Abstract
Conjugated linoleic acid (CLA) reduces adiposity in vivo. However, mechanisms mediating these changes are unclear. Therefore, we treated cultures of human adipocytes with trans-10, cis-12 (10,12) CLA, cis-9, trans-11 (9,11) CLA, or other trans fatty acids (FA) and measured indices of lipid metabolism. The lipid-lowering effects of 10,12 CLA were unique, as other trans FA did not reduce TG content to the same extent. Using low levels of [14C]-CLA isomers, it was shown that both isomers were readily incorporated into acylglycerols and phospholipids, albeit at lower levels than [14C]-oleic or [14C]-linoleic acids. When using [14C]-acetic acid and [14C]-pyruvic acid as substrates, 30 μM 10,12 CLA, but not 9,11 CLA, decreased de novo synthesis of triglyceride (TG), free FA, diacylglycerol, cholesterol esters, cardiolipin, phospholipids, and ceramides within 3–24 h. Treatment with 30 μM 10,12 CLA, but not 9,11 CLA, decreased total cellular lipids within 3 d and the ratio of monounsaturated FA (MUFA) to saturated FA, and increased C18:0 acyl-CoA levels within 24 h. Consistent with these data, stearoyl-CoA desaturase (SCD)-1 mRNA and protein levels were down-regulated by 10,12 CLA within 7–12 h, respectively. The mRNA levels of liver X receptor (LXR)α and sterol regulatory element binding protein (SREBP)-1c, transcription factors that regulate SCD-1, were decreased by 10,12 CLA within 5 h. These data suggest that the isomer-specific decrease in de novo lipid synthesis by 10,12 CLA is due, in part, to the rapid repression of lipogenic transcription factors that regulate MUFA synthesis, suggesting an anti-obesity mechanism unique to this trans FA.
Keywords: conjugated linoleic acid, adipocytes, lipid synthesis, stearoyl-CoA desaturase
Introduction
Conjugated linoleic acid (CLA) consists of a group of positional and geometric fatty acid (FA) isomers of linoleic acid (C18:2; cis-9, cis-12 octadecadienoic acid). Isomers of CLA are found naturally in ruminant meats and dairy products, and in relatively larger quantities in dietary supplements or fortified foods. The two major isomers of CLA, trans-10, cis-12 (10,12) and cis-9, trans-11 (9,11), have been reported to prevent obesity, diabetes, atherosclerosis, or cancer depending on the specific doses, isomers, and models used. There is a great deal of interest in CLA as a weight loss treatment, because of its ability to decrease body weight and body fat mass in many animal studies, and in some human studies (Reviewed in 1 and 2). Of the two major isomers, 10,12 CLA is responsible for its anti-obesity properties (3–7).
Potential mechanisms by which 10,12 CLA prevents obesity include 1) increasing energy expenditure and lipolysis, 2) inducing adipocyte apoptosis, and 3) deceasing adipogenesis or lipogenesis (Reviewed in 2). For example, CLA suppresses preadipocyte differentiation in animal (8–14) and human preadipocytes (5, 16), in part, via attenuating the expression or activity of peroxisome proliferator activated receptor (PPAR) γ, a transcription factor essential for adipogenesis. Indeed, rodents supplemented with 10,12 CLA had decreased expression of PPARγ and its target genes (14, 17–21). In mature, primary human adipocytes or murine 3T3-L1 adipocytes, 10,12 CLA decreases the expression or activity of PPAR (8, 22), and PPAR target genes and lipid content (15, 16). Other transcription factors involved in adipogenesis (e.g., CCAT/enhancer binding protein α, C/EBPα) or lipogenesis (e.g., sterol regulatory element binding protein 1c, SREBP-1c; liver X receptor α, LXRα) may also be impaired by CLA (5, 14–16, 19, 20). We previously showed that chronic 10,12 CLA treatment of differentiating primary human preadipocytes decreased the mRNA levels of the delta-9 desaturase stearoyl-CoA desaturase (SCD)-1, a SREBP-1c target gene, after 6 d and the monounsaturated fatty acid/saturated fatty acid (MUFA/SFA) ratio after 12 d of treatment, suggesting that down regulation of SCD-1 precedes reductions in the MUFA/SFA ratio (15). However, the effects and kinetics of CLA on regulators of lipid metabolism and de novo lipid metabolism in mature primary human adipocytes have not been reported.
Thus, we wanted to understand the specificity and kinetics of these anti-lipogenic actions of CLA isomers in human adipocytes; i.e., does CLA directly attenuate lipogenic transcription factors, which in turn alter lipid metabolism, or does CLA initially alter lipid metabolism, which in turn affects the expression or activity of these transcription factors. To address these questions, we compared the delipidating effects of 10,12 CLA to other trans FA. Furthermore, we examined the kinetics of CLA’s incorporation into lipid classes and its impact on de novo lipid synthesis and the expression of LXR, SREBP-1c and their target SCD-1 in newly differentiated human adipocytes.
Materials and Methods
Materials
All cell culture ware were purchased from Fisher Scientific (Norcross, GA, USA). Methanolic-HCl (3N) kit was purchased from Supelco (Belafonte, PA, USA), anhydrous methanol 99.8% from Aldrich (Steinheim, Germany), and the GC standard 68A from NU-CHEK-PREP, Inc. (Elysian, MN, USA). Antibodies for SCD-1 (N-20), donkey-anti goat (SC-2020), and GAPDH (FL-35) were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA, USA) and the anti-rabbit antibody from Promega (Madison, WI, USA). Western lightning chemiluminescence substrate was purchased from Perkin Elmer Life Science (Boston, MA, USA). One-step reverse transcription-polymerase chain reaction (RT-PCR) kit used in semi-quantitative mRNA analysis was purchased from Qiagen, Inc (Valencia, CA, USA). Immunoblotting buffers, precast gels and gene-specific primers were purchased from Invitrogen (Carlsbad, CA), and ribosomal 18S competimer technology internal standards and DNA-free were purchased from Ambion (Austin, TX). All other reagents and chemicals were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO) unless otherwise stated. All reagents and chemicals used to isolate lipids were of analytic grade or better.
Sources of FA
Isomers of CLA (+98% pure) were purchased from Matreya (Pleasant Gap, PA). [1-14C]-9,11 CLA and [1-14C]-10,12 CLA (specific activity = 53.4 mCi/mmol; radiochemical and isomeric purities >95%) were synthesized by the Service de Chimie Bioorganique et de Marquage, F-91191, Gif sur Yvette, France. They were prepared by stereoselective multistep synthesis involving sequential substitution of 1,2-dicholoro-ethene as previously described (23). [14C]-oleic acid and [14C]-linoleic acid (specific activities of 50 mCi/mmol), [14C]-acetate (specific activity of 57 mCi/mmol), and [14C]-pyruvate (specific activity of 15 mCi/mmol) were purchased from Amersham Biosciences (Buckinghamshire, UK). Unlabeled oleic acid, elaidic acid, vaccenic acid, and trans vaccenic acid were purchased from Sigma-Aldrich.
Methods
Culturing of human primary adipocytes
Abdominal white adipose tissue (WAT) was obtained from non-diabetic females, between the ages of 20–50 years old with a body mass index ≤ 30 during abdominoplasty with consent from the Institutional Review Board at the University of North Carolina at Greensboro. Tissue was digested using collagenase and stromal vascular (SV) cells were isolated as previously described (15). Experimental treatment of cultures containing ~50% preadipocytes and ~50% adipocytes occurred on day 6 of differentiation. Each experiment was done in duplicate and repeated at least once using a mixture of cells from 2–3 subjects unless otherwise indicated. These cells were used for data presented in Figures 6 and 7 due to the collaborative nature of this research.
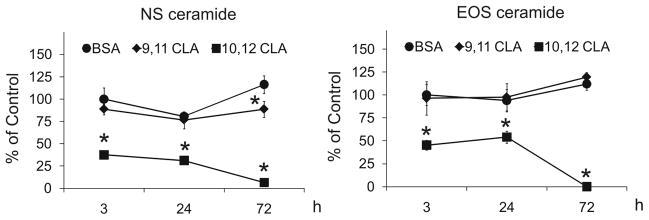
Figure 6. Trans-10, cis-12 CLA decreases de novo synthesis of ceramides.
