Abstract
Depolarization by the neurotransmitter GABA regulates adult neurogenesis. Here we show that interneurons of the neurogliaform cell family are a primary source of GABA for newborn neurons in mouse dentate gyrus. GABAergic depolarization occurs in concert with reduced synaptic inhibition of mature neurons, suggesting the local circuitry facilitates coordinated activation of new and pre-existing cells.
Neural stem cells in the adult dentate gyrus generate new neurons that integrate into the existing network and participate in brain functions. Neural activity in the neurogenic niche regulates neurogenesis via secreted factors, including direct depolarization of newborn cells by the transmitter GABA1. To identify the source of GABA mediating synaptic depolarization, we first tested whether stimulation of single interneurons was sufficient to generate synaptic currents in newborn granule cells (NGCs). Using proopiomelanocortin (POMC)-GFP reporter mice to identify NGCs at an early developmental stage when only sparse GABAergic inputs are present2, we serially tested multiple interneurons for connectivity with glutamate iontophoresis or loose patch stimulation (S Fig. 1). In a total of 111 NGCs, we tested 498 potential presynaptic partners and found a success rate of 2.3% (11/498). Presynaptic interneurons were located in the molecular layer (n = 2) and hilus (n = 9; Fig. 1a), suggesting that NGCs receive input from feed-forward and feedback circuits. Using the same approaches, the success rate for mature, non-GFP expressing granule cells was 10-fold higher (8/40). Unitary postsynaptic currents (uPSCs) were recorded in glutamate receptor antagonists and had latencies consistent with monosynaptic connections (S Fig. 2).
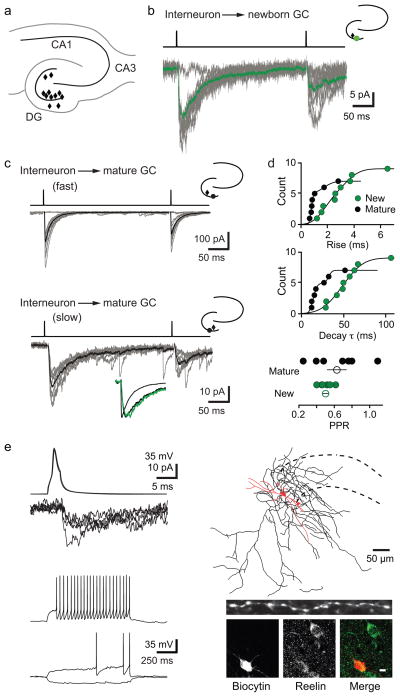
Figure 1. Ivy/NGs innervate NGCs.
a, Location of interneurons (diamonds) that innervated NGCs in acute brain slices. All procedures were approved by the UAB Institutional Animal Care and Use Committee.
b, Typical slow uPSCs in a NGC. Top, current injection protocol. Average postsynaptic response (green) is overlaid on individual uPSCs. Inset, location of pre- (black) and postsynaptic (green) cells.
c, uIPSCs in mature cells were either fast (top) or slow (bottom). Lower inset shows normalized currents from NGC (green) and mature cells (black) overlaid.
d, Rise times were fit with two Gaussian distributions with mean values of 0.78 (70%) and 1.7 ms in mature cells and 1.7 (57%) and 3.5 ms in NGCs. Decay τs were well fit with two Gaussian distributions with mean values of 14 (70%) and 32 ms in mature cells and a single distribution with a mean value of 48 ms in NGCs. PPD of uIPSCs in mature cells was more variable than in NGCs (300 ms interval). Error bars indicate S.E.M.
e, Interneuron action potentials and corresponding PSCs in a NGC (top, left) and interneuron firing pattern (bottom, left). Reconstruction of this presynaptic interneuron near the granule cell layer (dotted lines), with soma/dendrites in red (length, 820 μm) and axon in black (length, 8424 μm). Inset shows dense varicosities in a 50-μm length of axon. Post-hoc immunolabeling of reelin in the same interneuron (bottom, right). Scale bar, 10 μm.
