Abstract
Objective
To determine the function of T0901317 in combination treatment with cisplatin in ovarian cancer cells.
Methods
We screened the effects of three nuclear hormone receptor ligands on cell viability in a panel of ovarian cancer cells lines. T0901317 regulation of apoptosis and cell cycle regulators was determined when applied as a single agent, or in combination with cisplatin.
Results
Surprisingly, the LXR agonist T0901317 had no significant effects on a panel of seven ovarian cancer cell lines as a single agent. T0901317 does, however, significantly decrease cisplatin efficacy in at least three ovarian cancer cells lines. T0901317 reduces cisplatin induced apoptosis and reverses cisplatin induced expression of cell cycle regulators. T0901317 appears to work in an LXR, PXR, and FXR independent manner, as agonists of these nuclear hormone receptors did not show similar effects. Interestingly, in the A2780-cp drug resistant cell line, the effect of T0901317 is lost, suggesting that the pathways stimulated by T0901317 to reduce cisplatin efficacy could be inherently active features of the selected resistance.
Conclusions
Together, these data suggest that T0901317 inhibits cisplatin in some ovarian cancer cells. These data provide an avenue to investigate when T0901317 may be acting to promote tumor survival and drug resistance through control of apoptosis, and when it may be acting as an anti-tumor agent, as has been previously reported.
Keywords: T0901317, LXR, cisplatin, ovarian cancer, apoptosis
Introduction
Epithelial ovarian cancer is a deadly disease affecting thousands of women each year and is the most lethal of all the gynecologic cancers. Most patients are treated with an initial debulking surgery followed by platinum-taxane-based therapy, with 5-year survival approaching 50%. [1]. Recurrent disease is often both drug resistant and metastatic. Significant efforts have focused on developing new chemotherapeutic compounds that are effective either alone, or in combination with other available agents, such as taxol and platinum-based chemotherapeutics [2].
Recently, a number of members of the nuclear receptor (NR) superfamily have been shown to enhance chemotherapeutic efficacy. In particular, vitamin D receptor (VDR) [3-5], peroxisome proliferator-activated receptor gamma (PPARγ)[3, 6-9], and liver x receptors α and β (LXRs) [10-14] have been shown to mediate tumor proliferation in at least some systems. NRs regulate key factors and pathways that may be viable drug targets, and may also further our understanding of tumor biology. LXR ligands such as T0901317 have been investigated as possible adjuvant therapy in cancer cells, including ovarian cancer cells [10-14].
In this study, we tested three NR ligands on the efficacy of the commonly used chemotherapeutic agent, cis-diamminedichloroplatinum(II), cisplatin, and discovered that the LXR agonist, T0901317, either shows no significant effect on proliferation, or increases the IC50 of cisplatin in ovarian cancer cell lines. We determined that T0901317 increases the number of viable cells by decreasing cisplatin induced apoptosis. Other LXR ligands do not show similar effects, suggesting that T0901317 is inhibiting cisplatin by an LXR independent mechanism. In sum, T0901317 desensitizes some ovarian cancer cell lines to chemotherapeutics by driving progression through the cell cycle.