Cultures of newly differentiated human adipocytes were treated on day 6 of differentiation with BSA vehicle, 30 μM 9,11 CLA, or 30 μM 10,12 CLA for 3, 24, or 72 h. Cultures were then incubated with 2 uCi of [14C]-acetate for 4 h and harvested. Lipids were extracted (25), subjected to a mild alkaline hydrolysis to purify the amide-bound lipids, then separated twice by HPTLC in chloroform:methanol:acetic acid (190:9:1, v/v/v), incubated overnight on phospho-imager screens, and the lipid spots detected and quantified using a Typhoon scanner and ImageQuant TL. Means (± SEM, n=3) having an asterisks (*) are significantly (P<0.05) different than the BSA vehicle within a given time point. Data are representative of two independent experiments. Abbreviations used: Cer (NS), Non-hydroxy FA ceramide; Cer (EOS), Ceramide-containing sphingosine.
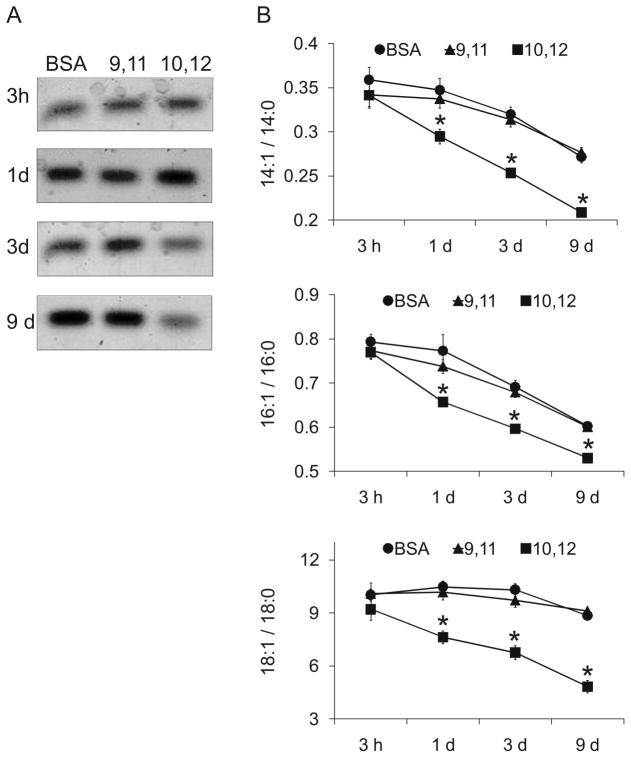
Figure 7. Acute treatment with trans-10, cis-12 CLA decreases the TG content and the MUFA/SFA ratio.
Cultures of newly differentiated, primary human adipocytes were treated on day 6 of differentiation with BSA vehicle, 30 μM 9,11 CLA, or 30 μM 10,12 CLA for 3 h, 1 d, 3 d, or 9 d, and then total lipids were extracted (25). A: Lipids were separated by HPTLC using a neutral lipid separation system consisting of hexane:diethylether:acetic acid (70:30:1). Data shown are representative of three individual experiments. B: Total lipids were saponified, methylated, and analyzed using GC. The FA composition of the total lipids was determined (% of total), from which the ratios of 14:1/14:0, 16:1/16:0, and 18:1/18:0 were calculated. 10,12 CLA means (± SEM, n=8) having an asterisks (*) are significantly (P<0.05) different than 9,11CLA and BSA vehicle within a time point.
Culturing of human Simpson–Golabi–Behmel Syndrome (SGBS) cells
SGBS is a rare X-linked disorder characterized by pre- and postnatal overgrowth. The cell strain exhibits a high capacity for adipose differentiation, resulting in mature fat cells which are biochemically and functionally similar to human primary adipocytes (24). They offer an advantage over human primary adipocytes, because they can be grown and differentiated in much greater quantities. For these reasons and due to the collaborative nature of this research, they were used in all of the experiments performed with the exception of data shown in Figures 6 and 7. They were generously provided by Dr. Martin Wabitsch. SGBS cells were grown to confluence in Dulbecoo’s Modified Eagle’s Medium/Nutrient Mixture F-12 Ham and supplemented with 10% bovine serum, 33 μM biotin, 17 μM pantothenate, 100 μg/ml streptomycin and 62 μg/ml penicillin. To induce differentiation, SGBS cells were repeatedly washed with PBS buffer and cultured in serum-free medium supplemented with 350 nM insulin, 200 pM triiodothyronine, 1 μM cortisol, 2 μM BRL 49653 (Rosiglitazone), 0.115 mg/ml 1-methyl-3- isobutylxanthine (IBMX), 0.25 mmol/L dexamethasone (DEX) and 0.01 mg/ml human transferrin for 4 d. After 4 d media was removed and replaced with the differentiation media in the absence of BRL 49653, IBMX, and DEX. All treatments of these cells started on day 6 of differentiation. These cells were used for data in Figures 1–5, 8, and 9.
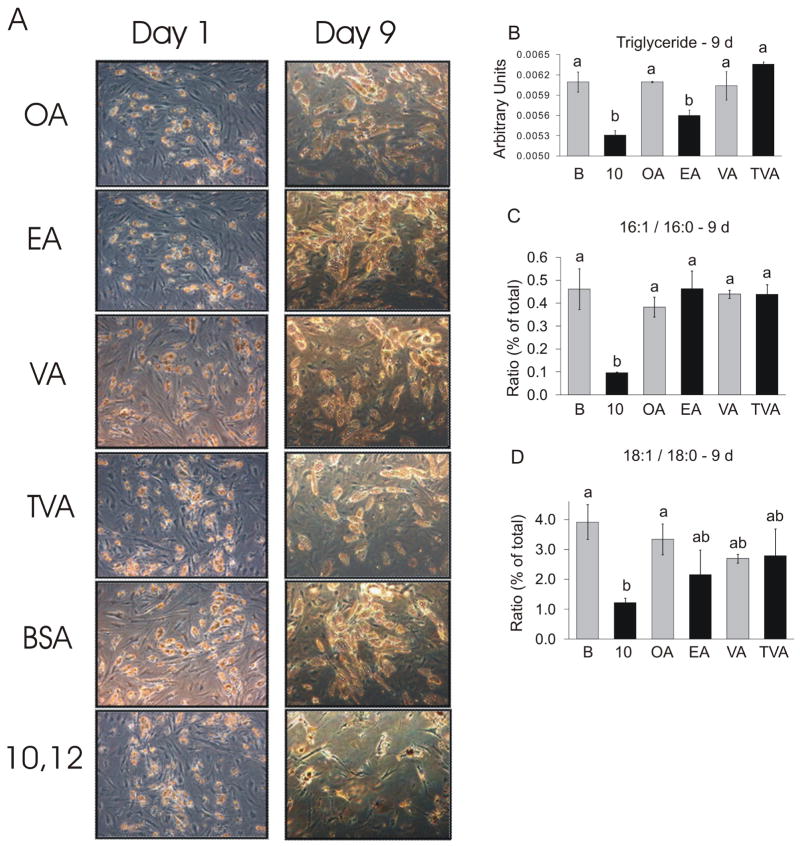
Figure 1. Chronic treatment with trans-10, cis-12 CLA, but not other trans FA, decreases TG content and the MUFA/SFA ratio.
Cultures of newly differentiated SGBS adipocytes were treated on day 6 of differentiation for 9 d with 30 μM oleic acid (OA; cis 9, C18:1), elaidic acid (EA; trans 9, C18:1), vaccenic acid (VA; cis 11, C18:1), trans vaccenic acid (TVA; trans 11, C18:1), BSA vehicle, or 10,12 CLA. A: On Day 0 and Day 9, cultures were visualized using a Leica DM IRBE inverted light microscope with a 10x objective. The pictures are representative of two independent experiments. Left panel, day 0; Right panel, day 9. B: Cultures were harvested and the lipids extracted (25) and spotted onto HPTLC plates and separated in hexane:diethylether: acetic acid (70:30:1 v/v). The separated spots were visualized by spraying the plate with a 3% copper-acetate/15% phosphoric acid solution and charred in a heating cabinet at 100 °C for 10–20 min and the quantification of the lipids was based on the intensities of the charred lipids (arbitrary units) using version 4.0.3 of Scion Image. Means (± SEM, n=2) not sharing a common superscript are significantly (P<0.05). C,D: The FA composition of the total lipids was determined (% of total), from which the ratios of 16:1/16:0, and 18:1/18:0 were calculated. Means (± SEM, n=2) not sharing a common superscript are significantly (P<0.05). Data are all panels are representative of two independent experiments.
Figure 5. Quantification of de novo synthesis of neutral and compound lipids.