Similar to focally evoked PSCs in NGCs2,3, uPSCs had small amplitudes (12.8 ± 2.9 pA, n = 9) with slow 20–80% rise times (3.5 ± 0.7 ms, n = 9) and decay τs (62.1 ± 7.0 ms, n = 9; Fig. 1b). In contrast, uIPSCs in mature granule cells displayed a range of kinetics that, on average, were faster (rise 1.3 ± 0.4 ms, p = 0.02; decay τ 19.4 ± 5.4 ms, p = 0.002, Fig. 1c). Cumulative Gaussian distributions of rise times and decay τs revealed that uIPSCs in mature cells were either fast or slow, whereas NGCs had only slow uPSCs (Fig. 1d). Similarly, paired-pulse depression (PPD) in mature cells had greater variability compared to NGCs (Fig. 1d). Together, these data indicate that stimulation of single interneurons is sufficient to generate slow PSCs in NGCs and that NGCs receive input from a more restricted population of interneurons than mature cells3.
Once a synaptic connection was identified, we examined the firing patterns of presynaptic interneurons using whole cell recording. Remarkably, 6/8 cells presynaptic to NGCs displayed a delayed firing pattern characteristic of Ivy and Neurogliaform interneurons (Ivy/NGs; Fig. 1e), including a long latency to fire at near threshold, modest spike frequency adaptation and relatively slow action potential kinetics4,5,6,7,8(S Table 1). Two interneurons were non-Ivy/NGs (S Fig.3). Ivy/NGs generate GABAB inhibition following single action potentials and GABAA IPSCs with strong paired-pulse depression4,5,6,7,8,9. Although we did not detect GABAB receptor-mediated currents in NGCs (unlike mature cells, unpublished observations), we measured strong paired-pulse depression of PSCs mediated by putative Ivy/NGs (PPD = 0.44 ± 0.04, n = 5 NGCs and 2 mature cells). As expected, interneurons presynaptic to mature cells were heterogeneous, including basket cells that generated fast uIPSCs (S Fig. 4) and delayed-spiking Ivy/NGs that generated slow uIPSCs (S Fig. 2c). Anatomical reconstruction of a delayed-spiking interneuron presynaptic to a NGC revealed thin aspinous primary dendrites and a dense axonal arbor covered with small boutons, with an average inter-bouton distance of 2.9μm8 (Fig 1e). This interneuron was also immunopositive for reelin (Fig. 1e). Furthermore, we found cells expressing NPY and nNOS, markers for Ivy/NGs in other brain regions, throughout the dentate6,7(S Fig. 5). The i) interneuron delayed-firing pattern, ii) robust synaptic depression, and iii) dense axonal arborization, along with iv) the unusual spatial-temporal GABA transient2,9–11 strongly argue that Ivy/NGs are the primary, but not necessarily exclusive, source of synaptic GABA signaling to NGCs.
