Abstract
Biomarkers capable of predicting the onset and severity of acute graft-vs.-host disease (GVHD) after allogeneic hematopoietic cell transplantation (HCT) would enable pre-emptive and risk-stratified therapy. Severe acute GVHD leads to gastrointestinal protein loss, resulting in hypoalbuminemia. We hypothesized that decreases in serum albumin at onset of acute GVHD would predict the risk of progression to severe acute GVHD. We identified 401 patients who developed acute GVHD grades II–IV after reduced-intensity allogeneic HCT and reviewed all available serum albumin values from 30 days before HCT to 45 days after initiation of treatment for acute GVHD. A ≥0.5 g/dL decrease in serum albumin concentration from pre-transplant baseline to the onset of treatment for acute GVHD predicted the subsequent development of grade III/IV acute GVHD (vs. grade II acute GVHD) with a sensitivity of 69% and a specificity of 73%. Overall mortality at 6 months after initiation of acute GVHD treatment was 36% vs. 17% for patients with and without ≥0.5 g/dL decreases in serum albumin, respectively (p=0.0009). We conclude that change in serum albumin concentration from baseline to initiation of acute GVHD treatment is an inexpensive, readily available, and predictive biomarker of GVHD severity and mortality after reduced-intensity allogeneic HCT.
INTRODUCTION
Acute graft-vs.-host disease (GVHD) is a frequent complication of allogeneic hematopoietic cell transplantation (HCT), affecting the skin, liver, and gastrointestinal tract and contributing to transplant-related morbidity and mortality. Approximately 50–60% of patients require systemic treatment for acute GVHD after allogeneic HCT [1]. Treatment of acute GVHD is effective in most patients, but approximately 5–15% develop severe and therapy-resistant acute GVHD associated with a poor prognosis. Since most patients with acute GVHD respond well to existing treatments, measures which prospectively identify the small group destined to develop severe acute GVHD might be more useful than global intensification of acute GVHD prophylaxis or treatment. Biomarkers capable of predicting both the clinical onset and the ultimate severity of acute GVHD before treatment initiation would enable pre-emptive and risk-stratified therapy.
Numerous biomarkers for acute GVHD have been proposed and described in the transplant literature, although none has yet entered clinical practice. An ideal biomarker would be predictive of both disease onset and prognosis, inexpensive, consistent across laboratories, and readily available with a rapid turnaround time to facilitate real-time clinical decision-making. Investigators in Genoa have described a prognostic score based on the clinical characteristics and a combination of standard serum chemistries measured at day +7 after HCT [2,3].Specific cytokines and proteins such as TNF-α, TGF-β, soluble IL-2 receptor, Fas, and VEGF have not had substantial predictive value as acute GVHD biomarkers when assayed individually [4]. More recently, investigators have applied proteomic approaches to develop panels of predictive biomarkers, with several groups reporting promising initial findings [5–8]. While proteomic approaches are invaluable in discovering novel markers of acute GVHD, the operating characteristics of putative biomarkers have not yet yielded clinically useful assays with adequate positive and negative predictive value in population-based studies.
Since the advent of ursodiol prophylaxis, liver acute GVHD has become rare, and midgut involvement is now predominantly responsible for the morbidity and mortality associated with acute GVHD [9]. Protein loss through the gut is characteristic of midgut acute GVHD [10]. We hypothesized that intestinal protein loss in patients destined to develop clinical acute GVHD would be reflected by a decrease in serum albumin. Since intestinal protein loss in acute GVHD precedes the development of mucosal erosion, ulceration, and denudation, we further hypothesized that changes in serum albumin concentration would allow early identification of patients whose acute GVHD would be characterized by midgut injury and a poor prognosis. Since conditioning chemoradiotherapy may also cause mucosal injury and protein loss, we limited our analysis to patients conditioned with reduced-intensity regimens which produce minimal regimen-related gut toxicity, thus isolating the relationship between acute GVHD and GI protein loss.
PATIENTS AND METHODS
Patient selection
We retrospectively reviewed the records of 708 consecutive patients who received a first allogeneic HCT after reduced-intensity conditioning according to Fred Hutchinson Cancer Research Center (FHCRC) institutional research protocols between December 16, 1997 and April 29, 2009. One of us (PJM) reviewed the clinical course of all patients and determined the date of initiation of systemic treatment for acute GVHD and the peak stage and grade of acute GVHD. These determinations were completed prior to, and independently of, the analysis described here.