Results
Screen of nuclear hormone receptor ligands on cisplatin efficacy
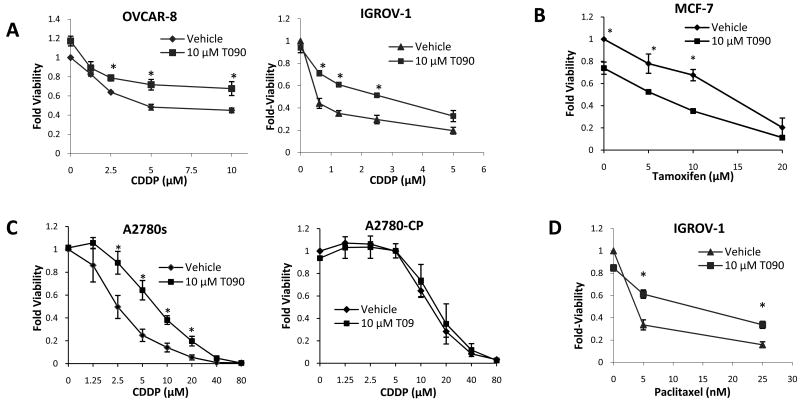
Six ovarian cancer cell lines (SKOV-3, IGROV-1, OVCAR-3, OVCAR-4, OVCAR-5, and OVCAR-8) were tested with combinations of cisplatin and either VDR agonist 1,25-dihydroxyvitamin D3 (D3), PPARγ agonist Rosiglitazone (Rosi), or LXR agonist T0901317. We also treated the breast cancer cell line, MCF-7, where the effects of these ligands have been previously reported [13, 15, 16]. Measurements of cell viability after four days of treatment revealed only small, mostly insignificant effects of D3 and Rosi on cisplatin efficacy (Table 1), but, surprisingly, we found that T0901317 treatment in combination with cisplatin reduced the efficacy of the drug in the cell lines IGROV-1 and OVCAR-8 (Figure 1A). Numerous previous reports suggest that T0901317 reduces proliferation of breast and prostate cancer cell lines [10, 13]. As expected, T0901317 treatment reduced the viability of MCF-7 cells in combination with Tamoxifen (Figure 1B). We focused on 10 μM T0901317 for most experiments, as we observed no significant effects at 1 μM and only modest effects at 5 μM T0901317 in these cell viability experiments.
Table 1. Viability Screen of Nuclear Receptor Ligands.
100 nM D3, 10 μM Rosi, and 10 μM T0901317 were tested in combination with tamoxifen in MCF-7 and cisplatin in all ovarian cell lines for four days, after which viability was measured by WST-1. “−−” indicates a significant reduction in viability of >20%, “−” indicates a mild reduction of <20%, “○” indicates no effect on viability, “+” indicates a mild increase in viability of <20%, and “++” indicates a significant increase in viability of >20%. “/” indicates that the compound was not tested.
| Cell Line | D3 | Rosi | T0901317 |
|---|---|---|---|
| IGROV-1 | ○ | ○ | + + |
| OVCAR-3 | − | − | − |
| OVCAR-4 | + | ○ | ○ |
| OVCAR-5 | ○ | − | ○ |
| OVCAR-8 | ○ | − | + + |
| SKOV-3 | ○ | ○ | + |
| MCF-7 | − − | ○ | − − |
| A2780 | / | / | + + |
| A2780-cp | / | / | ○ |
Figure 1. T0901317 Reduces Efficacy of cisplatin in Ovarian Cell Lines.
Cells were treated with the indicated drugs for four days and viability was measured by WST-1. (A) T0901317 (T09) reduces the efficacy of cisplatin (CDDP) in the ovarian cancer cell lines, OVCAR-8 and IGROV-1. (B) In the breast cancer cell line, MCF-7, T0901317 enhances chemotherapeutic efficacy. (C) In the ovarian cell line, A2780, T0901317 has the largest effect on cisplatin efficacy, but in the paired drug resistant line, A2780-cp, the effect on cisplatin efficacy is completely lost. (D) T0901317 also reduces the efficacy of paclitaxel in IGROV-1 cells. * p < 0.05 for the indicated treatment, as compared to vehicle treatment at the same concentration of chemotherapeutic from biological triplicates.
T0901317 treatment does not affect cisplatin efficacy in a chemo-resistant model
We tested the effects of T0901317 on cell viability in a commonly used model for drug resistance in ovarian cancer cells, the paired A2780 and A2780-cp cell lines. T0901317 did not affect cisplatin efficacy in the drug resistant line, A2780-cp (Figure 1C), suggesting that T0901317 stimulated pathways may be permanently altered as part of the selected resistance.
