Abstract
Background
Based on published data, it is widely believed and cited that rofecoxib use is associated with approximately a 50% reduction in significant gastrointestinal complications such as bleeding.
Methods
Data made available as part of litigation, including the VIGOR trial and an Azlheimers’ study, allow a reassessment of the reported benefits of rofecoxib in terms of a significant reduction in complicated GI events and in lower gastrointestinal bleeding.
Results
During the review process of the VIGOR study it was suggested that rofecoxib might have little benefit, with regards to GI toxicity, for rheumatoid arthritis patients not treated with corticosteroids. Reanalysis of the original Merck data set showed 9 complicated confirmed events in the rofecoxib group compared to 10 in the naproxen group among corticosteroid non-users and 7 vs. 27 among corticosteroid users so that the difference between rofecoxib and naproxen in the occurrence of confirmed complicated PUBs appeared to be entirely due to the effects within corticosteroid users. The claim that serious lower GI events were 54% lower with the use of the selective COX-2 inhibitor rofecoxib was stated to be based on an assessment blinded to treatment allocation. In fact the choice did not represent the original blinded analysis that showed a nonsignificant difference, but rather was based on an assessment after treatment allocation was disclosed.
Conclusion
Examination and reanalysis of unpublished data regarding rofecoxib has failed to confirm a safety advantage of rofecoxib over tNSAIDs in terms of complicated upper or lower GI events.
Keywords: Rofecoxib, gastrointestinal bleeding, NSAID complications, corticosteroids
Introduction
Improvements in health and quality of life are often dependent in part upon new medications that offer new or improved capabilities (e.g., blood pressure control), are safer, or more convenient. A “block-buster” drug generally fulfills a previously recognized or even an unrecognized “need”, is widely used, and very profitable for the company that develops it. In the last several decades, marketing has become an integral part of drug development, even in early phases, in assisting both in the identification of potential block-busters as well as helping to plan a research and development strategy. The design and reporting of pharmaceutical research involves both business and scientific decisions. As with all clinical research, in interpreting reported results, readers must consider the study design (i.e., choice of end points, differences in dosage, etc) as well as decisions regarding appropriate analyses. However, the reader can never discern information that was not revealed. The role of drug marketing has received considerable recent attention 1-3. The focus of that debate has been primarily related to authorship of clinical trial and review manuscripts, and disclosure of industry financial support 4 with considerable attention being focused on the marketing of the COX-2 inhibitor, rofecoxib, in part, because of the presence of court documents obtained as part of litigation related to rofecoxib and its potential cardiovascular effects, 4. More recent litigation has provided new opportunities to examine the reported safety claims made regarding the relationship between rofecoxib use and gastrointestinal complications.
Based on the published data, it is widely believed and cited that rofecoxib use is associated with approximately a 50% reduction in significant gastrointestinal complications such as bleeding 5-7. Data made available as part of recent litigation (State of Louisiana, ex rei., James D. Caldwell, Jr., Attorney General, Plaintiffs, v. Merck Sharp & Dohme Corp, Defendants. Case No. 05-3700, United States District Court Eastern District of Louisiana, April, 2010) suggested that the reported benefits of rofecoxib in terms of a significant reduction in complicated GI events and in lower gastrointestinal bleeding may deserve another look. Here, we focus on the five major manuscripts directly related to this topic, and related memos and analyses including the 1999 Langman et al. paper in which the results of 8 clinical trials with rofecoxib were published 8, the 2004 Watson et al. paper 9, and the underlying analysis contained in a memo from Qinfen Yu [“Combined analysis of PUBs in Vioxx studies (except Vigor) – Final update for the MK-vs-NSAIDs comparison dated 2/27/2003 (revised draft)”] that represent an update of the Langman et al. data, and three papers related to the original VIGOR trial (i.e., the trial itself and the two post hoc analyses that (i) stratified the risk of NSAID-related upper gastrointestinal critical events and (ii) evaluated serious lower gastrointestinal clinical events 10-12. The unpublished data and court documents referred to are available at (web site to be determined after acceptance). These studies represent the sum of the data examining possible reductions in complicated GI events and in lower gastrointestinal bleeding based on randomized controlled trials of rofecoxib.
Background
The wear and tear that comes with aging often results in painful musculoskeletal conditions prompting the widespread use of analgesic and anti-inflammatory agents among the elderly. For example, each year over 111 million prescriptions for NSAIDs are prescribed in the U.S. 13. NSAIDs are primarily used for reducing the pain and disability, and improving the quality of life, associated with pain and inflammation 14, 15. These benefits come at a price as the use of NSAIDs is associated with development of peptic ulcers that may be complicated by gastrointestinal bleeding or perforation. Recognized risk factors associated with ulcer complications among NSAID users include: age over 65 years, past medical history of ulcer disease or ulcer complications, past medical history of cardiovascular disease, high-dose NSAID therapy, co-administration of corticosteroids, NSAID-associated dyspepsia, and anticoagulant therapy 16.
For almost one-half century, the pharmaceutical industry regularly introduced new analgesic/anti-inflammatory compounds that have generally been touted as more effective, safer, or both. This search for a better/safer agent led to the discovery of the COX inhibitors that theoretically offered anti-inflammatory and analgesic effects with minimal or no serious gastrointestinal side effects. Celecoxib and rofecoxib were the first two specific COX-2 inhibitors that emerged for clinical use. The availability of these highly selective COX-2 inhibitors allowed testing of the hypothesis that, for the first time, relief of pain and inflammation could be separated from the gastrointestinal untoward effects of NSAIDs.
Clinical trials in patients with arthritis and pain showed that celecoxib and rofecoxib were equivalent but not superior to traditional NSAIDs, such as ibuprofen or naproxen, in terms of analgesia and anti-inflammatory activity. Were they safer? Proof that COX-2 selective agents were not toxic to the gastrointestinal tract (i.e., equivalent to placebo) required double blind placebo-controlled studies using reliable endpoints. Proof that an agent was safer than traditional NSAIDs requires substantial evidence of a meaningful reduction in significant clinical GI events (e.g., gastrointestinal bleeding or perforation). Clinical interpretation of such studies would also need to take into account the choice of comparator, as well as dose, in that traditional NSAIDs are known to differ in terms of gastrointestinal safety both in relation to the individual compound and the dosage administered 17-20. For example, an agent might appear to be safer than piroxicam but more toxic than ibuprofen, or similar in safety and efficacy to one dose of an NSAID but less toxic than a higher dose of the same NSAID. This known difference in traditional NSAIDs was responsible for the clinical adage to use the lowest dose for the shortest time.