The intensities of the HPTLC bands from [14C]-acetate incorporation into specific lipid spots in Figures 3 and 4 were quantified using a Typhoon scanner and ImageQuant TL and subjected to statistical analyses. 10,12 CLA means (± SEM, n=3) having an asterisks (*) are significantly (P<0.05) different than the BSA vehicle within a given time point. Data are representative of two independent experiments
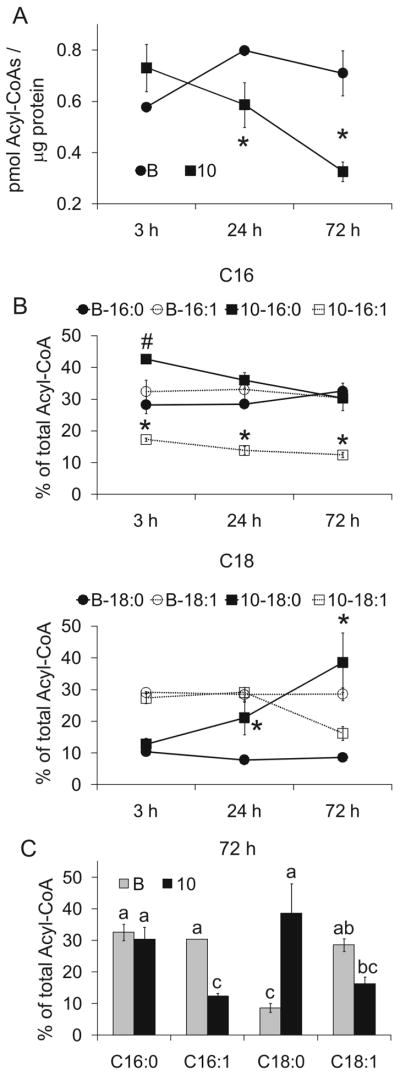
Figure 8. Trans-10, cis-12 CLA changes the rates of desaturation in acyl-CoAs.
Cultures of newly differentiated SGBS cells were treated on day 6 of differentiation with BSA vehicle (B) or 30 μM 10,12 CLA (10) for 3, 24, or 72 h. Cultures were then harvested and the acyl-CoAs were extracted and subsequently derivatized to etheno-acyl-CoAs, which were separated and detected by HPLC using a fluorescence detector. After being normalized to equal protein levels, the total amount of acyl-CoAs (panel A) and C16 and C18 acyl-CoAs (panel B) were calculated using the molecular weight of each individual FA + 767.5 (weight of CoA), compared to the C17:0-CoA internal standard. A: Means (± SEM, n=3) having an asterisks (*) are significantly (P<0.05) different than the BSA vehicle within a given time point. B: Means (± SEM, n=2) having an asterisks (*) or a pound sign (#) within a FA type (i.e., MUFA is an *; SFA is a #) are significantly different (P>0.05) from one another within a given time point. For panel C, means (± SEM, n=2) not sharing a common superscript are significantly (P<0.05). Data in all panels are representative of two independent experiments.
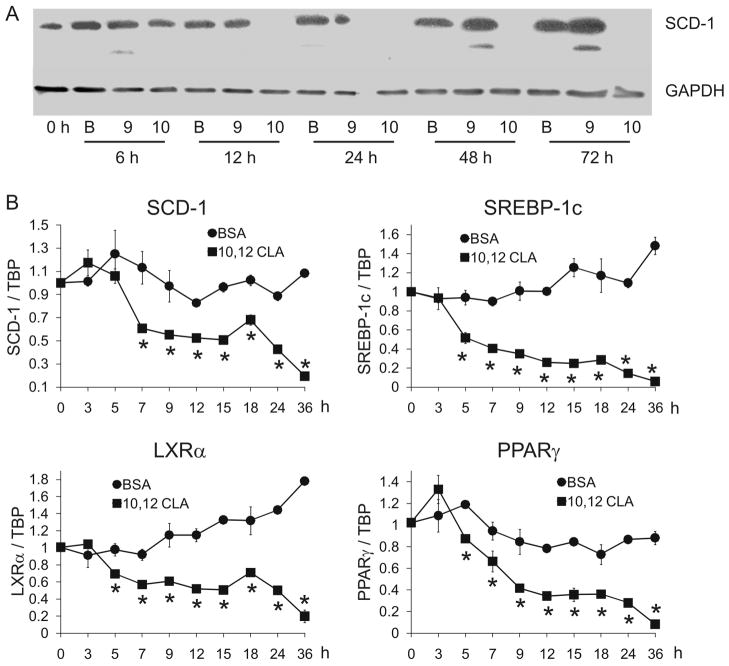
Figure 9. 10, 12 CLA decreases SCD-1 protein and mRNA levels.
Cultures of newly differentiated SGBS adipocytes were treated on day 6 of differentiation with BSA vehicle, 30 μM 9,11 CLA, or 30 μM 10,12 CLA over time. A: Cultures were treated with BSA, 9,11 CLA or 10,12 CLA for 6, 12, 24, 48, or 72 h. Cells were then harvested and SCD-1 protein and GAPDH levels were determined by immunoblotting. B: Cultures were treated for 3, 5, 7, 9, 12, 15, 18, 24, or 36 h with either a BSA vehicle or 10,12 CLA. Subsequently, cultures were harvested to determine the mRNA levels of SCD1, SREBP-1c, LXRa, PPARγ, and TATA-binding protein (TBP, load control) by qPCR. The expression level of a given gene was calculated after normalization to TBP expression, and expressed relative to Day 0 (confluent, noninduced) controls. Means (± SEM, n=2) having an asterisks (*) are significantly (P<0.05) different than the BSA vehicle within a given time point. Data shown in panels A and B are representative of two individual experiments.
Preparation of FA
Isomers of CLA, oleic acid, elaidic acid, vaccenic acid and trans vaccenic acid were complexed to FA-free- bovine serum albumin (BSA) (>98%, A7030, Sigma-Aldrich) at a 4:1 molar ratio using 1 mM BSA stocks. All cultures were continuously treated with FAs which were added fresh with each media change (every 3–4 d).
Complexing of [14C]-FA to BSA
10 nmol of cold FA (e.g. 10,12 CLA) was evaporated under nitrogen gas and 1 μCi of [14-C]-FA added and subsequently evaporated again. 10 μl of a 1.1 mM KOH solution was added and incubated for 10 min at 37°C, centrifuged at 2,000 rpm for 1 min and 90 μl of a 2.5 mM BSA stock was added, the solution was left at room temperature for 2–3 h and then overnight at 4°C and subsequently stored at −20°C.
[14C]-FA incorporation into lipid classes
Following treatments, cultures were incubated for multiple time points with 1 μCi of [14C]-9,11 CLA, [14C]-10,12 CLA, [14C]-oleic acid, or [14C]-linoleic acid, harvested, and the lipids extracted using the Bligh and Dyer method (25). Neutral and compound lipids were then separated by high performance thin layer chromatography (HPTLC) on silica gel 60 plates (Merck KGaA, 50 Darmstadt, Germany) and subsequently developed in (hexane:diethylether:acetic acid) (70:30:1 v/v) and chloroform/ethanol/water/triethyl-amine (30:35:7:35, v/v), respectively, incubated overnight on phospho-imager screens, and labelled lipids were detected and quantified using a Typhoon scanner and ImageQuant TL (GE Healthcare, Pittsburgh, PA).
De novo synthesis of neutral and compound lipids using [14C]-acetate and [14C]-pyruvate
After treatment, 100 μL of PBS containing 100 nM insulin and 2 μCi [14C]-acetate or 1 μCi [14C]-pyruvate were added to each plate and incubated for 240 min (based on results of a preliminary study). In all cultures, the labeling media was removed and the cells were washed in ice-cold Krebs-Ringer buffer, washed once with ice-cold PBS, harvested in 1 ml PBS, pH 7.4, and immediately frozen at −80°C. For neutral lipid detection, lipids were extracted [25], separated by HPTLC in hexane:diethylether:acetic acid (70:30:1 v/v), incubated overnight on phospho-imager screens, the lipid spots were detected and quantified using a Typhoon scanner and ImageQuant TL. For compound lipid detection, lipids were extracted [25], separated on boric acid (2.3%)-treated HPTLC plates in chloroform/ethanol/water/triethylamine (30:35:7:35, v/v), and then processed like the neutral lipid procedure. For ceramide detection, extracted lipids were subjected to a mild alkaline hydrolysis to purify the amide-bound lipids, then separated twice by HPTLC in chloroform:methanol:acetic acid (190:9:1 v/v/v), incubated overnight on phospho-imager screens, and detected as described above. Protein concentrations were determined using the QubitTM fluorometer (Invitrogen) and the Quant-iTTM protein assay kit as described by the manufacturer. Similar experiments were performed using 14C-pyruvate. Because the results from the 14C-acetate and 14C-pyruvate were similar, only data from the 14C-acetate are shown.