Effect of T0901317 on drug efficacy is not specific to cisplatin
To determine whether T0901317 inhibits cisplatin specifically, or also inhibits other anti-tumor drugs, we investigated combinations of T0901317 with paclitaxel. T0901317 reduced paclitaxel efficacy in IGROV-1 cells, similar to its effect on cisplatin efficacy, as measured by viability after four days of treatment (Figure 1D). This suggests that the mechanism of T0901317 action is likely not specific to the action of cisplatin, but rather that it may be influencing general survival and proliferation pathways.
The role of LXR in the T0901317 induced increase in cisplatin IC50
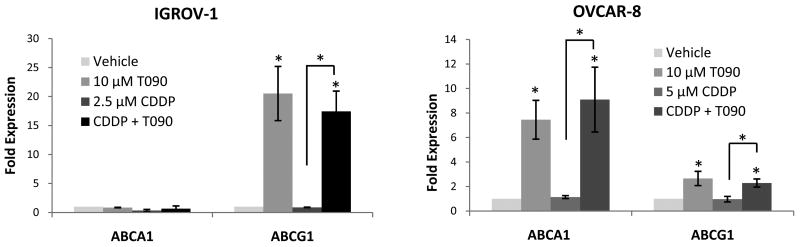
In order to test whether T0901317 was activating LXR in ovarian cancer cells, we measured transcript levels of two known LXR target genes, ABCA1 and ABCG1. In the T0901317 responsive IGROV-1 and OVCAR-8 cell lines, both ABCA1 and ABCG1 are significantly stimulated by T0901317 (Figure 2). In OVCAR-8 cells, ABCG1 increases a modest two-fold, while ABCA1 transcript levels increase nearly ten-fold. Interestingly, in IGROV-1 cells, T0901317 does not significantly stimulate ABCA1 transcript levels, but ABCG1 expression increases twenty-fold. These findings suggest that LXR is activated by T0901317 in these cell lines.
Figure 2. T0901317 Activates LXR.
T0901317 significantly upregulates expression of LXR targets. In IGROV-1, only the LXR target ABCG1 is upregulated, while in OVCAR-8 both ABCA1 and ABCG1 are upregulated after 24 hours of T0901317 treatment.* p < 0.05 for the treatment compared to vehicle, unless otherwise indicated.
To test whether the effect on viability was a result of T0901317's activation of LXR, we tested five additional LXR agonists, GW3965, 22(R)-hydroxycholesterol, and 25-hydroxycholesterol, desmosterol and stigmasterol [17] for effects on viability in combination with cisplatin treatment. Surprisingly, we found that none of these other ligands replicated T0901317's effect on cisplatin efficacy (Table 2). The chemical structures of all the ligands are shown in Supplemental Figure 1. Depending on the cell line, T0901317 had no effect, decreased or increased the number of viable cells. This raises the possibility that T0901317 affects the IC50 of cisplatin by an LXR independent mechanism.
Table 2. T0901317 Effect May Be LXR, PXR, and FXR Independent.
The indicated compounds were tested in combination with cisplatin in the ovarian cell line, A2780. “−−” indicates a significant reduction in viability of >20%, “−” indicates a mild reduction of <20%, “○” indicates no effect on viability, and “++” indicates a significant increase in viability of >20%.
| Cell Line | T0901317 | 22(R) | 25-OHC | GW3965 | CDCA | GW4064 | Hyper-forin | SR12813 | Desmo-sterol | Stigma-sterol |
|---|---|---|---|---|---|---|---|---|---|---|
| A2780 | + + | ○ | − − | − | ○ | ○ | − | ○ | ○ | ○ |
While T0901317 is widely used as an LXR agonist, it may stimulate other nuclear receptors as well. Two candidates are the pregnane X receptor (PXR) [18] and the farnesoid X receptor (FXR) [19]. Because a number of LXR ligands did not show similar effects as T0901317, we next attempted to determine whether the effect was mediated by one of these other receptors, for which T0901317 may serve as an agonist. We found, however, that no combination of FXR ligands (including chenodeoxycholic acid and GW4064) or PXR ligands (including hyperforin and SR12813) could replicate the effects of T0901317 on cell viability (Table 2). Therefore, it appears that T0901317's effect on cisplatin in these cell lines may be LXR, PXR, and FXR independent.