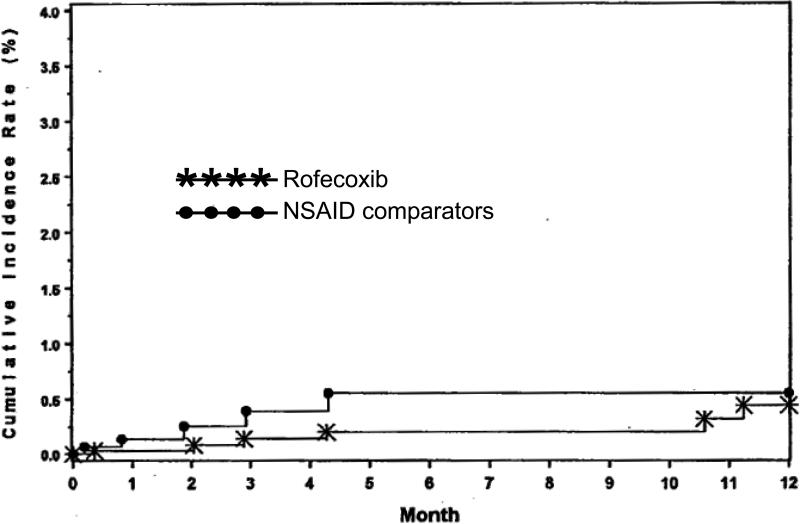
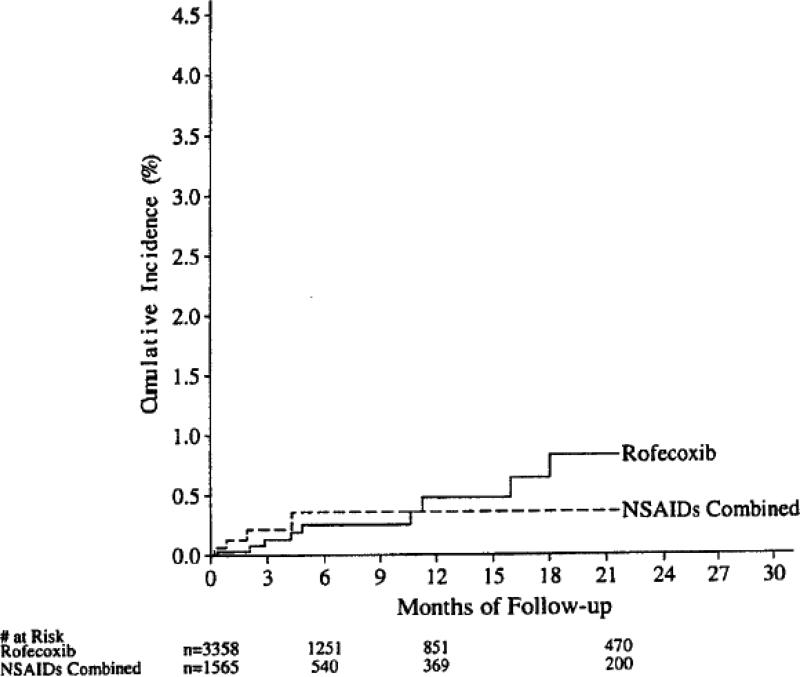
Initial studies of the safety and efficacy of rofecoxib focused on endoscopic ulcers as a measure of gastrotoxicity 8, 9, 21, 22. Endoscopic ulcers are a clear measure of gastrotoxicity, but have never been validated as a surrogate for significant clinical GI events 23. The clinical study report from protocol 069 (Clinical study report entitled “MK-0966 phase III gastrointestinal clinical event monitoring plan and case review committee procedures (protocol 069) dated October 22, 1998”) defined clinically important gastrointestinal events as “confirmed complicated” events (bleeding, perforation, or obstruction). They found a slight numerical advantage for rofecoxib that was not significant compared to NSAIDs in terms of confirmed complicated events (Figure 1A). The analyses were subsequently updated. Again, the differences were not significant but now the apparent slight advantage for rofecoxib had disappeared (Figure 1B) (from the 2/27/2003 memo from Qinfen Yu).
Figure 1A.
Cumulative incidence of confirmed upper-GI PUBs that were complicated, from 8 studies reported in the Clinical study report MK-0966, protocol 069, 22 October 1998
Figure 1B.
Cumulative incidence of confirmed upper-GI PUBs that were complicated, based on follow up of the 8 studies from protocol 069. Copied from a memo from Qinfen Yu, Subject: Combined analysis of PUBs in Vioxx studies (except Vigor) – Final update for the MK vs NSAID comparison, 2/27/2003.
In their unpublished clinical study report of data from 8 studies, Langman et al. defined a group of clinically complicated events to try to estimate the possible clinically important benefits. This group was defined as the presence of a gastric ulcer ≥3 cm in diameter, a duodenal ulcer ≥2 cm in diameter, stigmata of bleeding (active bleeding or visible vessel at endoscopy), obstruction due to an active gastric or duodenal ulcer, or confirmed clinically significant hemorrhage which was an upper GI hemorrhage with 1) significant bleeding/volume loss, and 2) transfusion of blood or packed red blood cells. Using these criteria they found that the cumulative incidence of confirmed significant events among rofecoxib users was not significantly reduced (i.e., cumulative follow up proportion affected was 0.45% with rofecoxib as compared to 0.55% for traditional NSAIDs; p-value = 0.26, using a log rank test). This specific outcome definition was not reported in any publication.
However, a number of reports suggested that rofecoxib was equivalent to placebo in terms of the development of endoscopic ulcers 8, 21, 22. For example, one study 21 concluded that “Rofecoxib at a dose of 25 mg (the highest dose recommended for osteoarthritis) satisfied prespecified criteria for equivalence to placebo”. Similarly, another article 22, concluded that “Rofecoxib, at doses 2-4 times the dose demonstrated to relieve symptoms of osteoarthritis, caused significantly less gastroduodenal ulceration than ibuprofen, with ulcer rates comparable to placebo”. Of interest, the rate of endoscopic ulcers among those receiving placebo and no gastric toxic drugs for 16 weeks was unexpectedly high (9.9% in 16 weeks) which led the authors of an accompanying editorial to raise concerns about the validity of the study 24.
Rofecoxib was subsequently proven to be significantly more gastrotoxic than placebo in terms of significant clinical events. For example, Merck protocol 078, completed in April 2003, was a 4 year randomized study of 1457 patients in which 25 mg of rofecoxib, or placebo, was tested for treatment of mild cognitive impairment and to prevent conversion to Alzheimer's disease. Suspected gastrointestinal perforations, ulcers or bleeds (GI PUBs) were reviewed by an independent expert adjudication board. In that study, both confirmed and unconfirmed complicated GI events were substantially more common among rofecoxib users than among those receiving placebo as demonstrated below.
Statistical analysis of the Alzheimer's trial protocol #078
The data, displayed below in Table 1, was obtained from Table 63 of the Clinical Study Report of the Alzheimer's Trial Protocol 078 prepared by Merck. An analysis of the incidence of confirmed complicated PUBs (perforations, ulcers, bleeds) in this trial yields the following results, based on observation of 10 confirmed complicated PUBs under rofecoxib (in 1375 patient years of follow-up) and 3 under placebo (in 1579 patient years): The estimated Incidence Rate Ratio (IRR) is 3.8, reflecting an almost quadrupling of event rates under rofecoxib as compared to placebo. The two-sided p-value for no difference in the rates between the two groups is 0.03 (based on an exact test), indicating that the rate is significantly higher with rofecoxib treatment than under placebo.