Techniques of hematopoietic cell transplantation
Patients and their respective donors were tested for HLA-A, HLA-B, and HLA-C by at least intermediate-resolution DNA typing and for HLA-DRB1 and HLA-DQB1 by high-resolution techniques. Conditioning regimens included low-dose total body irradiation (TBI) of 2–4 Gy with or without fludarabine 90 mg/m2 in 392 patients (98%) and cyclophosphamide 200 mg/kg with horse antithymocyte globulin 90 mg/kg in 8 patients (2%). One patient received no conditioning (Table 1). Supportive care, including antimicrobial and cytomegalovirus prophylaxis, was administered as described previously [11]. Post-grafting immunosuppression consisted of cyclosporine or tacrolimus combined with mycophenolate mofetil, as previously described [12–14]. Seventeen patients also received sirolimus as GVHD prophylaxis [14]. Hematopoietic growth factors were administered to the recipient only for persistent neutropenia after day +21. First-line treatment for acute GVHD consisted of corticosteroids at 1–2 mg/kg [15], while second-line and subsequent therapies (if needed) were prescribed at the discretion of the attending physician, subject to available institutional research protocols. Exogenous albumin was not routinely administered, and was used only as a supportive measure in cases of large-volume paracentesis.
Table 1.
Characteristics of 401 patients with acute GVHD grades II–IV after reduced-intensity allogeneic HCT.
| Age at HCT, years | |
| Median (range) | 55 (<1–75) |
|
| |
| Gender | |
| Male | 254 (63%) |
| Female | 147 (37%) |
|
| |
| Donor | |
| Related donor | 164 (41%) |
| Unrelated donor | 237 (59%) |
| HLA-matched | 173 |
| HLA-mismatched | 64 |
|
| |
| Transplant indication | |
| Acute myeloid leukemia | 104 |
| Non-Hodgkin lymphoma | 77 |
| Multiple myeloma | 56 |
| Chronic lymphocytic leukemia | 39 |
| Myelodysplastic syndrome | 25 |
| Primary ID/marrow failure | 25 |
| Acute lymphoblastic leukemia | 24 |
| Hodgkin lymphoma | 23 |
| Chronic myelogenous leukemia | 11 |
| Aplastic anemia | 7 |
| Solid tumor | 6 |
| Myeloproliferative disorder | 4 |
|
| |
| Conditioning regimen | |
| 2 Gy TBI | 51 |
| 2 Gy TBI + fludarabine 90 mg/m2 | 320 |
| 3 Gy TBI + fludarabine 90 mg/m2 | 14 |
| 4 Gy TBI + fludarabine 90 mg/m2 | 7 |
| CY 200 mg/kg + hATG 90 mg/kg | 8 |
| No conditioning | 1 |
|
| |
| Stem cell source | |
| G-PBMC | 370 (92%) |
| Bone marrow | 31 (8%) |
|
| |
| CD34+ cell dose, cells/kg | |
| Median | 7.82 × 106 |
|
| |
| Median time to acute GVHD onset | 35 days |
|
| |
| Peak acute GVHD grade | |
| II | 325 (81%) |
| III–IV | 76 (19%) |
Abbreviations: HCT, hematopoietic cell transplantation; HLA, human leukocyte antigen; ID, immunodeficiency; TBI, total body irradiation; CY, cyclophosphamide; hATG, horse antithymocyte globulin; G-PBMC, granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells; acute GVHD, acute graft-vs.-host disease.
Measurement of serum albumin concentration
Serum albumin levels were assayed at least once before HCT, at least once per week during the first 3 months after HCT, and more frequently as clinically indicated, per institutional standard practice. We reviewed all available serum albumin values from 30 days before HCT to 45 days after initiation of treatment for acute GVHD. Serum albumin was measured by clinical laboratories at the University of Washington Medical Center, the Seattle Cancer Care Alliance, and Seattle Children’s using SYNCHRON Systems machines and bichromatic digital endpoint methodology with bromcresol purple reagent.