Gas Chromatography (GC)
For measuring FA profiles, total lipids were saponified, methylated, and analyzed using GC. Briefly, cells were lysed by sonication and the protein concentration was determined. Before extraction, an internal standard C15:0 (pentadecanoic acid) was added. Total cellular lipids were extracted with chloroform/methanol (1:2 v/v) [25]. Aliquots of the lipid extracts were dried under nitrogen gas and converted to FA methyl esters (FAME) by using anhydrous 99.8% MeOH/Methanolic-HCl (3N) (2:1 v/v), heated at 60°C in oil bath for 20 min and identified by comparison to the 68A standard and vaccinate 18:1(n-7) (Larodan, Malmö, Sweden) using GC. GC was performed on a Chrompack CP 9002, equipped with a CPSIL88 FAME 0.25mm ID capillary column (Chrompack, 51 Middelburg, The Netherlands) and the oven temperature was programmed from 120°C to 220°C, with an increment of 3°C/min. The FA composition of the total lipids was determined (% of total), from which the ratios of 14:1/14:0, 16:1/16:0, and 18:1/18:0 were calculated.
HPLC
Cells were harvested, centrifuged, and the supernatant discarded and cells were stored at -80°C until acyl-CoA extraction. Identification of individual acyl-CoAs was performed using standard acyl-CoA mixtures as we described (26).
RNA isolation and real time qPCR
Total RNA was isolated from the cultures using Tri Reagent (Molecular Research Center, Inc, Cincinnati, OH) according to manufacturer’s protocol. RNA was extracted with phenol/1-bromo-3-chloropropane, and precipitated with ethanol, dried, and resuspended in H2O. Contaminating genomic DNA was removed by treatment with DNase (DNA-free; Ambion). First strand cDNA synthesis and real time quantitative PCR (qPCR) were carried out using the ABI PRISM 7700 Sequence Detection System (Applied Biosystems) as previously described (15). Primer sets for SCD-1, LXRα, SREBP-1c, PPARγ, and TATA-binding protein (TBP) are shown in Table 1.
Table 1.
Primers used
| Target gene | 5′-primer | 3′-primer |
|---|---|---|
| LXRα | TGAAGCGTCAAGAAGAGGAG | ATCAGTGAAGGAATCGCTTTCTG |
| PPARγ2 | AGCAAACCCCTATTCCATGCT | GGTGGTCACGAGCCCATTC |
| SCD-1 | CCAACACAATGGCATTCCAG | GGTGGTCACGAGCCCATTC |
| SREBP-1C | GGAGGGGTAGGGCCAACGGCCT | CATGTCTTCGAAAGTGCAATCC |
| TBP | GCCCGAAACGCCGAATAT | CCTCATGATTACCGCAGCAAA |
LXRα, Liver X-activated receptor; PPARγ2, Peroxisome Proliferator Activated Receptor γ2; SCD-, Stearoyl-CoA desaturase-1; SREBP, Sterol Regulatory Element Binding Protein; TBP, TATA binding protein
Statistics
Statistical analyses for data in Figures 2, 5, 6, 7B, and 8A, and 9B were performed testing the main effects of treatment time and FA type and the interaction of the two (time x FA) using two-way ANOVA (JMP Version 6.03, SAS Institute; Cary, NC). Statistical analyses for data in Figures 1B–D, 2, and 8B,C were conducted using a one-way ANOVA. Student’s t tests were used to compute individual pairwise comparisons of least square means (P<0.05). Data are expressed as the means ± S.E.M.
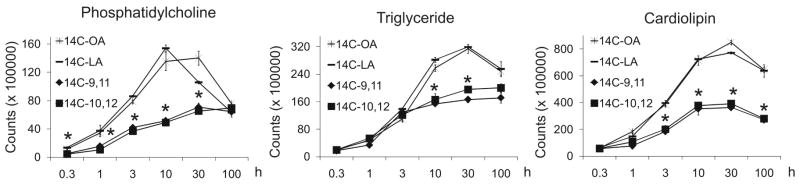
Figure 2. [14C]-10,12 CLA and [14C]-9,11 CLA incorporate equally into neutral and compound lipids.
Cultures of newly differentiated SGBS adipocytes were treated on day 6 of differentiation with 1 μCi of [14C]-9,11 CLA, [14C]-10,12 CLA, [14C]-9,11 oleic acid (OA), or [14C]-9,11 linoleic acid (LA) for 20 min, 1 h, 3 h, 10 h, 30 h, or 100 h. Cultures were then harvested and the lipids were extracted (25). Neutral and compound lipids were separated by HPTLC in hexane:diethylether:acetic acid (70:30:1, v/v) and chloroform/ethanol/water/triethyl-amine (30:35:7:35, v/v), respectively, incubated overnight on phospho-imager screens, and the spots for phosphatidylcholine, cardiolipin, and TG detected and quantified using a Typhoon scanner and ImageQuant TL, respectively. CLA means (± SEM, n=2) having an asterisks (*) are significantly (P<0.05) different from linoleic and oleic acid for a given time point. Data shown are representative of two individual experiments.
Results
Trans-10, cis-12 CLA, but not other trans FA, delipidates adipocytes
Previous studies have reported that trans FA such as elaidic acid (trans-9, C18:1) increase lipolysis (27) and inflammation (28) and reduce PPARγ expression (29) similar to that of 10,12 CLA. To examine if some or all of the effects of 10,12 CLA are due to the positioning of the trans carbon-carbon double bond in CLA, we treated newly differentiated cultures of SGBS cells with 30 μM elaidic acid (trans 9, C18:1), trans vaccenic acid (trans 11, C18:1), or 10,12 CLA. Cultures treated with 30 μM of oleic acid (cis 9, C18:1) or vaccenic acid (cis 11, C18:1) were included as controls for elaidic and trans vaccenic acid, respectively. Cultures treated with 10,12 CLA for 9 d appeared to contain less lipid (Fig. 1A), and had one of the lowest levels of TG compared to the other cultures (Fig. 1B), indicating that these cells were delipidated, as we previously reported for primary human in vitro-differentiated adipocytes (16, 22). Furthermore, 10,12 CLA decreased the C16:1/C16:0 (Fig. 1C) and C18:1/C18:0 ratios, (Fig. 1D), although only the C16:1/C16:0 ratio was significantly different than the other FAs. Taken together, these data suggest that the position of the trans carbon-carbon bond in 10,12 CLA provides a unique mechanism for reducing the TG content and the MUFA/SFA ratio in human adipocytes compared to other trans FA.
[14C]-10,12 CLA and [14C]-9,11 CLA incorporate equally into lipids
Although we previously demonstrated that CLA incorporates into neutral and phospholipids during human preadipocyte differentiation (15), the kinetics of CLA’s incorporation into specific lipid classes in mature adipocytes has not been determined. Thus, newly differentiated SGBS adipocytes were incubated on day 6 of differentiation with low levels of [14C]-9,11 CLA and [14C]-10,12 CLA, and their incorporation into specific lipid classes was determined by separating the lipid classes using HPTLC and determining their rate of incorporation into neutral and compound lipids. Cultures were also incubated with low levels of [14C]-oleic and [14C]-linoleic acid as FA controls. Both [14C]-isomers of CLA incorporated into phosphatidylcholine, cardiolipin, and TG at similar rates and amounts (Fig. 2). The rates of [14C]-oleic and [14C]-linoleic acid incorporation followed relatively similar patterns (Fig. 2), although they incorporated to a greater extent than the CLA isomers. These differences in incorporation into phosphatidylcholine, cardiolipin, and TG became apparent after 0.3, 3, and 10 h, respectively. Incorporation of [14C]-isomers of CLA into other phospholipid classes was not sufficient enough above background to be detected (data not shown). Thus, when given at low levels, CLA isomers readily incorporate into lipid classes equally, albeit at lower levels than oleic and linoleic acids.