Table 1.
Data on confirmed (and complicated) PUBS in the Alzheimers’ Trial Protocol #078.
| Placebo | Rofecoxib | ||
|---|---|---|---|
| Patients with confirmed complicated PUBS | 3 | 10 | |
| Patient-years at risk | 1579 | 1375 | |
| Rate (per 100 patient-years at risk) | 0.19 | 0.73 | |
| Incidence Rate Ratio (with the rate under rofecoxib in the numerator) 95% CI (exact) | 3.83 (0.99, 21.65) | ||
| Patients with confirmed PUBS | 4 | 14 | |
| Patient-years at risk | 1579 | 1375 | |
| Rate (per 100 patient-years at risk) | 0.25 | 1.02 | |
| Incidence Rate Ratio (with the rate under rofecoxib in the numerator) 95% CI | 4.02 (1.26, 16.77) |
An analogous analysis of all confirmed PUBs in the same patients (14 under Vioxx and 4 with placebo) yields an estimated IRR of 4.0. The two-sided p-value for no difference in the rates in the two groups is now 0.009 (again based on an exact test), indicating that the rate is significantly higher with rofecoxib treatment than under placebo
Thus, Merck protocol 078 clearly disproved the hypotheses regarding equivalence of rofecoxib to placebo. The data were not published until 2005 (i.e., after rofecoxib had been withdrawn from the market), and even then, only non-complicated PUBs were mentioned, and there was no accompanying statistical assessment of comparisons. As such, there was no apparent attempt to quickly correct the misconception fostered by earlier publications.
Subsequently, published randomized placebo controlled trials also collected outcome data and confirmed that rofecoxib was significantly more likely than placebo to lead to clinically important upper gastrointestinal events 25. For example, the APPROVe study (Adenomatous Polyp Prevention On Vioxx) demonstrated a significant increase in confirmed PUBs among rofecoxib users compared to those receiving placebo with an estimated relative risk of 4.9; 95% CI= 2.0-14.5 25. The relative risk of confirmed complicated ulcers was numerically similar (i.e., 3.8; 95% CI = 0.7-37.5) although not statistically significant (p-value = 0.14) due to the relatively infrequent occurrence of events.
Finally, large observational studies that included rofecoxib and considered an endpoint of gastrointestinal bleeding 19, 26-30 showed an increased risk with rofecoxib as compared to not using NSAIDs suggesting that rofecoxib caused gastrointestinal bleeding. It was also not possible to differentiate rofecoxib from traditional NSAIDs in terms of upper gastrointestinal bleeding, (i.e. rofecoxib offered no special advantage over tNSAIDs).
Rofecoxib compared to traditional NSAIDs as a cause of clinically important ulcer complications
Whether rofecoxib was less likely to cause clinically important ulcer complications than traditional NSAIDS was specifically evaluated in a randomized controlled trial, the VIGOR study 10. VIGOR involved 8,076 rheumatoid arthritis patients who were at least 50 years of age (or at least 40 years of age and receiving long-term glucocorticoid therapy). Subjects were randomized to receive either 50 mg of rofecoxib daily or 500 mg of naproxen twice daily. The primary end point was confirmed clinical upper gastrointestinal events (gastroduodenal perforation or obstruction, upper gastrointestinal bleeding, and symptomatic gastroduodenal ulcers). The final conclusions included that rofecoxib and naproxen had similar efficacy against rheumatoid arthritis and that “During a median follow- up of 9.0 months, 2.1 confirmed gastrointestinal events per 100 patient-years occurred with rofecoxib, as compared with 4.5 per 100 patient-years with naproxen (relative risk, 0.5; 95 percent confidence interval, 0.3 to 0.6; P<0.001)”.
The primary study outcome included “symptomatic” ulcers that differ from complicated events such as perforation, obstruction, and severe upper gastrointestinal bleeding, all of which have major clinical and potentially life threatening implications. Symptomatic ulcers are a “soft” endpoint that can potentially be manipulated (i) in terms of the definition of what constitutes an ulcer and (ii) because their identification depends in part upon the threshold for “significant” symptoms that prompt endoscopy. Thus, symptomatic ulcers can represent a combination of clinical and endoscopic ulcers. In previous studies, the endoscopic ulcer model had shown that rofecoxib was generally less likely than traditional NSAIDs (tNSAIDs) to cause endoscopic ulcers such that inclusion of endoscopic ulcers in the symptomatic ulcer category could potentially tilt the results in favor of rofecoxib 8, 21, 22. In the VIGOR protocol, patients were asked questions concerning the occurrence of symptoms at each contact, including those made by phone, potentially resulting in a low threshold for performing endoscopy and thus enhancing the detection of “symptomatic” endoscopic ulcers. Rofecoxib had also been shown to be associated with a lower incidence of GI symptoms than the tNSAID comparators which could also increase the chance of bias in terms of the number of procedures in which endoscopic ulcers were discovered and labeled as symptomatic ulcers.
Not withstanding these caveats, rofecoxib was shown to be superior to naproxen at the doses given using what we believe to be the most reliable endpoint of clinically important occurrences (i.e., complicated confirmed events such as perforation, obstruction, and severe upper gastrointestinal bleeding). The rates for these events were 0.6 per 100 patient-years with rofecoxib compared to 1.4 per 100 patient-years with naproxen (IRR = 0.4; 95 percent confidence interval, 0.2 to 0.8; p-value = 0.005). Thus, 50 mg of rofecoxib appeared superior to 1,000 mg of naproxen with regard to serious gastrointestinal outcomes, even using the most clinically relevant end point, and the one least subject to bias or manipulation.
However, the overall analysis may not provide the clearest interpretation of the observed group difference. In particular, was this difference generally applicable or possibly attributable to a predefined subgroup? One of the predefined subgroups in VIGOR was concomitant corticosteroid users, a group known to be associated with an increased risk of serious upper GI events 31, 32. The published manuscript reported a difference in the rate of occurrence of all PUBS between baseline corticosteroid users and non-users, and reported relative comparisons of rofecoxib and naproxen stratified on baseline corticosteroid use: “Patients with no steroid therapy at baseline (relative risk, 0.7; 95 percent confidence interval, 0.4 to 1.2), and patients with steroid therapy at base line (relative risk, 0.4; 95 percent confidence interval, 0.2 to 0.6)” 10. This suggests the possibility that the benefit (i.e., the reduced risk associated with treatment with rofecoxib as compared to naproxen) may have been limited to those patients who received glucocorticoid therapy. This aspect of the role of steroid therapy was raised by a reviewer of the original publication who noted “The steroid finding is worthy of comment, even if the interaction is not statistically significant (the power for interactions is low). It may be that the new agent has little benefit for those not treated with steroids”. The authors responded, “While there was not a significant reduction in relative risk of confirmed PUBs in the subgroup not taking steroids at baseline, VIGOR was only designed to detect a significant reduction overall, and not within any subgroup. There were, however, reductions in relative risk in this subgroup albeit not significant. Whenever many subgroups are examined, it becomes likely that there will be no apparent effect in some of them purely by chance. That is why the test for interaction, although underpowered as you rightly pointed out, is the appropriate way to assess subgroup effects. When there is no treatment-by-subgroup interaction, the more reliable estimate of treatment effect within a subgroup is the effect in the entire cohort. It is quite remarkable, given these caveats, that there were reductions in relative risk in all the subgroups examined, and that all of the reductions were significant with the one exception of patients not taking baseline steroids”.
