two- compartment model was used to fit the data. Nevertheless, our population parameters can be compared with the parameters reported by Schnider et al.,2 who have derived pharmacokinetic parameters with a two compartment model using computer-controlled infusion of lidocaine in chronic pain patients. The clearance (CL1= 0.022 l/kg/min) reported by Schnider et al.2 is higher than the value found in the current study (CL1 = 0.0042 l/kg/min). Besides, a smaller V1 (0.062 l/kg) was derived in the current study when compared with the V1(0.10 l/kg) reported by Schnider et al.2 These differences may be related to dissimilarities in patient characteristics and infusion time (only 2 hours in the study by Schneider et al). Moreover, congestive heart failure, which can reduce the clearance of lidocaine, is common in CABG patients. Of note, the results of our study are similar to that of Vozeh et al.,5 who demonstrated a 24% reduction in V1 and 46% reduction in elimination clearance of lidocaine in patients with congestive heart failure. A final limitation of our study is that it was not designed to assess the effect of CPB on the kinetics of lidocaine. However, Holley et al.17 have studied the effects of CPB on lidocaine disposition. Kinetic studies were performed before surgery, 15 min after CPB, and 1 day after CPB following an intravenous bolus of 100 mg lidocaine. The results demonstrated lidocaine kinetics was unchanged 15 min and 1 day after CPB. On the other hand, Morrell et al.18 have found a change of lidocaine kinetics during CPB and suggested the alteration may be attributed largely to decreased binding to plasma protein following hemodilution. Also, Davies et al.19 have shown the changing concentrations of α1-acid glycoprotein, a binding protein of lidocaine, will significantly affect free drug concentration in the perioperative period. Their findings suggest that free fraction would be underestimated in the first postoperative day and would be overestimated later in the postoperative period by measurement of total drug concentrations.
In summary, our study demonstrates that a two compartment pharmacokinetic model best describes the plasma concentrations of lidocaine infusion in patients undergoing heart surgery with cardiopulmonary bypass. The inclusion of body weight as a covariate on clearance (CL1) and central compartment (V1) improves the final model suggesting that dosing should be weight-based. Our results also indicate prolonged infusions of lidocaine may decrease clearance and therefore, the infusion rate should be decreased by 20% after 24 hours to prevent toxicity. An ideal lidocaine infusion protocol designed to maintain lidocaine levels below 5μg/ml is a bolus of 1 mg/kg, followed by an infusion of 50 μg/kg/min for the first hour, 25 μg/kg/min for the second hour, 12 μg/kg/min for the next 22 hours, and then 10 μg/kg/min for 24 to 48 hours.
Acknowledgments
This work was supported in part by grants #9970128N (Dr Newman) from the American Heart Association, #M01-RR-30 from the National Institutes of Health, and by the Division of Cardiothoracic Anesthesiology and Critical Care Medicine, Department of Anesthesiology, Duke University Medical Center.
Footnotes
Name(s) of institution(s) in which work was done; Duke University Medical Center & Mackay Memorial Hospital
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yung-Wei Hsu, Department of Anesthesiology, Mackay Memorial Hospital, Taipei, Taiwan.
Jacques Somma, Department of Anesthesiology, Hospital Laval de Québec, Quebec, Canada.
Mark Newman, Department of Anesthesiology, Duke University Medical Center, Durham, NC 27710.
Joseph P. Mathew, Department of Anesthesiology, Duke University Medical Center, Durham, NC 27710.