Trans-10, cis-12 CLA decreases de novo lipid synthesis
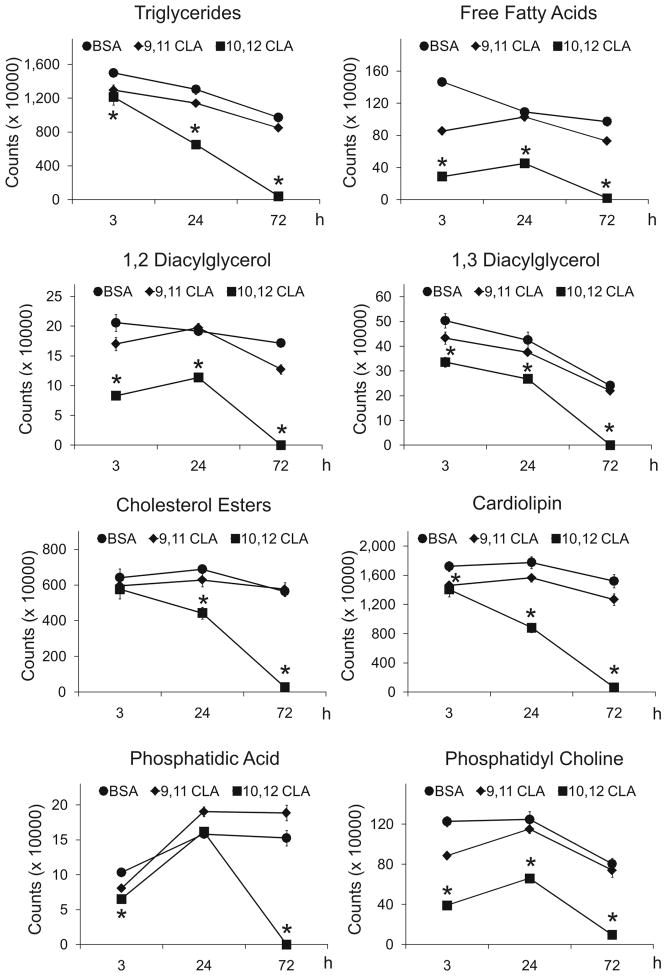
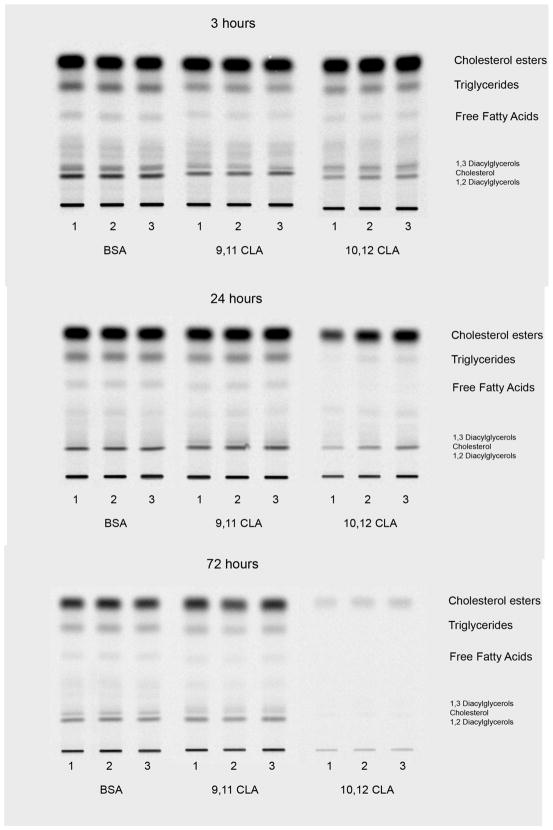
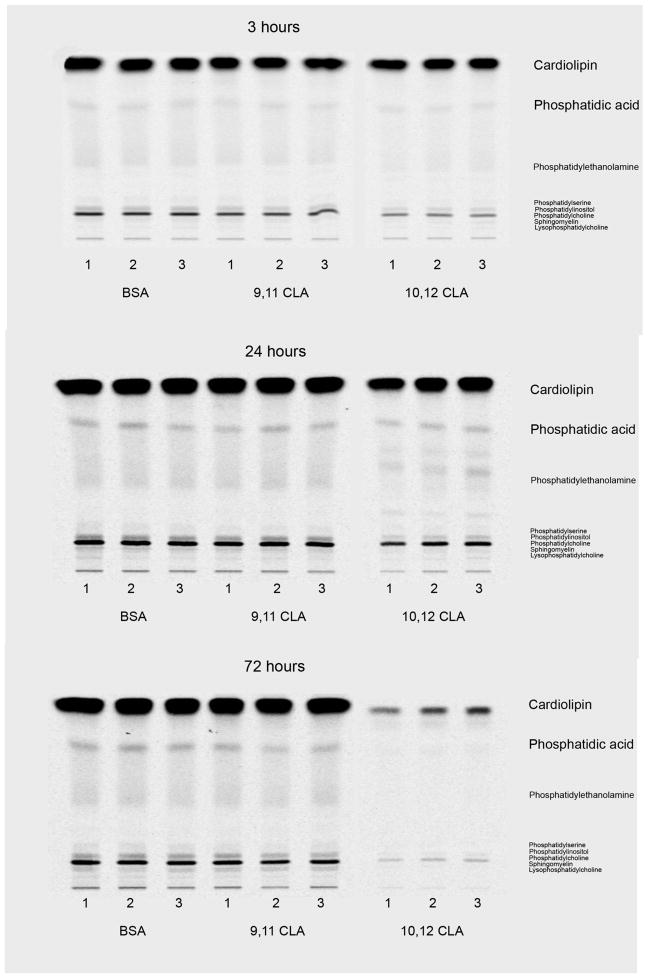
To investigate the influence of 10,12 CLA on de novo lipid synthesis, cultures of newly differentiated SGBS cells were treated on day 6 of differentiation with BSA, 30 μM 9,11 CLA, or 30 μM 10,12 CLA for 3, 24, or 72 h. Subsequently, cultures were incubated for 4 h, based on the results of a pilot study (data not shown), with either [14C]-acetate or [14C]-pyruvate as substrates. Because the results from both labelled substrates were relatively similar, only data from the [14C]-acetate studies are shown here. Treatment for 3–24 h with 30 μM 10,12 CLA, but not 9,11 CLA, decreased de novo synthesis of neutral lipids (Fig. 3) including cholesterol esters, TG, free FA, cholesterol, and diacylglyerols, and compound lipids (Fig. 4) including cardiolipin, phosphatidic acid, and phospholipids compared to the BSA vehicle. Quantification and statistical analyses of some of these lipid spots on the HPTLC plates are shown in Figure 5. Similarly, de novo synthesis of non-hydroxy ceramide (NS) and ceramide-containing sphingosine (EOS), ceramide-containing lipids made from SFA, were lower in cultures treated with 10,12 CLA for 3, 24, and 72 h compared to 9,11 CLA and BSA (Fig. 6). Collectively, these data demonstrate that 10,12 CLA markedly reduces de novo synthesis of neutral, phosphoglycerol, and complex lipids within 3–24 h in an isomer-specific manner.
Figure 3. Trans-10, cis-12 CLA decreases de novo synthesis of neutral lipids.
Cultures of newly differentiated SGBS adipocytes were treated on day 6 of differentiation with BSA, 30 μM 9,11 CLA, or 30 μM 10,12 CLA for 3, 24 or 72 h. Cultures were then incubated with 2 uCi of [14C]-acetate for 240 min and harvested. Lipids were extracted [25], separated by HPTLC in hexane:diethylether:acetic acid (70:30:1 v/v), incubated overnight on phospho-imager screens, the lipid spots were detected. The first three lanes for each treatment represent three different cell culture plates (n=3), and are representative of two independent experiments.
Figure 4. Trans-10, cis-12 CLA decreases de novo synthesis of compound lipids.
Cultures of newly differentiated SGBS adipocytes were treated on day 6 of differentiation with vehicle (BSA), 30 μM 9,11 CLA, or 30 μM 10,12 CLA for 3, 24 or 72 h. Cultures were then incubated with 2 uCi of [14C]-acetate for 4 h and harvested. Lipids were extracted (25), separated on 2.3% boric acid-treated HPTLC plates in chloroform/ethanol/water/triethylamine (30:35:7:35, v/v), incubated overnight on phospho-imager screens, and the lipid spots detected. The first three lanes for each treatment represent three different cell culture plates (n=3), and are representative of two independent experiments.
Trans-10, cis-12 CLA decreases the lipid content and MUFA/SFA ratio
Although we previously demonstrated that chronic treatment with 10,12 CLA reduced the lipid content and MUFA/SFA ratio of differentiating human preadipocytes (15) and in mature human adipocytes (Fig. 1), we did not know the kinetics of this suppression. Therefore, newly differentiated primary adipocytes were treated on day 6 of differentiation with BSA (vehicle), 9,11 CLA (isomer control), or 10,12 CLA and harvested after 3 h, 1 d, 3 d, or 9 d of treatment. Trans-10, cis-12 CLA reduced the TG levels (Fig. 7A) after 3 d and the MUFA/SFA ratio (Fig. 7B) after 1 d of treatment compared to 9,11 CLA and the BSA vehicle. Whereas 10,12 CLA decreased total acyl-CoAs (Fig. 8A) after 24–72 h of treatment, it increased the percentage of C18:0 acyl-CoA (Fig. 8B,C), which has been implicated in causing insulin resistance (30).