“Further evidence that the appropriate estimate of effect within this subgroup is the overall cohort estimate is provided by the prespecified combined analysis of 8 double-blind, efficacy studies in patients with osteoarthritis, as cited in our manuscript (reference 25). This analysis demonstrated a 50% reduction in the incidence of clinically important upper GI events with rofecoxib as compared to nonselective NSAIDs. None of the patients in this analysis were taking steroids at baseline, yet the 49% estimate of the reduction in relative risk was remarkably similar to the 54% reduction in confirmed clinical upper GI events seem in the total VIGOR cohort”.
“For these reasons, one cannot conclude that there is a lack of benefit of rofecoxib in the steroid subgroup. The effect within the subgroups of patients not taking steroids at baseline shou1d be considered no different than the effect within any other subgroup in which the interaction effect was not significant”
Subsequently, the journal requested additional changes for the revised version of the manuscript regarding the corticosteroid issue stating “First please provide a new paragraph for the discussion with regard to the effects among steroid users and nonusers. This issue is of sufficient potential clinical importance – for example, for patients with rheumatoid arthritis who use steroids – and biologic plausibility that it can not simply be mentioned in a sentence in the results without further comment. The points you make in your letter of August 20, 2000 notwithstanding, you should acknowledge the points raised by reviewer D. These are the possibility that the benefit in the patient population you studied is mainly limited to the users of steroids and that the use of a selective COX-2 inhibitor may be more important in this higher risk group.” The authors responded, “Pages 19-20 (which are attached) include the suggested revisions to the discussion section. We have highlighted that there were numerical differences in risk reduction in the steroid user/non-user subgroups. While in this study there was no treatment by subgroup interaction, we have pointed out that the study was not powered to assess the differences between subgroups and therefore the possibility exists that patients on steroids will derive greater benefit compared with nonusers. We strongly believe that this addition accurately reflects the data in this study.” “On page 15, we have added the results of an additional subgroup analysis which evaluated the risk reduction in patients who had no other GI risk factors as presently described in the manuscript (age <65, not on corticosteroid use at baseline, no prior hx of GI bleed and negative for H. Pylori at baseline). There was a marked reduction in events even in the lowest risk subgroup which includes patients not using corticosteroids.”
The authors chose to deal with these prespecified subgroups by stating “The relative risks in these subgroups and the other prespecified subgroups (defined according to sex, race or ethnic group, and location of study center) were not significantly different, indicating that there was no significant interaction between the treatments and the subgroups”. In addition, in the response to the reviewer who believed that the corticosteroid finding was worthy of comment again, the authors noted that the finding was not further investigated or of concern in that the interaction was non-significant. Considering that these were prespecified subgroups and one had clearly been shown to be important in terms of the clinically important GI events, it seems that further analysis should have been implemented and reported. We return to this further below.
The VIGOR trial was subsequently accepted for publication 10. The authors then undertook a post hoc analysis of the gastrointestinal outcomes 11. The data analysis plan included subgroups with prior history of PUBs, study region, age group, ethnic group, gender, and use of systemic corticosteroids at baseline, and was to include analyses of the incidence of confirmed plus unconfirmed PUBs. An email from the principal author to the other potential authors stated that the three most clinically relevant baseline risk factors were age, prior events and corticosteroids. In discussing the results of the analyses, an email from Merck notes “The other issue is more complicated. Your raising it sparked my memory that in the final model, we did have one significant interaction that was with baseline steroid use. In the univariate model that we discussed in the NEJM article, the interaction wasn't significant, (close though [0.07]) but in this multi-variate model, it was, p=0.043. This is the only significant interaction, and it is a quantitative interaction and so one we typically dismiss. I'm attaching a revision, therefore, to the manuscript since I think we are bound to mention it. ... I am still concerned about this issue in comparison to the NEJM article. Maybe the other authors can weigh in.”. Shortly thereafter an email from Chris Brett at Merck to the principal author stated “Deborah and I believe that since changes have been made since the 8/6/01 version that went into review, and these changes deal with the sensitive issue of interaction with baseline steroid use, that this change is significant enough to warrant Merck reviewers seeing the revised manuscript. I am proposing that we pull the one currently in circulation and submit this one instead”. The principal author had stated in the same email correspondence “I agree with your points regarding the steroid paragraph in the Discussion and the fact that we didn't show a significant treatment-by-subgroup interaction for the steroid group. However, in terms of negative impact on rofecoxib, even if steroids are only a risk factor in nonselective NSAIDs, it wouldn't mean that coxibs don't still improve safety in steroid or nonsteroid users (isn't that correct)? Thus, although I agree, we need to substantially tone it down? I thought we might leave it in (see below) because it is kind of interesting. However, it's not that important to the paper and a revised paragraph may be a little confusing to readers. So if you and other Merck colleagues think it should come out, I'll just take out the whole paragraph”.
The final manuscript contained a statement “there was a quantitative interaction (P = 0.043) between treatment and baseline use of corticosteroids (i.e., although the risk of an event with rofecoxib was reduced in patients who did and did not use corticosteroids at baseline, the reduction was significantly greater in baseline corticosteroid users)”. Their accompanying table (Table 2 in their paper 11) showed that the absolute risk reduction (per 100 patient years) was 0.94 for those not using corticosteroids at base line and 3.56 for those using corticosteroids at baseline. However, much was missing from this discussion, namely the role of baseline corticosteroid use on confirmed complicated PUBS that makes the entire situation much clearer. Although not discussed in the entire debate between authors, reviewers and editors, among corticosteroid non-users there were 9 confirmed complicated events among rofecoxib users compared to 10 among naproxen users. Among corticosteroid users there were 7 such events among rofecoxib users vs. 27 events among naproxen users respectively, as indicated in Table 2. The next section discusses the analysis of this previously unexamined data.
Table 2.