Statistical analysis
Baseline albumin was defined as the minimum albumin value measured within 30 days before HCT. Albumin at onset of GVHD treatment was defined as the highest albumin value occurring within 2 days of the start of treatment. Overall survival was estimated by the Kaplan-Meier method, and cumulative incidences of non-relapse mortality (NRM) and relapse were calculated according to methods previously described [16]. All deaths occurring in the absence of documented relapsed malignancy were considered NRM. Cox regression models were used to estimate hazard ratios. Relapses which occurred before the onset of treatment were accommodated in these analyses by assigning the day of relapse to the first day after treatment. Receiver operating characteristic (ROC) curves and associated parameters were derived from logistic regression models. Initial models used albumin at the start of GVHD therapy or the change in albumin from baseline as single predictors. Subsequent models added HLA-matching (HLA-matched vs. HLA-mismatched), donor relation (related vs. unrelated), time to onset of acute GVHD, patient age, and total serum bilirubin, blood urea nitrogen, creatinine, and platelet count at the initiation of acute GVHD therapy. The latter six variables were entered as continuous variables; creatinine and bilirubin were truncated at a maximum value of 5.0.
RESULTS
Of 708 consecutive transplant recipients, 401 (57%) developed acute GVHD grades II–IV requiring systemic treatment and were included in this analysis (Table 1). In these 401 patients with acute GVHD grades II–IV, acute GVHD peaked at grade II in 325 patients (81%) and grades III–IV in the remaining 76 patients (19%). Most patients had HLA-matched donors, while 16% had HLA-mismatched unrelated donors. The majority of patients (371, 93%) were conditioned with 2 Gy TBI with or without fludarabine. Mobilized peripheral blood mononuclear cell grafts were used in 370 patients (92%), while the remaining 31 patients (8%) received bone marrow grafts. Patients initiated systemic treatment for acute GVHD at a median of 35 days after HCT (range, 5–175 days). Three hundred fifty-two patients (88%) had sufficient albumin data available to calculate both a baseline value (between day −30 and day −1 before HCT) and a value at treatment initiation (within 2 days before or after initiation of systemic treatment for acute GVHD).
Serum albumin concentration and severity of acute GVHD
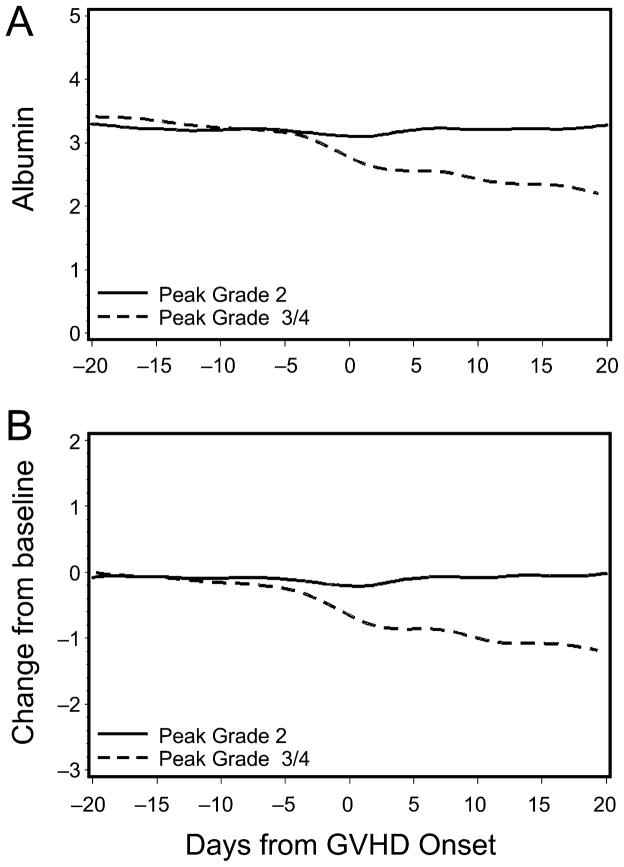
Before the initiation of treatment for acute GVHD, patients whose acute GVHD was ultimately graded as III/IV showed sharp decreases in mean serum albumin concentrations, both in absolute terms and relative to pre-transplant baseline albumin concentrations (Figure 1). This decrease from baseline first became apparent approximately 5 days before starting systemic treatment for acute GVHD. In contrast, patients whose acute GVHD never exceeded grade II showed no change from baseline in mean serum albumin concentration.
Figure 1. Change in absolute (A) and relative (B) serum albumin concentration in patients with peak grade 2 acute GVHD (solid line) versus peak grade 3/4 acute GVHD (dashed line).