Trans-10, cis-12 CLA decreases SCD-1 protein and mRNA levels
Given the important role that SCD-1 plays in desaturating long chain SFA into MUFA needed for lipid storage and metabolism, we examined the impact of CLA isomers on SCD-1 mRNA and protein levels in mature adipocytes. SGBS adipocytes were treated on day 6 of differentiation with BSA, 30 μM 9,11 CLA, or 30 μM 10,12 CLA, and harvested after 6–72 h for protein or 3–36 h for mRNA expression. Trans-10, cis-12 CLA reduced the protein (Fig. 9A) and mRNA levels (Fig. 9B) of SCD-1 after 12 and 7 h of treatment, respectively, compared to 9,11 CLA or BSA vehicle. The mRNA levels of two transcription factors that are essential for the transcriptional regulation of SCD-1, SREBP-1c and LXR, were decreased after 5 h of treatment with 10,12 CLA compared to BSA vehicle and 9,11 CLA (Fig. 9B). The mRNA levels of PPARγ, another important regulator of lipogenesis in adipocytes, were similarly decreased by 10,12 CLA. Taken together, these data support the hypothesis that 10,12 CLA decreases lipid metabolism not only by rapidly suppressing the mRNA levels of PPARγ, but also LXRα and SREBP-1c and their target gene SCD-1, a desaturase required for the synthesis of MUFA essential for neutral and compound lipid synthesis.
Discussion
The purpose of this study was to determine the extent to which CLA isomers impacted de novo lipid synthesis in human adipocytes. We found that the lipid-lowering effect of 10,12 CLA does not appear to be due to a “structural trans effect”, because several other trans FA did not delipidate adipocytes or alter the MUFA/SFA ratio to the extent of 10,12 CLA. Alternatively, these effects may be due to the position of the trans double bond in the C18:2 structure of CLA. Consistent with these data, we found that 10,12 CLA, but not 9,11 CLA, suppressed de novo lipid synthesis within 3–24 h of treatment. This suppression does not appear to be due to impaired incorporation of 10,12 CLA, as both CLA isomers equally incorporate into lipids, albeit at lower levels than other unsaturated FA. Notably, we demonstrated that 10,12 CLA decreases the MUFA/SFA ratio within 24 h and the mRNA and protein levels of SCD-1, a delta-9 desaturase essential for MUFA synthesis, metabolism, and TG storage, within 7 h in an isomer-specific manner (see Table 2 for the kinetics of these effects). Furthermore, the mRNA levels of LXRα and SREBP-1c were similarly decreased by 10,12 CLA within 5 h. Taken together, these data suggest that 10,12 CLA rapidly reduces SCD-1 abundance and activity in human adipocytes as it does in murine adipocytes (12, 31–33), possibly by reducing the levels of LXRα, SREBP-1c, and PPARγ that regulate its expression or activity. The rapid 10,12 CLA-mediated reduction in the expression of these lipogenic transcription factors and SCD-1 is likely responsible, in part, for the reduction in MUFA, which could impair neutral and compound lipid synthesis, metabolism, or storage.
Table 2.
Kinetics of 10,12 CLA-mediated suppression of transcription factors or enzymes that regulate lipogenesis and lipid metabolism in human adipocytes.
| ↓Regulators of Lipogenesis by CLA | ↓Lipid Metabolism by CLA | ||||
|---|---|---|---|---|---|
| mRNA or Protein | De novo synthesis | MUFA/SFA Ratio | Total Lipids | ||
| 5–12 h | 3 h | 24 h | 72 h | 24 h | 72 h |
| LXRa | TG | CE | PA | C14:1/C14:0 | Total lipids |
| SREBP-1c | DAG | C16:1/C16:0 | |||
| PPARγ | FFA | C18:1/C18:0 | |||
| SCD-1 | PC | ||||
| CL | |||||
| CER | |||||
Abbreviations used: LXRa, liver X receptor a; SREBP-1c, sterol regulatory element binding protein-1c; PPARγ, peroxisome proliferator activated receptor γ; SCD-1, stearoyl CoA desaturase-1; TG, triglyceride; DAG, diacylglycerol; FFA, free fatty acid; PC, phosphatidylcholine; CL, cardiolipin; CER, ceramide; CE, cholesterol ester; PA, phosphatidic acid; TL, total lipids.
These and previously published data suggest that 10,12 CLA lowers the lipid content of adipocytes by rapidly 1) decreasing SCD-1 activity, thereby reducing the MUFA needed for neutral and compound lipid synthesis (reviewed in 34), 2) decreasing PPARγ activity, thereby reducing the expression of adipogenic and lipogenic proteins needed for lipid biosynthesis (reviewed in 2), or 3) increasing inflammatory lipid metabolites or signals that antagonize glucose and FA uptake and subsequent metabolism (reviewed in 2).
Stearoyl-CoA desaturase-1 is a central lipogenic enzyme (reviewed in 34), and Ntambi first proposed that inhibition of SCD-1 by 10,12 CLA in mice (12, 31, 32) and in humans (33) is key to the anti-lipogenic effects of 10,12 CLA. Stearoyl-CoA desaturase-1 and diacylglycerol transferase-2 (DGAT2) have been shown to co-localize in the ER and be important for TG synthesis (35). Dietary and endogenous palmitate (C16:0) and stearate (C18:0) are desaturated by SCD-1 and channeled to DGAT2 for the final step in TG synthesis in the ER. This close association between SCD-1 and DGAT2 enhance the efficiency of TG synthesis (35). However, 10,12 CLA’s reduction in body fat mass was similar in SCD-1 knockout and wild type mice, suggesting that CLA’s antiobesity properties are independent of SCD-1 in mice (36).
We previously demonstrated that long-term treatment (i.e., 12 d) of differentiating primary human preadipocytes with 10,12 CLA decreased the MUFA/SFA ratio and the mRNA levels of SCD-1 (15). Reductions in SCD-1 mRNA were detected after 6 d of treatment in differentiating human preadipocytes (15). We found in the current study that 10,12 CLA reduced the mRNA levels of SCD-1, LXRα, and SREBP-1c within 5–7 h (Fig. 9B) in mature human adipocytes. Similarly, 10,12 CLA decreased the mRNA expression of SREBP-1c in pig preadipocytes (37). Conversely, neither 9,11 CLA nor 10,12 CLA affected SREBP-1a or SREBP-1c mRNA levels or the precursor protein in a bovine mammary cell line, whereas the abundance of the mature fragment of SREBP-1a and SREBP-1c protein was significantly reduced by 10,12 CLA, but not 9,11 CLA (38). Moreover, 10,12 CLA reduced the mRNA expression for critical genes in the lipid synthesis pathway, whereas 9,11 CLA had no effect. Collectively, these data suggest that 10,12 CLA might also affect the SREBP-1 pathway at the level of proteolytic cleavage rather than by affecting the availability of the precursor protein.
Alternatively, 10,12 CLA could reduce lipogenesis by inhibiting transporters of glucose (i.e., GLUT4) or FA (i.e., aP2) or esterification enzymes regulated by PPARγ [4, 15, 16, 22, 39], thereby limiting the de novo synthesis of SFA for desaturation by SCD-1. Less SFA substrate for SCD-1 would limit its activity (reviewed in 34). In agreement with this hypothesis, 10,12 CLA reduced PPARγ mRNA levels within 5 h (Fig. 9B, which could contribute to reduced PPARγ activity. Consistent with these data, we have previously shown that 10,12 CLA reduces PPARγ mRNA [15, 16], protein (22, 39–41), or activity (22, 40) in human adipocytes, and co-supplementation with the PPARγ agonist Rosiglitazone attenuates this effect along with preventing insulin resistance and delipidation by 10,12 CLA (22). In vivo, Belury’s group has shown that Rosiglitazone attenuates many of the adverse effects of CLA treatment including insulin resistance, lipodystrophy, or adiponectin depletion (21, 42).
We speculated that 10,12 CLA produces lipid-borne metabolites like ceramide [reviewed in 43] that antagonize PPARγ and insulin sensitivity, because increased PPARγ activity is antagonized by pro-inflammatory mediators like NFκB (44, 45), and our previous finding demonstrating that NFκB inhibitors or siRNA targeting p65 attenuates 10,12 CLA’s suppression of PPARγ and insulin resistance (39). However, 10,12 CLA decreased overall de novo synthesis of several lipid classes including ceramides, TG, and cholesterol esters. Therefore, although 10,12 CLA decreased the ratio of MUFA/SFA, it did not increase the levels of ceramide, a SFA-derived inflammatory lipid that causes insulin resistance (reviewed in 43). Consistent with these data, we found that treatment of human adipocytes with ceramide inhibitors (i.e., myriosin, fumonisin B1) did not attenuate 10,12 CLA-mediated induction of inflammatory genes or suppression of adipogenic/lipogeneic genes (data not shown). Thus, ceramide does not appear to play a role in 10,12 CLA-mediated inflammation or insulin resistance. In addition, 10,12 CLA reduced total acyl-CoA levels and increased the amount of C18:0 acyl-CoA, which has been implicated in insulin resistance due to impaired oxidation (30).