Data on confirmed complicated PUBS in the VIGOR study.
| No Baseline Steroid Use | Baseline Steroid Use | |||
|---|---|---|---|---|
| N Patients with confirmed complicated PUBS Patient-years at risk Rate (per 100 patient-years at risk) Incidence Rate Ratio (with the rate under Naproxen in the numerator) 95% CI p-value of exact (homogeneity) test, comparing the IRRs across the two groups defined by baseline steroid use |
Vioxx | Naproxen | Vioxx | Naproxen |
| 1803 | 1776 | 2244 | 2253 | |
| 9 | 10 | 7 | 27 | |
| 1184 | 1178 | 1513 | 1516 | |
| 0.76 | 0.85 | 0.46 | 1.78 | |
| 1.12 | 3.85 | |||
| (0.41, 3.11) | (1.64, 10.47) | |||
| p = 0.047 | ||||
Reanalysis of the steroid use prespecified subgroups
We reanalyzed the data using the VIGOR data files provided by Merck. Summary patient year information, stratified by baseline steroid use, was obtained from Table 12.3-6 of the Clinical Study Report for the trial. Individual patient follow-up data was taken from Merck data files. In the VIGOR trial comparing rofecoxib and naproxen there were 37 confirmed complicated PUBs under naproxen treatment (in 2694 patient years of follow-up) and 16 under rofecoxib (in 2697 patient years). These data yield an estimated Incidence Rate Ratio (IRR) of 2.32, reflecting a more than doubling of the event rates under naproxen, discussed earlier. The two-sided p-value for no difference in the rates in the two groups is 0.004 (based on an exact test) 33, suggesting that the rate is significantly higher with naproxen treatment than under rofecoxib.
A stratified analysis of this data by baseline use of corticosteroids presents a more informative interpretation of these results. For those individuals without baseline corticosteroid use, the estimated IRR is now only 1.12, whereas for those with baseline corticosteroid use, the estimated IRR is 3.85 (Table 1). There is thus very strong effect modification of the impact of naproxen use (as compared to rofecoxib) by baseline corticosteroid use. The (interaction) p-value for testing the same treatment effect (rofecoxib as compared to naproxen) in the two groups (corticosteroid and corticosteroid non-users) is 0.047 33, indicating that the noted large difference in IRRs is unlikely to be attributable to chance. The two-sided p-value for no difference in event rates comparing naproxen and rofecoxib amongst the corticosteroid users is 0.0005 (again using an exact test)33. However, amongst corticosteroid non-users, the analogous two-sided p-value is 0.82, indicating that there is absolutely no evidence of a difference in rates in this group of individuals. In summary, the difference between rofecoxib and naproxen in the occurrence of confirmed complicated PUBs appears to be entirely due to the effects within corticosteroid users.
Reanalyses Using the Cox Proportional Hazards Model
The VIGOR study report indicated that analyses should be based on patient level data on time-to-events (here, confirmed complicated PUBS), employing the Cox proportional hazards model to estimate regression effects 34. We repeated the above analysis using exactly this approach on the original data. The results are almost identical to the summary data findings reported above: The full (unstratified) data yield an estimated Hazard Ratio (HR) of 2.31, again reflecting a more than doubling of the event rates under naproxen. A stratified analysis of this data by baseline use of corticosteroids yields an estimated HR of 1.12 for corticosteroid non-users, whereas for those with baseline corticosteroid use, the estimated HR is 3.84. The (interaction) p-value for comparing these treatment effects (rofecoxib as compared to naproxen) in the two groups (corticosteroid and corticosteroid non-users) is 0.048, indicating that the noted large difference in HRs is unlikely to be attributable to chance. The two-sided p-value for no difference in event rates comparing naproxen and rofecoxib amongst the corticosteroid users is 0.002. However, amongst corticosteroid non-users, the analogous two-sided p-value is 0.81, indicating that there is no evidence of a difference in rates in this group of individuals.
There is one advantage to our original grouped analysis in that most of the tests are exact and therefore do not rely on large sample approximations (as with the standard Cox model assessment of p-values); but the results are so close that this is essentially irrelevant.
In the VIGOR statistical protocol, it is stated that when an interaction is found across one of the subgroups to be considered, the final analysis would ignore this so long as the direction of the risk of the event (here against naproxen) is the same in both subgroups. This is not a defensible strategy when one of the subgroups shows no evidence of a difference in risk whereas there is a large difference in the other subgroup, exactly the situation here where the data provides no evidence of a benefit of using rofecoxib, in terms of a reduced risk of confirmed complicated PUBS, amongst patients with no baseline corticosteroid use, a considerable fraction of the sampled population.
In summary, the difference in the occurrence of confirmed complicated PUBs between rofecoxib and naproxen appears to be entirely due to the effects within baseline corticosteroid users. This conclusion is supported by both a grouped analysis of incidence rates and a Cox proportional hazards analysis.
Lower gastrointestinal events and rofecoxib
The post hoc analysis of lower gastrointestinal events was presented in a separate paper 12 that concluded that “Serious lower GI events occurred at a rate of 0.9% per year in rheumatoid arthritis patients taking the nonselective NSAID naproxen, accounting for nearly 40% of the serious GI events that developed in these patients. Serious lower GI events were 54% lower with the use of the selective COX-2 inhibitor rofecoxib”. The analysis describes that “a list of serious GI events and criteria for the events were developed post hoc in a blinded manner” and “all review of the data was performed blinded to treatment allocation”. In early December 2001 the initial analysis was done using the blinded data and the endpoint criteria agreed by the authors. That analysis showed 20 vs. 11 events (naproxen vs. rofecoxib; p-value = 0.11). For clinical lower GI events the results were 26 vs. 14; p-value = 0.061. On December 4, the principal author asked for the treatment allocation table which was received on December 5. He then returned a new list in which 5 patients had been deleted and asked for an analysis which was returned that same afternoon. The new analysis was then for 24 events vs. 11 (naproxen vs. rofecoxib); p-value = 0.032). These data were used in the subsequent abstract and manuscript in which the methods section stated that “serious lower GI clinical events were assessed blinded to treatment allocation”. The fact that the choice did not represent the original blinded analysis, but was a subgroup chosen after treatment allocation was unblinded, was not disclosed in either the abstract or the manuscript.
Discussion
Rofecoxib was ultimately withdrawn from the market because of an established increased risk for cardiovascular events. The GI safety of rofecoxib has however largely remained unquestioned. Access to previously confidential communications has indicated that one of the reviewers of the VIGOR paper noticed that the benefit of rofecoxib in the VIGOR trial might have been largely restricted to those using concomitant corticosteroids. During the review process, Merck e-mails expressed concern that the Journal might commission the critical reviewer to write an editorial suggesting that the benefit was restricted to that subgroup. Although his/her concerns required several revisions of the manuscript, it was subsequently published and without an accompanying editorial. Our reanalysis of the original data set suggests that the reviewer's suspicion may have been correct as we found that the difference between rofecoxib and naproxen in the occurrence of confirmed complicated upper gastrointestinal events appeared to lie entirely within the group of corticosteroid users.
The failure of rofecoxib to conclusively confirm safety superiority to high dose tNSAIDs was mirrored in the results with two other selective COX-2 inhibitors (celecoxib and etoricoxib) both of which failed to show a significant decrease in important events in double blind randomized controlled trials. For example, in the CLASS trial (celecoxib) a significant GI benefit was reported at the 6 month analysis but significance was lost by the 12 month follow-up 35. The MEDAL trial which randomized 34,701 patients to the COX-2 inhibitor etoricoxib also reported no difference in complicated events among patients randomized to the COX-2 inhibitor or the nonselective nonsteroidal anti-inflammatory drug, diclofenac 4.