T0901317 inhibits apoptosis when treated in combination with cisplatin
To determine whether T0901317 induced reduction of cisplatin efficacy is mediated through the inhibition of apoptosis, we tested the effect of T0901317 on cisplatin induced apoptosis in OVCAR-8 and IGROV-1 cells using two independent assays. Figure 3A shows apoptosis as measured by DAPI staining cells after 48 hours of either vehicle treatment, 2.5 μM cisplatin treatment, 10 μM T0901317 treatment, or a combination of both cisplatin and T0901317 treatment. Cells were scored as either apoptotic or non-apoptotic based on nuclear morphology and the fraction of apoptotic cells in each condition is charted, showing a significant decrease in apoptotic fraction in the co-treated conditions compared to cisplatin treatment alone (Figure 3A). We also measured caspase 3/7 activity over a range of cisplatin concentrations, and observe a significant reduction of caspase activation after 48 hours with T0901317 co-treatment, compared to cisplatin alone (Figure 3B). This assay also allowed us to show a dose dependent reduction in apoptosis in the A2780 cell line, with significant effects at both 2 μM and 10 μM T0901317. These assays suggest that T0901317 significantly inhibits cisplatin induced apoptosis.
Figure 3. T0901317 Treatment Reduces cisplatin-induced Apoptosis.
48 hour co-treatment of T0901317 with cisplatin causes a significant reduction in apoptosis compared to cisplatin treatment alone, as measured by two independent assays. (A) DAPI staining of nuclei and classification of cells as either apoptotic or non-apoptotic indicates that T0901317 significantly reduces apoptosis when treated in combination with cisplatin. (B) Measurement of apoptosis by quantification of caspase 3 and caspase 7 activity also indicates reduced levels of apoptosis with co-treatment of T0901317 and cisplatin when compared to cisplatin treatment alone. In the more T0901317-sensitive cell line, A2780, there is a dose dependent reduction of apoptosis. * p < 0.05 in the indicated condition when compared to vehicle treatment.
T0901317 hinders cisplatin induced cell cycle arrest
Cisplatin also induces changes in expression of key cell cycle checkpoint proteins, leading to cell cycle arrest. The effects of T0901317 on the expression of key factors regulating cell cycle progression were investigated by Western blotting analysis. A2780 cells were treated with either, vehicle control, 2.5 μM cisplatin, 10 μM T0901317, or 2.5 μM cisplatin and 10 μM T0901317 together, for 48 hours and protein was collected. T0901317 treated cells showed increased levels of CDK4, Cyclin D1, and Cyclin D3 protein in the absence or presence of cisplatin (Figure 4). The formation of CDK-cyclin catalytic complexes is essential for G1/S transition [20, 21, 22]. These data suggest T0901317 promotes entry into the cell cycle even in the presence of cisplatin.
Figure 4. T0901317 regulates the balance between cell cycle inhibitors and drivers.