Based on our previous results and results from this work we suggest that, because of its unique structure, 10,12 CLA or its lipid-borne metabolites initially (e.g., within 5–7 h) alters the activity of lipogenic transcription factors and their respective downstream targets. Our previous results indicate that this could occur through activation of inflammatory signals such as NFκB [39, 41] that antagonize LXRα, PPARγ, and possibly also SREBP-1. This leads to decreased activity of lipogenic enzymes essential for FA desaturation and incorporation into neutral and compound lipids within 3–24 h. These 10,12 CLA-mediated changes ultimately reduce total cellular lipids within 72 h. Alternatively, 10,12 CLA could also directly affect the activity of desaturases and lipogenic enzymes; however, the kinetics of our data summarized in Table 2 are consistent 10,12 CLA acting primarily by attenuating the activity of lipogenic transcription factors and their targets, which in turn decrease de novo lipid metabolism and lipid accumulation, and not vice-versa.
Acknowledgments
Supported by grants from the NIH NIDDK/ODS (5R01-DK063070) to MM and SM, the North Carolina Agriculture Research Service (NCARS 06520) to MM, and the NIH-NIDDK (F31DK084812-02) to KM.
Supported by grants from the NIH NIDDK/ODS (5R01-DK063070) to MM and SM, the North Carolina Agriculture Research Service (NCARS 06520) to MM, and the NIH-NIDDK (F31DK084812-02) to KM. No conflicts of interest are declared. The authors’ responsibilities were as follows: TO, conducted studies in Figures 1–6, 8–9 and prepared the first draft of this manuscript; NF, daily direction of the research conducted by TO; SC, conducted the cell experiments in shown in Figure 7, which were sent to TO for subsequent analyses; KM, prepared and conducted statistical analyses of data shown in Figures 1–2, 5–10, and conducted experiment with ceramide inhibitors (data not shown); SG, conducted experiments with ceramide inhibitors (data not shown); OL, synthesized and provided the [14C]-CLA isomers; MW, provided the SGBS cells; SM, co-mentor for TO and CO-I on NIH CLA grant; and MM, PI of NIH CLA grant and revised the manuscript with input from TO and co-authors.
Abbreviations
- ACBP
acyl-CoA binding protein
- aP2/FABP4
adipocyte fatty acid binding protein 4
- BCA
bicinchoninic acid
- BCP
1-bromo-3-chloropropane
- BSA
bovine serum albumin
- CLA
conjugated linoleic acid
- DMEM
Dulbeco’s modified eagles medium
- DMSO
dimethyl sulfoxide
- EA
elaidic acid
- EOS
ceramide-containing sphingosine
- FA
fatty acids
- FBS
fetal bovine serum
- GC
gas chromatography
- GPDH
glycerol-3-phosphate dehydrogenase
- HBSS
Hanks balanced salt solution
- HEPES
n[2-hydroxyethyl]piperazine-n′-[2-ethansulfonic acid]
- HPTLC
high performance thin layer chromatography
- LXR
liver X receptor
- MUFA
monounsaturated fatty acid
- NS
non-hydroxy ceramide
- OA
oleic acid
- ORO
oil red O
- PBS
phosphate buffered saline
- PMSF
phenylmethylsulfonyl fluoride
- PPAR
peroxisome proliferator-activated receptor
- PPRE
peroxisome proliferator response element
- PVDF
polyvinylidene difluoride
- RT-PCR
reverse transcription- polymerase chain reaction
- RXR
retinoid X receptor
- SCD-1
stearoyl-CoA desaturase-1
- SDS
sodium dodecyl sulfate
- SFA
saturated fatty acid
- SGBS
Simpson-Golabi-Behmel Syndrome
- SREBP-1C
sterol regulatory element binding protein
- SV
stromal vascular
- TBP
TATA binding protein
- TLC
thin layer chromatography
- TG
triglyceride
- TVA
trans vaccenic acid
- VA
vaccenic acid
- WAT
white adipose tissue
Footnotes
No conflicts of interest are declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whigham LD, Watras AC, Schoeller DA. Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Am J Clin Nutr. 2007;85:1203–11. doi: 10.1093/ajcn/85.5.1203. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy A, Martinez K, Schmidt S, Mandrup S, Lapoint K, McIntosh M. Anti-obesity mechanisms of action of conjugated linoleic acid. J Nutr Biochem. 2010;21:171–79. doi: 10.1016/j.jnutbio.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park Y, Storkson J, Albright K, Liu W, Pariza M. Evidence that trans-10, cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids. 1999;34:235–41. doi: 10.1007/s11745-999-0358-8. [DOI] [PubMed] [Google Scholar]
- 4.Brown JM, Halverson YD, Lea-Currie R, Geigerman C, McIntosh M. Trans-10, cis-12, but not cis-9, trans-11, conjugated linoleic acid attenuates lipogenesis in primary cultures of stromal vascular cells from human adipose tissue. J Nutr. 2001;131:2316–21. doi: 10.1093/jn/131.9.2316. [DOI] [PubMed] [Google Scholar]
- 5.House RL, Cassady JP, Eisen EJ, Eling TE, Collins JB, Grissom SF, et al. Functional genomic characterization of delipidation elicited by trans-10, cis-12-conjugated linoleic acid (t10c12-CLA) in a polygenic obese line of mice. Physiol Genomics. 2005;21:351–61. doi: 10.1152/physiolgenomics.00244.2004. [DOI] [PubMed] [Google Scholar]
- 6.Brandebourg TD, Hu CY. Isomer-specific regulation of differentiating pig preadipocytes by conjugated linoleic acids. J Anim Sci. 2005;83:2096–105. doi: 10.2527/2005.8392096x. [DOI] [PubMed] [Google Scholar]
- 7.Raff M, Tholstrup T, Toiubro S, Bruun J, Lund P, Straarup E, et al. Conjugated linoleic acids reduce body fat in healthy postmenopausal women. J Nutr. 2009;139:1347–52. doi: 10.3945/jn.109.104471. [DOI] [PubMed] [Google Scholar]
- 8.Miller JR, Siripurkpong P, Hawes J, Majdalawieh A, Ro HS, McLeod RS. The trans-10, cis-12 isomer of conjugated linoleic acid decreases adiponectin assembly by PPARgamma-dependent and PPARgamma-independent mechanisms. J Lipid Res. 2008;49:550–62. doi: 10.1194/jlr.M700275-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Evans M, Geigerman C, Cook J, Curtis L, Kuebler B, McIntosh M. Conjugated linoleic acid suppresses triglyceride accumulation and induces apoptosis in 3T3-L1 preadipocytes. Lipids. 2000;35:899–910. doi: 10.1007/s11745-000-0599-6. [DOI] [PubMed] [Google Scholar]
- 10.Brodie AE, Manning VA, Ferguson KR, Jewell DE, Hu CY. Conjugated linoleic acid inhibits differentiation of pre- and post- confluent 3T3-L1 preadipocytes but inhibits cell proliferation only in preconfluent cells. J Nutr. 1999;129:602–6. doi: 10.1093/jn/129.3.602. [DOI] [PubMed] [Google Scholar]
- 11.Satory DL, Smith SB. Conjugated linoleic acid inhibits proliferation but stimulates lipid filling of murine 3T3-L1 preadipocytes. J Nutr. 1999;129:92–7. doi: 10.1093/jn/129.1.92. [DOI] [PubMed] [Google Scholar]
- 12.Choi Y, Kim YC, Han YB, Park Y, Pariza MW, Ntambi JM. The trans-10,cis-12 isomer of conjugated linoleic acid downregulates stearoyl-CoA desaturase 1 gene expression in 3T3-L1 adipocytes. J Nutr. 2000;130:1920–4. doi: 10.1093/jn/130.8.1920. [DOI] [PubMed] [Google Scholar]
- 13.Granlund L, Juvet LK, Pedersen JI, Nebb HI. Trans10, cis12-conjugated linoleic acid prevents triacylglycerol accumulation in adipocytes by acting as a PPARgamma modulator. J Lipid Res. 2003;44:1441–52. doi: 10.1194/jlr.M300120-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Kang K, Liu W, Albright KJ, Park Y, Pariza MW. Trans-10,cis-12 CLA inhibits differentiation of 3T3-L1 adipocytes and decreases PPAR gamma expression. Biochem Biophys Res Commun. 2003;303:795–9. doi: 10.1016/s0006-291x(03)00413-3. [DOI] [PubMed] [Google Scholar]