We also call into question the reported beneficial effects of rofecoxib in reducing the risk of lower GI bleeding which are, in retrospect, not the result of a subgroup selected blindly but rather of another subgroup that was selected after treatment allocation was disclosed (i.e., the published secondary analysis reporting that treatment with rofecoxib was associated with significantly fewer hospitalizations for lower GI events, was based on unblinded data and the endpoint was subsequently modified in favor of rofecoxib).
We believe the real issue is one of trust. It has long been recognized that NSAID therapy, despite it many benefits, carries a definite risk in terms of gastrointestinal complications. The ability to identify a strategy that would provide the benefits of NSAIDs at reduced risk is clearly an opportunity. Unfortunately, there are many ways in which study design, analysis, and in choosing which data to present may obfuscate the primary issue 1-3.
This is particularly true in this context because individual tNSAIDs vary widely in their propensity to cause clinically significant GI events and for many NSAIDs there is also a strong dose response effect with regard to risk. NSAIDs also appear similar in terms of their ability to reduce pain and inflammation requiring that companies seek marketing advantages from other factors such as safety. The safety issues surrounding NSAIDs are complicated in that they can involve many different systems, predominantly gastrointestinal, renal, and cardiovascular especially in relation to hypertension and myocardial ischemia. Gastrointestinal safety is probably the most difficult to assess in that there are no standardized and validated surrogates for prevention of clinically important events (reviewed in 23). The fact that NSAIDs vary in their propensity to cause acute topical gastrointestinal injury facilitates the ability to design studies showing differences that may not be meaningful in terms of actual safety 36, 37 making it imperative that the clinician look carefully at not only the drugs but also the doses and formulations.
The data from 8 clinical trials with rofecoxib were published in one paper 8, subsequently updated (from the 2/27/2003 memo from Qinfen Yu and manuscript 9. These manuscripts presented the data as rofecoxib (at various doses) compared to all tNSAIDs (20 studies) despite the fact that the tNSAIDs potentially varied markedly in terms of risk. The average dose of rofecoxib was not high being approximately 25 mg. In contrast, in the majority of studies, the tNSAID (87%) was “high” dose (e.g., 1000 mg of naproxen [the most commonly used], 2400 mg ibuprofen, and 150 mg diclofenac) that placed the patient into their highest ulcer risk groups 8, 9. Such high doses were more than thought needed to control pain and/or inflammation for the majority of the patients with osteoarthritis of mild to moderate severity (ARA functional status I or II) 38..
Of interest, the data for nabumetone, one of the tNSAIDs often thought to be less gastrotoxic, were not provided in either manuscript although 930 patients, a number similar to those receiving diclofenac and ibuprofen, had received the drug 8, 9. The nabumetone comparison data were provided in a memo of 2/27/2003 regarding “the final update for the MK vs. NSAIDs comparison”. That analysis showed that there were zero confirmed PUBs among the 930 subjects receiving nabumetone (194 patient years) (1500 mg once daily) compared to 2 among the 1013 receiving rofecoxib (248 patient years) (12.5 mg daily) . The data for the combination of misoprostol and diclofenac (909 patients, 100 mg) vs. rofecoxib (12.5 mg) were also not reported and there were no confirmed PUBs among approximately 450 patients receiving either misoprostol plus diclofenac or rofecoxib; each group followed for an average of 66.5 patient years. The diclofenac plus misoprostol data was not reported separately but was instead combined with diclofenac (150 mg, 2048 patients) vs. rofecoxib at doses ranging from 5 to 25 mg).
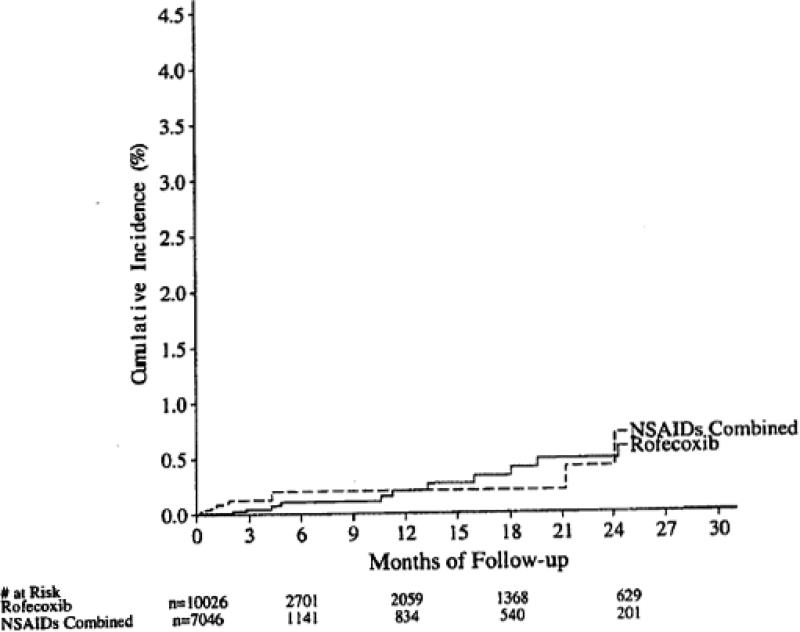
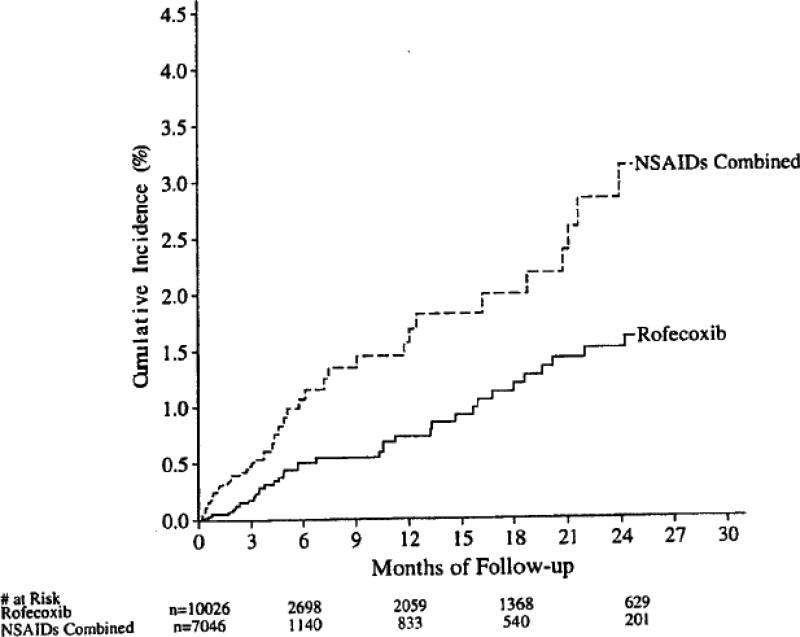
The final conclusion of the Watson et al. summary paper was “Treatment with rofecoxib was associated with a statistically significantly (p < 0.001) lower incidence of PUBs than was treatment with NSAIDs. The difference was maintained in subgroups of patients with risk factors, as well as in those with no risk factors, for PUBs”; this finding was illustrated by a graphic showing the cumulative incidence of confirmed PUBS based on the combined data from the active treatment periods 9 (Figure 3). In contrast, as noted above, examination of the clinically most important variable, confirmed complicated PUBs – bleeding, perforation, obstruction, showed no advantage for rofecoxib (Figure 2).