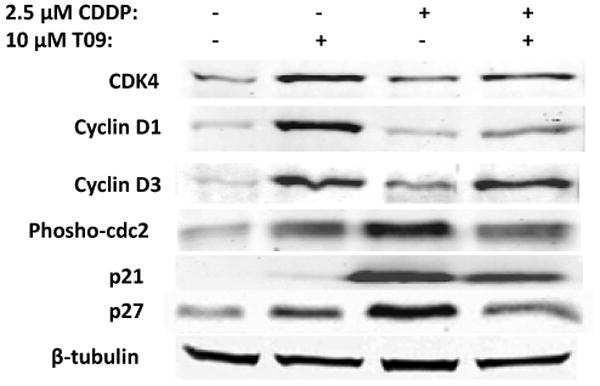
Whole cell lysates from A2780 were subjected to immunoblotting. T0901317 as a single agent or in combination with cisplatin increases the expression of cell cycle drivers, CDK4, Cyclin D1, and Cyclin D3. T0901317 reduces expression of p21, p27 and Tyr15 phosphorylated cdc2 in combination with cisplatin compared to the cisplatin treated condition. A representative β-tubulin blot is shown as a loading control.
Cell cycle inhibitors are commonly regulated in cancer cell lines in response to various agents. Dephosphorylation of the kinase, cdc2, at tyrosine 15 (Tyr15) is a critical step for its activation, and for entry into mitosis, and high levels of Tyr15 phosphorylation indicate cell cycle arrest [23]. Cisplatin treatment increased levels of phosphorylated cdc2 compared to vehicle. However, in cells co-treated with T0901317 and cisplatin, the level of Tyr15-phosphorylated cdc2 was considerably lower than in cisplatin treated cells (Figure 4). Platinum therapeutics have previously been shown to increase the expression of p27 Kip1 and p21 Waf1/Cip1, leading to cell cycle inhibition [24, 25]. Figure 4 shows similar increase in p21 and p27 expression upon cisplatin treatment. However, T0901317 co-treatment significantly reduced expression of both p21 and p27, compared to cisplatin treatment alone (Figure 4). These observations are consistent with p27 and p21 acting as inhibitors of the cell cycle, as shown by increased expression induced by cisplatin and reduced expression by T0901317 co-treatment (Figure 4), when cells are more viable (Figure 1). In sum, these data suggest that T0901317 treatment is capable of not only driving the cell cycle, but also of reducing the magnitude of cisplatin-induced cell cycle arrest.
Discussion
The synthetic compound, T0901317, is an LXR agonist commonly used to test LXR function. The influence of T0901317 and LXR in mediating cancer cell proliferation has been tested in many cell lines, including ovarian cancer cells [26-28]. We aimed to test how T0901317 may enhance cisplatin efficacy as a combination treatment. However, unlike previous reports [26, 28], we observe that T0901317 has very little influence on cell growth when used alone, but significantly increases the IC50 of both cisplatin and paclitaxel it is added in combination. In the A2780-cp cell line, selected for drug resistance, the effect of T0901317 is abrogated, suggesting that T0901317-regulated pathways are important contributors to cisplatin sensitivity in the parental A2780 cell line. Together, these data suggest that T0901317 is not an effective anti-tumor therapeutic for some ovarian cancer cells, but may instead be harmful, as it appears to actually reduce the efficacy of cisplatin and paclitaxel.
To investigate the mechanisms of T0901317's influence on the cell cycle in ovarian cancer cell lines, we probed the protein levels of cell cycle and survival regulators. These data suggest an apparent paradox of T0901317 activity. CDK4, cyclin D1, and cyclin D3 are all significantly increased upon T0901317 treatment, consistent with T0901317 increasing the number of viable cells. At the same time, cell cycle inhibitors such as p27 and p21, which often are up-regulated from toxic compounds such as cisplatin [24, 25], are significantly increased. When co-treated with cisplatin, T0901317 significantly increases the expression of CDK4, cyclin D1, and cyclin D3, likely promoting the observed survival and reduced apoptosis. These data suggest that T0901317 is activating both pro-growth and pro-apoptotic signals, but that its action leads to a net decrease in cisplatin efficacy on some ovarian cancer cells.