Albumin concentration (ordinate) is in grams per deciliter.
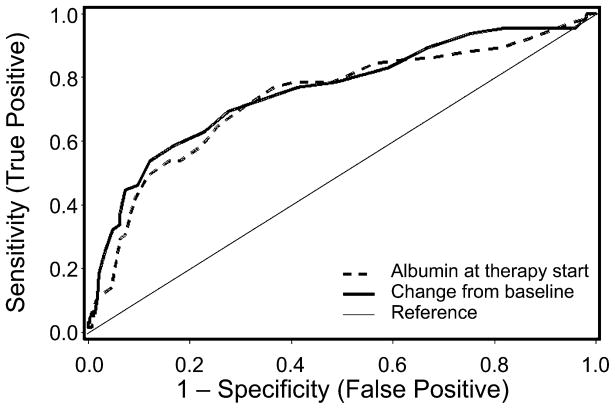
Receiver-operator characteristic (ROC) curves demonstrated that at the time of treatment initiation for acute GVHD, a decrease in serum albumin concentration of ≥0.5 g/dL from baseline optimally discriminated between patients who subsequently developed severe acute GVHD and those whose acute GVHD peaked at grade II, with an area under the ROC curve of 0.76 (Figure 2). A decrease from baseline serum albumin concentration of ≥0.5 g/dL at the time of treatment initiation predicted progression to severe acute GVHD with a sensitivity of 69% and a specificity of 73%, with reference to those patients whose acute GVHD peaked at grade II.
Figure 2. Receiver-operator characteristic (ROC) curves for serum albumin as a predictor of peak grade 3/4 acute GVHD.
Dashed line represents ROC of absolute serum albumin concentration at initiation of systemic therapy for acute GVHD. Solid line represents ROC of change in serum albumin concentration from baseline at initiation of systemic therapy for acute GVHD.
Serum albumin and mortality
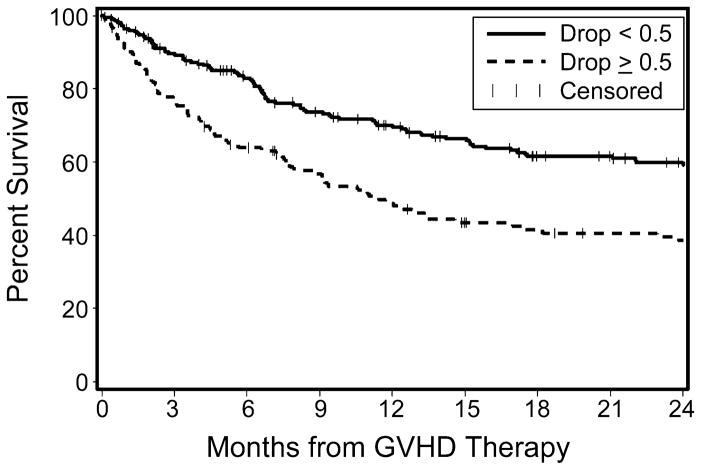
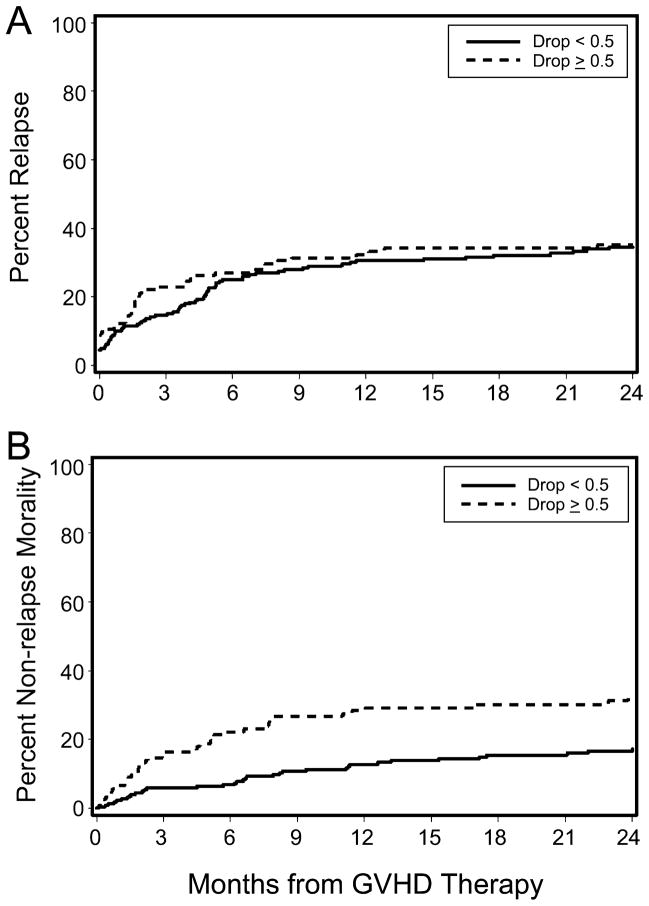
Decreases in serum albumin concentration before acute GVHD onset also correlated with the risk of overall mortality. Patients with a serum albumin decrease from baseline of ≥0.5 g/dL before beginning GVHD treatment showed an increased risk of mortality at 6 months after treatment initiation, persisting through the follow-up period (Figure 3). At 6 months after starting acute GVHD treatment, overall survival (OS) was 83% vs. 64% for patients without and with a pre-treatment albumin decrease of ≥0.5 g/dL, respectively (mortality hazard ratio 1.64, 95% confidence interval 1.2–2.2, p=0.0009). This 19% absolute increase in mortality was driven by non-relapse deaths. Rates of relapsed malignancy were similar in the two groups, whereas non-relapse mortality (NRM) was significantly higher in the group with a pre-treatment albumin decrease (Figure 4). The cumulative incidence of NRM in patients with a ≥0.5 g/dL decrease in serum albumin concentration was 27% at 6 months after starting treatment, compared to 7% in the group without a substantial pre-treatment albumin decrease (HR 2.01, 95% confidence