Figure 3.
Cumulative incidence of confirmed PUBs based on the active treatment period of the combined data from 20 treatment trials. Copied from a memo from Qinfen Yu, Subject: Combined analysis of PUBs in Vioxx studies (except Vigor) – Final update for the MK vs NSAID comparison, 2/27/2003.
Figure 2.
Cumulative incidence of confirmed complicated PUBs based on the active treatment period of the combined data from 20 treatment trials. Copied from a memo from Qinfen Yu, Subject: Combined analysis of PUBs in Vioxx studies (except Vigor) – Final update for the MK vs NSAID comparison, 2/27/2003.
Recommendations for the future
In our opinion, the reporting of benefits of rofecoxib with regard to serious GI events was inappropriately optimistic regarding the gastrointestinal safety of rofecoxib in relation to tNSAIDs. The tendency to lump NSAIDs into two categories, tNSAIDs and selective COX-2 inhibitors is in itself misleading since tNSAIDs vary greatly in their propensity to cause damage. Lumping tends to obscure a strong dose response effect in terms of risk. We suggest that the summary provide the actual comparison (i.e., while 50 mg rofecoxib may be superior to 1000 mg of naproxen in terms of gastrointestinal safety it may be the equivalent or even inferior to a lower dose (e.g., 220 mg of naproxen twice a day). Formulation must also be taken into account (e.g., recent studies have suggested that enteric coated or slow release formulations are more likely to cause significant bleeding 39, and studies of low dose enteric coated aspirin suggest that the enteric coated formulation is associated with acute small bowel injury 40-42.
We agree with Beejay and Wolfe, the authors of an editorial regarding the safety of rofecoxib that “it is imperative that a decrease in ulcer complications (i.e., hemorrhage, perforation, and gastric outlet obstruction) be shown before establishing the safety of any new NSAID”. Except for possibly the subgroup of rheumatoid arthritis patients taking corticosteroids and high dose naproxen, available data do not show any significant safety advantage for rofecoxib.
Acknowledgments
Support and Potential Conflicts of Interest
This material is based upon work supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs. Dr. Graham is supported in part by Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center and DK067366 and CA116845. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA or NIH. Dr. Graham is consultant for Novartis in relation to vaccine development for treatment or prevention of H pylori infection, and is a paid consultant for Otsuka Pharmaceuticals, manufacturer of the 13C-urea breath test and an unpaid member of the executive committee of the PRECISION trial which is supported by Pfizer and is designed to compare the cardiovascular safety of celecoxib, naproxen, and ibuprofen in cardiovascular higher risk patients. Dr. Graham was a consultant at the request of plaintiffs (the State of Louisiana) in litigation against Merck & Co Inc related to rofecoxib. The funds for this work were directed to the Baylor College of Medicine to be used to support College activities and for research. Dr. Graham received no support for work on this paper.
Dr. Chan is a consultant to Pfizer, Eisai, Takeda, and Otsuka. He has received grants from Pfizer and has been paid lecture fees (including service on speakers’ bureaus) by Pfizer, Astra Zeneca, and Takeda. Dr Chan received no support for work on this paper.
Dr. Jewell was compensated for his original work as a consultant at the request of plaintiffs in litigation against Merck & Co Inc related to rofecoxib, but not for work on this paper.
All unpublished legal documents used in this article are available at (we will either place them at a web site or as a supplement to the article).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angell M. How they deceive us and what to do about it. Random house; New York: 2004. The truth about the drug companies. [Google Scholar]
- 2.Moynihan R, Cassels A. How the world's biggest pharmaceutical companies are turning us all into patients. Nation Books; New York: 2005. Selling sickness. [Google Scholar]
- 3.Petersen M. How the pharmaceutical companies transformed themselves into slick marketing machines and hooked the nation on prescription drugs. Sarah Crichton Books; New York: 2008. Our daily med. [Google Scholar]
- 4.Ross JS, Hill KP, Egilman DS, et al. Guest authorship and ghostwriting in publications related to rofecoxib: a case study of industry documents from rofecoxib litigation. JAMA. 2008;299:1800–1812. doi: 10.1001/jama.299.15.1800. [DOI] [PubMed] [Google Scholar]
- 5.Rostom A, Muir K, Dube C, et al. Gastrointestinal safety of cyclooxygenase-2 inhibitors: a Cochrane Collaboration systematic review. Clin Gastroenterol Hepatol. 2007;5:818–28. 828. doi: 10.1016/j.cgh.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Chan FK, Graham DY. Review article: prevention of non-steroidal anti-inflammatory drug gastrointestinal complications--review and recommendations based on risk assessment. Aliment Pharmacol Ther. 2004;19:1051–1061. doi: 10.1111/j.1365-2036.2004.01935.x. [DOI] [PubMed] [Google Scholar]
- 7.Graham DY, Chan FK. NSAIDs, risks, and gastroprotective strategies: current status and future. Gastroenterology. 2008;134:1240–1246. doi: 10.1053/j.gastro.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Langman MJ, Jensen DM, Watson DJ, et al. Adverse upper gastrointestinal effects of rofecoxib compared with NSAIDs. JAMA. 1999;282:1929–1933. doi: 10.1001/jama.282.20.1929. [DOI] [PubMed] [Google Scholar]
- 9.Watson DJ, Yu Q, Bolognese JA, et al. The upper gastrointestinal safety of rofecoxib vs. NSAIDs: an updated combined analysis. Curr Med Res Opin. 2004;20:1539–1548. doi: 10.1185/030079904x3078. [DOI] [PubMed] [Google Scholar]
- 10.Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343:1520–8. 2. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 11.Laine L, Bombardier C, Hawkey CJ, et al. Stratifying the risk of NSAID-related upper gastrointestinal clinical events: results of a double-blind outcomes study in patients with rheumatoid arthritis. Gastroenterology. 2002;123:1006–1012. doi: 10.1053/gast.2002.36013. [DOI] [PubMed] [Google Scholar]
- 12.Laine L, Connors LG, Reicin A, et al. Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology. 2003;124:288–292. doi: 10.1053/gast.2003.50054. [DOI] [PubMed] [Google Scholar]
- 13.Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology. 2001;120:594–606. doi: 10.1053/gast.2001.21907. [DOI] [PubMed] [Google Scholar]
- 14.Griffin MR. Epidemiology of nonsteroidal anti-inflammatory drug-associated gastrointestinal injury. Am J Med. 1998;104:23S–29S. doi: 10.1016/s0002-9343(97)00207-6. discussion 41S- [DOI] [PubMed] [Google Scholar]
- 15.MacDonald TM. Epidemiology and pharmacoeconomic implications of non-steroidal anti-inflammatory drug-associated gastrointestinal toxicity. Rheumatology (Oxford) 2000;39(Suppl 2):13–20. doi: 10.1093/rheumatology/39.suppl_2.13. [DOI] [PubMed] [Google Scholar]
- 16.Tielemans MM, Eikendal T, Jansen JB, et al. Identification of NSAID users at risk for gastrointestinal complications: a systematic review of current guidelines and consensus agreements. Drug Saf. 2010;33:443–453. doi: 10.2165/11534590-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Griffin MR, Piper JM, Daugherty JR, et al. Nonsteroidal anti-inflammatory drug use and increased risk for peptic ulcer disease in elderly persons. Ann Intern Med. 1991;114:257–263. doi: 10.7326/0003-4819-114-4-257. [DOI] [PubMed] [Google Scholar]
- 18.Garcia Rodriguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994;343:769–772. doi: 10.1016/s0140-6736(94)91843-0. [published erratum appears in Lancet 1994 Apr 23;343(8904):1048].