The observation that myriad LXR ligands do not affect ovarian cancer cells in the same fashion as T0901317 suggests that T0901317 is not working through LXR. LXR is expressed in these cell lines and T0901317 stimulates the up-regulation of known LXR targets, suggesting LXR signaling is activated. However, none of the other LXR ligands showed similar significant effects on cell viability and drug response, suggesting that T0901317 may not be reducing chemotherapy efficacy through an LXR mediated mechanism. PXR and FXR agonists also showed no significant effects, suggesting that T0901317 affects drug response in ovarian cell lines by an LXR, PXR, and FXR independent mechanism. SiRNA knock-down of LXRα and LXRβ did not affect the number of viable OVCAR-8 cells (data not shown), further suggesting that LXR activity does not have significant effects on cell proliferation in OVCAR-8. The concentration of T0901317 required to yield the effect on cisplatin efficacy is also indicative of LXR independence. Typically, T0901317 induces activation of LXR at concentrations <1 μM. We have seen no significant effects of T0901317 at concentrations this low (data not shown), suggesting that the effects presented here, seen at treatments between 1 and 10 μM, are likely independent of LXR.
Together, these data suggest that T0901317 increases ovarian cancer cell survival and drug resistance, likely in an LXR independent manner. These data differ from previous reports of anti-proliferative effects of T0901317 where very high concentrations were used [12, 26]. At concentrations above 20 μM, we also observe T0901317 anti-proliferative effects. We observe T0901317 protective effects in the 1-10 μM range. We have performed these experiments using multiple lots of T0901317 and have observed very consistent data from both relatively early and late passage cells. Because we see T0901317 killing MCF-7 cells, we believe that the T0901317 we are using is effective to reduce the number of viable cells for some cell lines, as previously reported [13]. We also observe T0901317 stimulating p27 and p21 expression, similar to previous reports [26], but these changes do not seem to manifest as changes in the number of viable cells in the 1-10 μM range. These earlier studies of T0901317's effect on ovarian cancer cells did not address its effect in combination with cisplatin, where we observe a significant reduction in efficacy, suggesting that T0901317 has different activities at low and high concentrations.
We have identified new effects of T0901317 in mediating cytotoxic drugs in ovarian cancer cells. Future efforts to investigate these concentration dependent effects may uncover LXR dependent and independent pathways mediating ovarian cancer cell growth and apoptosis, and may lead to new mechanisms controlling chemotherapy. Identifying these key factors and mechanisms mediating T0901037 and LXR phenotypes will be critical to translating these observations into advances in the clinical treatment of ovarian cancer.
Materials and Methods
Materials
SKOV-3 and OVCAR-3 cell lines were purchased from the American Type Culture Collection. IGROV-1, OVCAR-4, OVCAR-5, OVCAR-8 cell lines were purchased through the National Cancer Institute DTP tumor repository program, A2780 and A2780-cp lines were generously provided by Dr. Barbara Vanderhyden (University of Ottawa, Ottawa, Ontario). Cells were grown in DMEM (Cellgro) with 10% FBS, 1% Penicillin, and 1% Streptavidin (Thermo-Fisher) added, except for OVCAR-3 cells, which were grown in 20% FBS. Cisplatin, CDCA, GW3965, GW4064, hyperforin, Paclitaxel, SR12813, 22(R)-hydroxycholesterol, and 25-hydroxycholesterol were purchased from Sigma-Aldrich. T0901317, Rosiglitazone, and GW9622 were purchased from Axxora, and 1,25-dihydroxyvitamin D3 was purchased from Enzo Life Sciences.
Cell Viability Assays
Cells were plated in 96 well plates and treated with the indicated concentrations of drug 24 hours later. 96 hours after treatment, viability was measured using WST-1 (Roche) according to manufacturer's protocol.