Reference List
- 1.Dyck JB, Wallace MS, Lu JQ, et al. The pharmacokinetics of lignocaine in humans during a computer-controlled infusion. Eur J Pain. 1997;1:141–148. doi: 10.1016/s1090-3801(97)90072-0. [DOI] [PubMed] [Google Scholar]
- 2.Schnider TW, Gaeta R, Brose W, et al. Derivation and cross-validation of pharmacokinetic parameters for computer-controlled infusion of lidocaine in pain therapy. Anesthesiology. 1996;84:1043–1050. doi: 10.1097/00000542-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Rowland M, Thomson PD, Guichard A, et al. Disposition kinetics of lidocaine in normal subjects. Ann N Y Acad Sci. 1971;179:383–398. doi: 10.1111/j.1749-6632.1971.tb46915.x. [DOI] [PubMed] [Google Scholar]
- 4.Kuipers JA, Boer F, de RA, et al. Modeling population pharmacokinetics of lidocaine: should cardiac output be included as a patient factor? Anesthesiology. 2001;94:566–573. doi: 10.1097/00000542-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Vozeh S, Berger M, Wenk M, et al. Rapid prediction of individual dosage requirements for lignocaine. Clin Pharmacokinet. 1984;9:354–363. doi: 10.2165/00003088-198409040-00005. [DOI] [PubMed] [Google Scholar]
- 6.Thomson PD, Melmon KL, Richardson JA, et al. Lidocaine pharmacokinetics in advanced heart failure, liver disease, and renal failure in humans. Ann Intern Med. 1973;78:499–508. doi: 10.7326/0003-4819-78-4-499. [DOI] [PubMed] [Google Scholar]
- 7.Mathew JP, Mackensen GB, Phillips-Bute B, et al. Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke. 2009;40:880–887. doi: 10.1161/STROKEAHA.108.531236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehra P, Caiazzo A, Maloney P. Lidocaine toxicity. Anesth Prog. 1998;45:38–41. [PMC free article] [PubMed] [Google Scholar]
- 9.Benowitz NL, Meister W. Clinical pharmacokinetics of lignocaine. Clin Pharmacokinet. 1978;3:177–201. doi: 10.2165/00003088-197803030-00001. [DOI] [PubMed] [Google Scholar]
- 10.Waller ES. Pharmacokinetic principles of lidocaine dosing in relation to disease state. J Clin Pharmacol. 1981;21:181–194. doi: 10.1002/j.1552-4604.1981.tb05698.x. [DOI] [PubMed] [Google Scholar]
- 11.Oellerich M, Armstrong VW. The MEGX test: a tool for the real-time assessment of hepatic function. Ther Drug Monit. 2001;23:81–92. doi: 10.1097/00007691-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki T, Fujita S, Kawai R. Precursor-metabolite interaction in the metabolism of lidocaine. J Pharm Sci. 1984;73:136–138. doi: 10.1002/jps.2600730140. [DOI] [PubMed] [Google Scholar]
- 13.Thomson AH, Elliott HL, Kelman AW, et al. The pharmacokinetics and pharmacodynamics of lignocaine and MEGX in healthy subjects. J Pharmacokinet Biopharm. 1987;15:101–115. doi: 10.1007/BF01062338. [DOI] [PubMed] [Google Scholar]
- 14.LeLorier J, Grenon D, Latour Y, et al. Pharmacokinetics of lidocaine after prolonged intravenous infusions in uncomplicated myocardial infarction. Ann Intern Med. 1977;87:700–706. doi: 10.7326/0003-4819-87-6-700. [DOI] [PubMed] [Google Scholar]
- 15.Drayer DE, Lorenzo B, Werns S, et al. Plasma levels, protein binding, and elimination data of lidocaine and active metabolites in cardiac patients of various ages. Clin Pharmacol Ther. 1983;34:14–22. doi: 10.1038/clpt.1983.122. [DOI] [PubMed] [Google Scholar]
- 16.Halkin H, Meffin P, Melmon KL, et al. Influence of congestive heart failure on blood vessels of lidocaine and its active monodeethylated metabolite. Clin Pharmacol Ther. 1975;17:669–676. doi: 10.1002/cpt1975176669. [DOI] [PubMed] [Google Scholar]
- 17.Holley FO, Ponganis KV, Stanski DR. Effects of cardiac surgery with cardiopulmonary bypass on lidocaine disposition. Clin Pharmacol Ther. 1984;35:617–626. doi: 10.1038/clpt.1984.85. [DOI] [PubMed] [Google Scholar]
- 18.Morrell DF, Harrison GG. Lignocaine kinetics during cardiopulmonary bypass. Optimum dosage and the effects of haemodilution. Br J Anaesth. 1983;55:1173–1177. doi: 10.1093/bja/55.12.1173. [DOI] [PubMed] [Google Scholar]
- 19.Davies RF, Dube LM, Mousseau N, et al. Perioperative variability of binding of lidocaine, quinidine, and propranolol after cardiac operations. J Thorac Cardiovasc Surg. 1988;96:634–641. [PubMed] [Google Scholar]