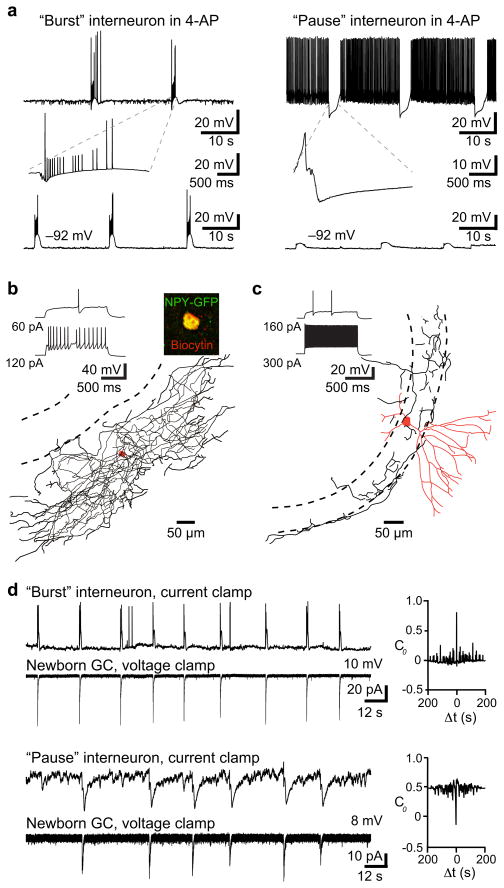
The K+ channel blocker 4-AP generates low frequency giant GABA-mediated PSCs that depolarize NGCs2(S Fig. 6 and S Fig. 7) presumably due to rhythmic firing of interneurons12. Our data thus predicts that 4-AP triggers rhythmic low-frequency firing of Ivy/NGs. Current clamp recordings from interneurons revealed principally two types of responses in the presence of 4-AP: either a “burst” or “pause” pattern of spiking (n = 35; Fig. 2a). All interneurons with a late-spiking phenotype characteristic of Ivy/NGs (Fig.2b) exhibited the burst pattern with firing at 0.04 ± 0.003 Hz (n = 9/9). In contrast, most non-late spiking interneurons were inhibited at a similar frequency in the presence of 4-AP (“pause” 0.04 ± 0.003 Hz; n = 18/26; Fig. 2a and 2c). A few non-late spiking interneurons showed the burst pattern in 4-AP (n = 6/26) and two did not have identifiable patterns. These results are also consistent with the idea that late-spiking Ivy/NGs are presynaptic to NGCs.
Figure 2. Activity patterns in 4-AP support Ivy/NG innervation.
a, Two distinct interneuron firing patterns in 4-AP (100 μM). Left, example of a “burst” interneuron that fired spikes and spikelets at 0.04 Hz. Burst shown on an expanded timescale (inset). Right, example of a “pause” interneuron that was inhibited at 0.04 Hz. Somatic hyperpolarization altered the tonic firing rate of all interneurons, but did not change the low frequency burst or pause patterns. Some pause interneurons did not fire spontaneously at resting potential (as in d).
b, Example of an interneuron with a delayed firing pattern that showed bursting in 4-AP (same cell as in a). The dense axonal arbor (length, 13,664 μm) and expression of NPY-GFP (inset) is consistent with Ivy/NGs5,7,8.
c, Example of a non-late spiking interneuron that was inhibited at 0.04 Hz. The high-frequency firing, fast action potential kinetics and morphology suggest it was a basket cell (dendrite length 2,493 μm; partial axon length 3,320 μm).
d, Both “bursts” (upper pair) and “pauses” (lower pair) were correlated with giant PSCs in NGCs. Right, cross-correlation analysis showed a positive correlation with interneuron bursts (4/11 pairs; C0 = 0.57 ± 0.05; see methods) and a negative correlation with pauses (7/11; C0 = −0.65 ± 0.05).
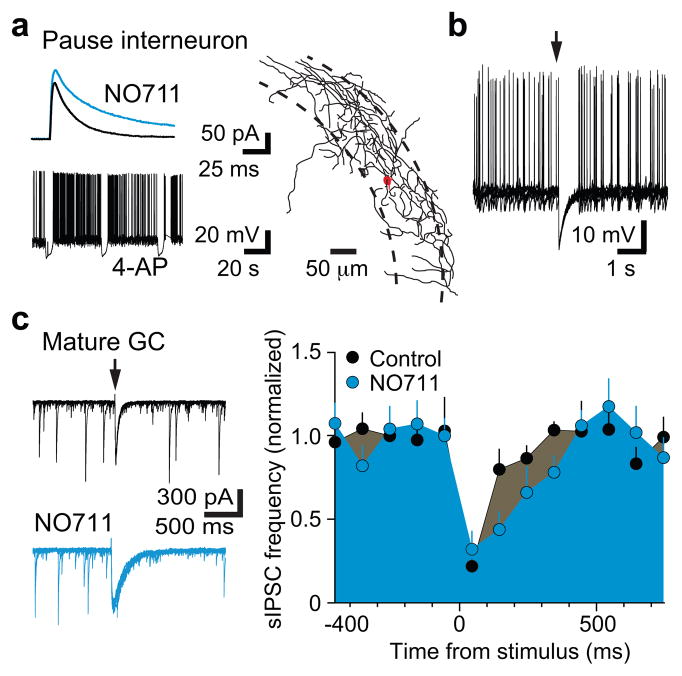
Interestingly, simultaneous recordings of interneurons and NGCs in 4-AP (n = 11) revealed that both bursts and pauses were correlated with giant PSCs in NGCs (Fig. 2d). Bursting in Ivy/NGs could, therefore, contribute to the pause in other interneurons through synaptic inhibition, if Ivy/NGs innervate interneurons as well as mature and NGCs (S Fig. 8). Focal stimulation in the molecular layer generates slow, NO711 sensitive PSCs in NGCs and mature cells2, presumably arising predominantly from Ivy/NGs8 (Fig. 1). We found that focal simulation also evoked slow, NO711 sensitive IPSCs in interneurons