Apoptotic Assays
Cells were plated, and, after 24 hours, were treated with the indicated concentrations of drug. For 4′,6-Diamidino-2-Phenylindole, (DAPI) staining assays the cells were stained with DAPI (Invitrogen) 48 hours after drug treatment, then imaged and counted by eye. For caspase activity measurements, Caspase-Glo 3/7 kit (Promega) was used according to manufacturer's protocol.
mRNA transcript level measurements
mRNA transcript levels were measured by real time quantitative polymerase chain reaction. RNA was isolated using Trizol (Invitrogen), and prepped using miRNeasy kit (Qiagen) according to manufacturer's protocol. Total RNA was then reverse transcribed using Superscript III (Invitrogen) and quantified and normalized using Picogreen (Invitrogen). Real time PCR was performed with SYBR green master mix (Applied Biosystems) in a 7900HT Fast Real-Time PCR thermal cycler (Applied Biosystems). Delta delta CT between treatment and vehicle was calculated using calnexin, which is not altered by drug treatment.
Immunoblotting
Anti-bodies against Cyclin D1, Cyclin D3, CDK4, p15 INK4B, p27 Waf1/Cip1, and phospho-cdc2 (Tyr15) were purchased from Cell Signaling. Anti-body against β-tubulin was purchased from Abcam. Secondary anti-bodies were purchased from Li-Cor Biosciences, and blots were visualized and quantified using a Li-Cor Odyssey Infrared Imaging scanner.
Statistical Analysis
P-values for DAPI analysis were calculated using Fisher's exact test. All other p-values were calculated using two-tailed, unpaired t tests. All error bars indicate +/- one standard deviation from all biological replicates.
Supplementary Material
Acknowledgments
We thank Barbara Vanderhyden for her kind gift of A2780 cells. This work was supported in part by Brown University start-up fund, a NHGRI K22 (7K22HG002488) Genome Scholar Award (A.S.B.) and NIH grant 5P41RR001395 (A.S.B).
Footnotes
The authors declare that there are no conflicts of interest.
References
- 1.ACS Cancer Facts and Figures. 2010 Available from: http://ww2.cancer.org/downloads/STT/Cancer_Facts_and_Figures_2010.pdf.
- 2.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3(7):502–16. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 3.Sertznig P, et al. Peroxisome proliferator-activated receptor (PPAR) and vitamin D receptor (VDR) signaling pathways in melanoma cells: promising new therapeutic targets? J Steroid Biochem Mol Biol. 2010;121(1-2):383–6. doi: 10.1016/j.jsbmb.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 4.McCullough ML, Bostick RM, Mayo TL. Vitamin D gene pathway polymorphisms and risk of colorectal, breast, and prostate cancer. Annu Rev Nutr. 2009;29:111–32. doi: 10.1146/annurev-nutr-080508-141248. [DOI] [PubMed] [Google Scholar]
- 5.Peterlik M, Grant WB, Cross HS. Calcium, vitamin D and cancer. Anticancer Res. 2009;29(9):3687–98. [PubMed] [Google Scholar]
- 6.Broadhead ML, Dass CR, Choong PF. Cancer cell apoptotic pathways mediated by PEDF: prospects for therapy. Trends Mol Med. 2009;15(10):461–7. doi: 10.1016/j.molmed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Menendez JA. Fine-tuning the lipogenic/lipolytic balance to optimize the metabolic requirements of cancer cell growth: molecular mechanisms and therapeutic perspectives. Biochim Biophys Acta. 2010;1801(3):381–91. doi: 10.1016/j.bbalip.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Mansure JJ, Nassim R, Kassouf W. Peroxisome proliferator-activated receptor gamma in bladder cancer: a promising therapeutic target. Cancer Biol Ther. 2009;8(7):6–15. doi: 10.4161/cbt.8.7.7853. [DOI] [PubMed] [Google Scholar]
- 9.Ondrey F. Peroxisome proliferator-activated receptor gamma pathway targeting in carcinogenesis: implications for chemoprevention. Clin Cancer Res. 2009;15(1):2–8. doi: 10.1158/1078-0432.CCR-08-0326. [DOI] [PubMed] [Google Scholar]