- 15.Brown M, Sandberg-Boysen M, Skov S, Morrison R, Storkson J, Lea-Currie R, et al. Isomer-specific regulation of metabolism and PPAR by conjugated linoleic acid (CLA) in human preadipocytes. J Lipid Res. 2003;44:1287–300. doi: 10.1194/jlr.M300001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown J, Boysen M, Chung S, Fabiyi O, Morrison R, Mandrup S, et al. Conjugated linoleic acid (CLA) induces human adipocyte delipidation: autocrine/paracrine regulation of MEK/ERK signaling by adipocytokines. J Biol Chem. 2004;279:26735–47. doi: 10.1074/jbc.M401766200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poirier H, Shapiro JS, Kim RJ, Lazar MA. Nutritional supplementation with trans-10,cis-12 conjugated linoleic acid induces inflammation of white adipose tissue. Diabetes. 2006;55:1634–40. doi: 10.2337/db06-0036. [DOI] [PubMed] [Google Scholar]
- 18.LaRosa PC, Miner J, Xia Y, Zhou Y, Kachman S, Fromm ME. Trans-10, cis-12 conjugated linoleic acid causes inflammation and delipidation of white adipose tissue in mice: a microarray and histological analysis. Physiol Genomics. 2006;27:282–94. doi: 10.1152/physiolgenomics.00076.2006. [DOI] [PubMed] [Google Scholar]
- 19.Granlund L, Pedersen JI, Nebb HI. Impaired lipid accumulation by trans10, cis12 CLA during adipocyte differentiation is dependent on timing and length of treatment. Biochim Biophys Acta. 2005;1687:11–22. doi: 10.1016/j.bbalip.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 20.LaRosa PC, Riethoven JJ, Chen H, Xia Y, Zhou Y, Chen M, et al. Trans-10, cis-12 conjugated linoleic acid activates the integrated stress response pathway in adipocytes. Physiol Genomics. 2007;31:544–53. doi: 10.1152/physiolgenomics.00156.2007. [DOI] [PubMed] [Google Scholar]
- 21.Liu LF, Purushotham A, Wendel AA, Belury MA. Combined effects of rosiglitazone and conjugated linoleic acid on adiposity, insulin sensitivity, and hepatic steatosis in high-fat-fed mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1671–G82. doi: 10.1152/ajpgi.00523.2006. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy A, Chung S, LaPoint K, Fabiyi O, McIntosh MK. Trans-10, cis-12 conjugated linoleic acid antagonizes ligand-dependent PPARgamma activity in primary cultures of human adipocytes. J Nutr. 2008;138:455–61. doi: 10.1093/jn/138.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loreau O, Maret A, Charigny JM, Sebedio JL, Noel JP. Sequential substitution of 1,2-dichloro-ethene: a convenient stereoselective route to (9Z,11E)-, (10E,12Z)- and (10Z,12Z)-[1-14C] conjugated linoleic acid isomers. Chem Phys Lipids. 2001;110:57–67. doi: 10.1016/s0009-3084(00)00229-2. [DOI] [PubMed] [Google Scholar]
- 24.Wabitsch M, Brenner RE, Melzner I, Braun M, Moller P, Heinze E, et al. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int J Obes Relat Metab Disord. 2001;25:8–15. doi: 10.1038/sj.ijo.0801520. [DOI] [PubMed] [Google Scholar]
- 25.Bligh E, Dyer W. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 26.Just M, Faergeman NJ, Knudsen J, Beck-Nielsen H, Gaster M. Long-chain Acyl-CoA is not primarily increased in myotubes established from type 2 diabetic subjects. Biochim Biophys Acta. 2006;1762:666–72. doi: 10.1016/j.bbadis.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Cromer KD, Jenkins TC, Thies EJ. Replacing cis octadecenoic acid with trans isomers in media containing rat adipocytes stimulates lipolysis and inhibits glucose utilization. J Nutr. 1995;125:2394–99. doi: 10.1093/jn/125.9.2394. [DOI] [PubMed] [Google Scholar]
- 28.Mozaffarian D, Pischon T, Hankinson SE, Rifai N, Joshipura K, Willett WC, et al. Dietary intake of trans fatty acids and systemic inflammation in women. Am J Clin Nutr. 2004;79:606–12. doi: 10.1093/ajcn/79.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saravanan N, Haseeb A, Ehtesham NZ, Ghafoorunissa Differential effects of dietary saturated and trans-fatty acids on expression of genes associated with insulin sensitivity in rat adipose tissue. Eur J Endocrinol. 2005;153:159–65. doi: 10.1530/eje.1.01946. [DOI] [PubMed] [Google Scholar]
- 30.Lowell B, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–87. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 31.Lee K, Pariza M, Ntambi J. Conjugated linoleic acid decreases hepatic stearoyl-CoA desaturase mRNA expression. Biochem Biophys Res Commun. 1998;248:817–21. doi: 10.1006/bbrc.1998.8994. [DOI] [PubMed] [Google Scholar]
- 32.Park Y, Storkson J, Ntambi J, Cook M, Sih C, Pariza M. Inhibition of hepatic stearoyl-CoA desaturase activity by trans-10, cis-12 CLA and its derivatives. Biochem Biophys Acta. 2000;1486:285–92. doi: 10.1016/s1388-1981(00)00074-3. [DOI] [PubMed] [Google Scholar]
- 33.Choi Y, Park Y, Pariza M, Ntambi J. Regulation of stearoyl-CoA desaturase activity by trans-10, cis-12 CLA in HepG2 cells. Biochem Biophys Res Commun. 2001;284:689–93. doi: 10.1006/bbrc.2001.5036. [DOI] [PubMed] [Google Scholar]
- 34.Ntambi J, Miyazaki M. Regulation of stearoyl-CoA desaturase and role in metabolism. Prog Lipid Res. 2004;43:91–104. doi: 10.1016/s0163-7827(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 35.Man WC, Miyazaki M, Chu K, Ntambi J. Co localization of SCD1 and DGAT2: implying preference for endogenous monounsaturated fatty acids in triglyceride synthesis. J Lipid Res. 2006;47:1928–39. doi: 10.1194/jlr.M600172-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Kang K, Miyazaki M, Ntambi J, Pariza M. Evidence that the antiobesity effect of CLA is independent of effects on stearoyl-CoA desaturase 1 expression and enzyme activity. Biochem Biophys Res Commun. 2004;315:532–37. doi: 10.1016/j.bbrc.2004.01.087. [DOI] [PubMed] [Google Scholar]
- 37.Brandebourg TD, Hu CY. Isomer-specific regulation of differentiating pig preadipocytes by conjugated linoleic acids. J Anim Sci. 2005;83:2096–105. doi: 10.2527/2005.8392096x. [DOI] [PubMed] [Google Scholar]
- 38.Peterson DG, Matitashvili EA, Bauman DE. The inhibitory effect of trans-10, cis-12 CLA on lipid synthesis in bovine mammary epithelial cells involves reduced proteolytic activation of the transcription factor SREBP-1. J Nutr. 2004;134:2523–27. doi: 10.1093/jn/134.10.2523. [DOI] [PubMed] [Google Scholar]
- 39.Chung S, Brown JM, Provo JN, Hopkins R, McIntosh M. Conjugated linoleic acid promotes human adipocyte insulin resistance through NF B-dependent cytokine production. J Biol Chem. 2005;280:38445–456. doi: 10.1074/jbc.M508159200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy A, Overman A, Martinez K, LaPoint K, Hopkins R, Chuang CC, et al. Conjugated linoleic acid (CLA)-mediated inflammation and insulin resistance in human adipocytes are attenuated by resveratrol. J Lipid Res. 2009;50:225–32. doi: 10.1194/jlr.M800258-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kennedy A, Martinez K, Chung S, LaPoint K, West T, Hopkins R, et al. Inflammation and insulin resistance induced by trans-10, cis-12 conjugated linoleic acid are dependent on intracellular calcium levels in primary cultures of human adipocytes. J Lipid Res. 2010;51:1906–17. doi: 10.1194/jlr.M005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Purushotham A, Wendel A, Liu C, Belury M. Maintenance of adiponectin attenuates insulin resistance induced by conjugated linoleic acid in mice. J Lipid Res. 2007;48:444–52. doi: 10.1194/jlr.M600393-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Holland W, Summer S. Sphingolipids insulin resistance, and metabolic disease; new insights from in vivo manipulation of sphingolipid metabolism. Endrocrine Rev. 2008;229:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pascual G, Fong A, Ogawa S, Gamliel A, Li AC, Perissi V, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPARgamma. Nature. 2005;437:759–63. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ricotte M, Glass C. PPARs and molecular mechanisms of transrepression. Biochem Biophys Acta. 2007;1771:926–35. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]