interval 1.3–3.0, p=0.001). Rates of relapsed or progressive malignancy at 6 months in the two groups were 27% among patients with an albumin decrease ≥0.5 g/dL and 25% among those with stable albumin concentrations (HR 1.25, 95% confidence interval 0.9–1.8, p=0.23). These figures include progression rates of 9% and 4%, respectively, before initiation of systemic therapy for acute GVHD. A ≥0.5 g/dL decrease from baseline serum albumin concentration predicted NRM at 6 months with a sensitivity of 64% and a specificity of 69%.
Figure 3. Overall survival from initiation of therapy for acute GVHD.
Solid line represents survival in patients with an insignificant albumin decrease (<0.5 g/dL), while dashed line represents survival in patients with an albumin decrease of ≥0.5 g/dL.
Figure 4. Cumulative incidences of relapse (A) and non-relapse mortality (B), stratified by change in serum albumin at initiation of acute GVHD treatment.
We analyzed whether low pre-transplant albumin concentration was an independent risk factor for NRM. Low baseline albumin was defined as a pre-transplant albumin concentration of ≤3.0 g/dL, representing the lowest quartile of baseline values. Low baseline serum albumin alone was not significantly predictive of increased NRM (HR 1.41, 95% confidence interval 0.9–2.3, p=0.16). Incorporating low baseline serum albumin concentration into a predictive model for NRM had essentially no effect on its association with albumin decrease (adjusted HR 2.08, 95% confidence interval 1.4–3.1, p=0.0005).
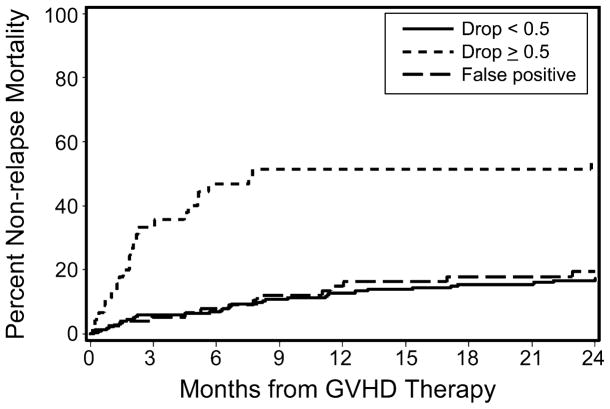
We attempted to confirm that the increased risk of NRM in patients with an albumin decrease was driven primarily by acute GVHD by retrospectively separating these patients according to their ultimate peak acute GVHD grade. In patients whose acute GVHD peaked at grade II, pre-treatment decreases in serum albumin did not predict NRM. The association between NRM and decreased albumin was confined solely to those patients who developed grade III/IV acute GVHD (Figure 5). This finding supports the role of serum albumin as a biomarker for midgut acute GVHD and for acute-GVHD-specific mortality, since the predictive power of decreasing albumin as a biomarker of mortality derives entirely from its association with severe midgut acute GVHD.