identified as “burst” (n = 3; not shown) or “pause” (n = 5; Fig. 3b) with subsequent 4-AP application, consistent with previous findings that Ivy/NGs innervate other interneurons subtypes5,8,13 and generate a slow synaptic GABA transient that is tightly regulated by GABA transporters9,10. Additionally, the slow synaptic IPSP in interneurons was sufficient to inhibit spontaneous action potential firing (Fig. 3c). Thus, GABAergic Ivy/NGs may link NGC depolarization with disinhibition of mature cells. Mature cells receive strong inhibition from perisomatic synapses that give rise to spontaneous IPSCs (sIPSCs)14, so we tested how activation of putative Ivy/NGs alter sIPSCs onto mature cells. Molecular layer stimulation and the resulting slow IPSC was indeed accompanied by a robust reduction in sIPSCs (Fig. 3d), similar to suppression of fast inhibition described in CA115. Suppression of sIPSCs in mature cells was prolonged by NO711 (Fig. 3d) and evident following giant IPSCs triggered by 4-AP (not shown), consistent with GABA release from Ivy/NGs.
Figure 3. Disinhibition of mature cells by Ivy/NGs predicts coordinated activity.
a, The GAT1 inhibitor NO711 (2 μM) increased the amplitude (114 ± 4% of control, p = 0.02) and decay phase (half width = 161 ± 16% of control, p = 0.05; area = 200 ± 22% of control, p = 0.01) of slow IPSCs in pause interneurons, supporting innervation by Ivy/NGs9,10. Inset, pause pattern of firing during subsequent 4-AP application. The axon of the pause cell shown in (b) and (c) revealed it targeted the perisomatic region.
b, Spontaneous interneuron firing induced by somatic depolarization was inhibited by the slow IPSP (from 1.0 ± 0.4 Hz to 0.1 ± 0.1 Hz, p = 0.05; n = 5). Arrow indicates time of synaptic stimulation. Six traces are overlaid.
c, Left, sIPSCs in mature granule cells were reduced after molecular layer stimulation (arrow). Eight traces overlaid. Right, NO711 significantly prolonged the suppression of sIPSC (half-width increased from 136 ± 20 ms to 222 ± 35 ms, n = 6, p < 0.02). Error bars indicate S.E.M.
Here we show that NGCs are primarily innervated by Ivy/NGs, an interneuron subtype that also innervate other interneurons. Thus, the local circuitry promotes coordinated activity: correlated depolarization of NGCs with disinhibition of mature granule cells. Unlike most interneuron subtypes that target precise subcellular domains of principal cells, Ivy/NGs mediate a form of GABA volume transmission with less spatial selectivity via densely spaced axonal terminals that often lack morphologically defined postsynaptic structures11. This form of transmission may be optimized for trophic signaling to NGCs in a dynamic period of dendrite motility and growth that limits synapse stabilization. Together these results demonstrate that Ivy/NGs are poised to mediate activity-dependent regulation of neurogenesis and coordinate activation of newborn and pre-existing granule cells.