- 10.Chuu CP, et al. Modulation of liver X receptor signaling as novel therapy for prostate cancer. J Biomed Sci. 2007;14(5):543–53. doi: 10.1007/s11373-007-9160-8. [DOI] [PubMed] [Google Scholar]
- 11.Villablanca EJ, et al. Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat Med. 2010;16(1):98–105. doi: 10.1038/nm.2074. [DOI] [PubMed] [Google Scholar]
- 12.Scoles DR, et al. Liver X receptor agonist inhibits proliferation of ovarian carcinoma cells stimulated by oxidized low density lipoprotein. Gynecol Oncol. 2010;116(1):109–16. doi: 10.1016/j.ygyno.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 13.Vedin LL, et al. The oxysterol receptor LXR inhibits proliferation of human breast cancer cells. Carcinogenesis. 2009;30(4):575–9. doi: 10.1093/carcin/bgp029. [DOI] [PubMed] [Google Scholar]
- 14.Gong H, et al. Estrogen deprivation and inhibition of breast cancer growth in vivo through activation of the orphan nuclear receptor liver X receptor. Mol Endocrinol. 2007;21(8):1781–90. doi: 10.1210/me.2007-0187. [DOI] [PubMed] [Google Scholar]
- 15.Kim KY, Kim SS, Cheon HG. Differential anti-proliferative actions of peroxisome proliferator-activated receptor-gamma agonists in MCF-7 breast cancer cells. Biochem Pharmacol. 2006;72(5):530–40. doi: 10.1016/j.bcp.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Welsh J. Induction of apoptosis in breast cancer cells in response to vitamin D and antiestrogens. Biochem Cell Biol. 1994;72(11-12):537–45. doi: 10.1139/o94-072. [DOI] [PubMed] [Google Scholar]
- 17.Yang C, et al. Sterol intermediates from cholesterol biosynthetic pathway as liver X receptor ligands. J Biol Chem. 2006;281(38):27816–26. doi: 10.1074/jbc.M603781200. [DOI] [PubMed] [Google Scholar]
- 18.Mitro N, et al. T0901317 is a potent PXR ligand: implications for the biology ascribed to LXR. FEBS Lett. 2007;581(9):1721–6. doi: 10.1016/j.febslet.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 19.Houck KA, et al. T0901317 is a dual LXR/FXR agonist. Mol Genet Metab. 2004;83(1-2):184–7. doi: 10.1016/j.ymgme.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Reed SI. Control of the G1/S transition. Cancer Surv. 1997;29:7–23. [PubMed] [Google Scholar]
- 21.Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79(4):551–5. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81(3):323–30. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 23.Norbury C, Blow J, Nurse P. Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. EMBO J. 1991;10(11):3321–9. doi: 10.1002/j.1460-2075.1991.tb04896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitsuuchi Y, et al. The phosphatidylinositol 3-kinase/AKT signal transduction pathway plays a critical role in the expression of p21WAF1/CIP1/SDI1 induced by cisplatin and paclitaxel. Cancer Res. 2000;60(19):5390–4. [PubMed] [Google Scholar]
- 25.He G, et al. Upregulation of p27 and its inhibition of CDK2/cyclin E activity following DNA damage by a novel platinum agent are dependent on the expression of p21. Br J Cancer. 2006;95(11):1514–24. doi: 10.1038/sj.bjc.6603448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rough JJ, et al. Anti-proliferative effect of LXR agonist T0901317 in ovarian carcinoma cells. J Ovarian Res. 2010;3:13. doi: 10.1186/1757-2215-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim KH, et al. Inhibitory effect of LXR activation on cell proliferation and cell cycle progression through lipogenic activity. J Lipid Res. 2010;51(12):3425–33. doi: 10.1194/jlr.M007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuu CP, Lin HP. Antiproliferative effect of LXR agonists T0901317 and 22(R)-hydroxycholesterol on multiple human cancer cell lines. Anticancer Res. 2010;30(9):3643–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.