- 19.Garcia Rodriguez LA, Barreales TL. Risk of upper gastrointestinal complications among users of traditional NSAIDs and COXIBs in the general population. Gastroenterology. 2007;132:498–506. doi: 10.1053/j.gastro.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Henry D, Lim LL, Garcia Rodriguez LA, et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta- analysis. BMJ. 1996;312:1563–1566. doi: 10.1136/bmj.312.7046.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawkey C, Laine L, Simon T, et al. Comparison of the effect of rofecoxib (a cyclooxygenase 2 inhibitor), ibuprofen, and placebo on the gastroduodenal mucosa of patients with osteoarthritis: a randomized, double-blind, placebo-controlled trial. The Rofecoxib Osteoarthritis Endoscopy Multinational Study Group. Arthritis Rheum. 2000 Feb;43(2):370–7. doi: 10.1002/1529-0131(200002)43:2<370::AID-ANR17>3.0.CO;2-D. 2000;43:370-377. [DOI] [PubMed] [Google Scholar]
- 22.Laine L, Harper S, Simon T, et al. A randomized trial comparing the effect of rofecoxib, a cyclooxygenase 2-specific inhibitor, with that of ibuprofen on the gastroduodenal mucosa of patients with osteoarthritis.Rofecoxib Osteoarthritis Endoscopy Study Group. Gastroenterology. 1999;117:776–783. doi: 10.1016/s0016-5085(99)70334-3. [DOI] [PubMed] [Google Scholar]
- 23.Graham DY. Endoscopic ulcers are neither meaningful nor validated as a surrogate for clinically significant upper gastrointestinal harm. Clin Gastroenterol Hepatol. 2009;7:1147–1150. doi: 10.1016/j.cgh.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beejay U, Wolfe MM. Cyclooxygenase 2 selective inhibitors: panacea or flash in the pan? Gastroenterology. 1999;117:1002–1005. doi: 10.1016/s0016-5085(99)70358-6. [DOI] [PubMed] [Google Scholar]
- 25.Lanas A, Baron JA, Sandler RS, et al. Peptic ulcer and bleeding events associated with rofecoxib in a 3-year colorectal adenoma chemoprevention trial. Gastroenterology. 2007;132:490–497. doi: 10.1053/j.gastro.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Mamdani M, Rochon PA, Juurlink DN, et al. Observational study of upper gastrointestinal haemorrhage in elderly patients given selective cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs. BMJ. 2002;325:624–629. doi: 10.1136/bmj.325.7365.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hippisley-Cox J, Coupland C, Logan R. Risk of adverse gastrointestinal outcomes in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ. 2005;331:1310–1316. doi: 10.1136/bmj.331.7528.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin nonsteroidal anti-inflammatory drugs, aspirin and combinations. Gut. 2006;55:1731–1738. doi: 10.1136/gut.2005.080754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castellsague J, Holick CN, Hoffman CC, et al. Risk of upper gastrointestinal complications associated with cyclooxygenase-2 selective and nonselective nonsteroidal antiinflammatory drugs. Pharmacotherapy. 2009;29:1397–1407. doi: 10.1592/phco.29.12.1397. [DOI] [PubMed] [Google Scholar]
- 30.Laporte JR, Ibanez L, Vidal X, et al. Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents. Drug Saf. 2004;27:411–420. doi: 10.2165/00002018-200427060-00005. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez-Diaz S, Rodriguez LA. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Arch Intern Med. 2000;160:2093–2099. doi: 10.1001/archinte.160.14.2093. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez-Diaz S, Rodriguez LA. Steroids and risk of upper gastrointestinal complications. Am J Epidemiol. 2001;153:1089–1093. doi: 10.1093/aje/153.11.1089. [DOI] [PubMed] [Google Scholar]
- 33.STATA Statistical software. StataCorp; College Station, Texas: 2009. [Google Scholar]
- 34.Cox DR. Regression models and life-tables (with discussion). J Royal Statistical Society, Series B. 1972;34:178–220. [Google Scholar]
- 35.Henry D, McGettigan P. Selective COX-2 inhibitors: a promise unfulfilled? Gastroenterology. 2007;132:790–794. doi: 10.1053/j.gastro.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Graham DY. Nonsteroidal anti-inflammatory drugs, Helicobacter pylori, and ulcers: where we stand. Am J Gastroenterol. 1996;91:2080–2086. [PubMed] [Google Scholar]
- 37.Graham DY, Smith JL, Holmes GI, et al. Nonsteroidal anti-inflammatory effect of sulindac sulfoxide and sulfide on gastric mucosa. Clin Pharmacol Ther. 1985;38:65–70. doi: 10.1038/clpt.1985.136. [DOI] [PubMed] [Google Scholar]
- 38.Saag K, van der Heijde D, Fisher C, et al. Rofecoxib, a new cyclooxygenase 2 inhibitor, shows sustained efficacy, comparable with other nonsteroidal anti-inflammatory drugs: a 6-week and a 1-year trial in patients with osteoarthritis. Osteoarthritis Studies Group. Arch Fam Med. 2000;9:1124–1134. doi: 10.1001/archfami.9.10.1124. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez EL, Patrignani P, Tacconelli S, et al. Variability of risk of upper gastrointestinal bleeding among nonsteroidal anti-inflammatory drugs. Arthritis Rheum. 2010 doi: 10.1002/art.27412. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe T, Sugimori S, Kameda N, et al. Small bowel injury by low-dose enteric-coated aspirin and treatment with misoprostol: a pilot study. Clin Gastroenterol Hepatol. 2008;6:1279–1282. doi: 10.1016/j.cgh.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Smecuol E, Pinto Sanchez MI, Suarez A, et al. Low-dose aspirin affects the small bowel mucosa: results of a pilot study with a multidimensional assessment. Clin Gastroenterol Hepatol. 2009;7:524–529. doi: 10.1016/j.cgh.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 42.Shiotani A, Haruma K, Nishi R, et al. Randomized, double-blind, pilot study of geranylgeranylacetone versus placebo in patients taking low-dose enteric-coated aspirin. Low-dose aspirin-induced small bowel damage. Scand J Gastroenterol. 2010;45:292–298. doi: 10.3109/00365520903453182. [DOI] [PubMed] [Google Scholar]