Supplementary Material
Supplementary Figure 1. Strategy for identifying presynaptic interneurons
Supplementary Figure 2. Comparison of synaptic latency in newborn and mature cells
Supplementary Figure 3. Non-Ivy/NGs presynaptic to NGCs
Supplementary Figure 4. Basket cells innervate mature granule cells
Supplementary Figure 5. NPY+/nNOS+ interneurons in dentate gyrus
Supplementary Figure 6. 4-AP drives giant GABAergic currents in NGCs from juvenile and adult mice
Supplementary Figure 7. 4-AP drives simultaneous giant GABAergic currents in NGC and mature cells
Supplementary Figure 8. Proposed innervation pattern of Ivy/NGs
Supplementary Table 1. Intrinsic properties of interneurons presynaptic to NGCs
Acknowledgments
The authors thank members of the Wadiche labs and Anastassios Tzingounis for helpful comments, Alison Margolies and Chuansheng Zhao for technical assistance. This work was supported by NIH T32GM08111-23 (SJM), RO1NS064025 (LOW) and P30-HD38395.
Footnotes
Author Contributions: All authors contributed to each aspect of this work.
References
- 1.Ge S, et al. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markwardt SJ, Wadiche JI, Overstreet-Wadiche LS. J Neurosci. 2009;29:15063–15072. doi: 10.1523/JNEUROSCI.2727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esposito MS, et al. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamas G, Lorincz A, Simon A, Szabadics J. Science. 2003;299:1902–1905. doi: 10.1126/science.1082053. [DOI] [PubMed] [Google Scholar]
- 5.Price CJ, et al. J Neurosci. 2005;25:6775–6786. doi: 10.1523/JNEUROSCI.1135-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuentealba P, et al. Neuron. 2008;57:917–929. doi: 10.1016/j.neuron.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tricoire L, et al. J Neurosci. 2010;30:2165–2176. doi: 10.1523/JNEUROSCI.5123-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong C, Szabadics J, Tamas G, Soltesz I. J Comp Neurol. 2011;519:1476–1491. doi: 10.1002/cne.22577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karayannis T, et al. J Neurosci. 2010;30:9898–9909. doi: 10.1523/JNEUROSCI.5883-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szabadics J, Tamas G, Soltesz I. Proc Natl Acad Sci U S A. 2007;104:14831–14836. doi: 10.1073/pnas.0707204104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olah S, et al. Nature. 2009;461:1278–1281. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michelson HB, Wong RK. J Physiol. 1994;477:35–45. doi: 10.1113/jphysiol.1994.sp020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olah S, et al. Front Neural Circuits. 2007;1:4. doi: 10.3389/neuro.04.004.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soltesz I, Smetters DK, Mody I. Neuron. 1995;14:1273–1283. doi: 10.1016/0896-6273(95)90274-0. [DOI] [PubMed] [Google Scholar]
- 15.Banks MI, White JA, Pearce RA. Neuron. 2000;25:449–457. doi: 10.1016/s0896-6273(00)80907-1. [DOI] [PubMed] [Google Scholar]
- 16.Cowley MA, et al. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 17.Overstreet LS, et al. J Neurosci. 2004;24:3251–3259. doi: 10.1523/JNEUROSCI.5173-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Strategy for identifying presynaptic interneurons
Supplementary Figure 2. Comparison of synaptic latency in newborn and mature cells
Supplementary Figure 3. Non-Ivy/NGs presynaptic to NGCs
Supplementary Figure 4. Basket cells innervate mature granule cells
Supplementary Figure 5. NPY+/nNOS+ interneurons in dentate gyrus
Supplementary Figure 6. 4-AP drives giant GABAergic currents in NGCs from juvenile and adult mice
Supplementary Figure 7. 4-AP drives simultaneous giant GABAergic currents in NGC and mature cells
Supplementary Figure 8. Proposed innervation pattern of Ivy/NGs
Supplementary Table 1. Intrinsic properties of interneurons presynaptic to NGCs