Figure 5. Non-relapse mortality from time of initiation of acute GVHD treatment.
“False-positive” line represents patients with an albumin decrease of ≥0.5 g/dL, but who did not progress to grade 3/4 acute GVHD. Non-relapse mortality is increased only in patients with an albumin decrease and severe acute GVHD, suggesting that the prognostic value of albumin decrease is linked entirely to its ability to predict severe acute GVHD.
Analysis of covariates
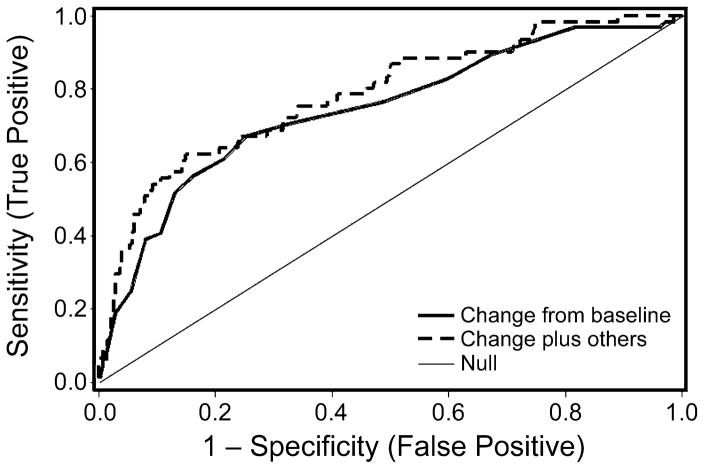
We attempted to improve the predictive power of falling serum albumin by incorporating other clinical and laboratory parameters that might also predict acute GVHD severity or NRM. We analyzed data on donor type (related vs. unrelated), degree of HLA disparity, time to acute GVHD onset [17], patient age, and total serum bilirubin, blood urea nitrogen, creatinine, and platelet count at acute GVHD treatment initiation for the study cohort. Of these factors, only HLA mismatch and time to acute GVHD onset were weakly associated with progression to grade III/IV acute GVHD. When these factors were combined with serum albumin into a predictive model, the area under the ROC curve improved slightly from 0.76 to 0.79 (Figure 6). Thus, the inclusion of these additional clinical parameters did not substantially improve the prediction of acute GVHD severity.
Figure 6. Receiver-operator characteristic curve for serum albumin decrease ≥0.5 g/dL alone (solid line) and in combination with other clinical factors (dashed line) in predicting progression to grade 3/4 acute GVHD.
The initial ROC curve (solid line) has an area under the curve of 0.76. The incorporation of other clinical variables (donor type, degree of HLA disparity, time to acute GVHD onset, patient age, and bilirubin, creatinine, and platelet count at acute GVHD treatment initiation) resulted in an ROC curve with an area under the curve of 0.79 (dashed line).
DISCUSSION
Decreases in serum albumin precede the clinical onset of severe acute GVHD and predict overall mortality in patients treated for acute GVHD after reduced-intensity allogeneic HCT. Serum albumin measurement is standardized, inexpensive, and widely available with rapid turnaround. This biomarker can readily be applied to aid real-time clinical decision-making: when a patient develops acute GVHD requiring systemic treatment, serum albumin concentration can be measured and compared to the pre-transplant baseline value. If the serum albumin concentration has decreased by ≥0.5 g/dL from baseline, then the patient is at increased risk of severe midgut GVHD and NRM. Because this information is available at the time of treatment initiation, high-risk patients could be stratified to receive more aggressive immunosuppressive therapy upfront, ideally suppressing graft-vs.-host reactions before irreversible mucosal injury occurs.
Serum albumin concentration is influenced by numerous factors, including nutritional status and hepatic synthetic function. Hypoalbuminemia resulting from decreased protein synthesis develops gradually, as the half-life of serum albumin is approximately 19 days [18]. The steep and sudden decline seen in this patient cohort is characteristic of rapid protein loss from the midgut, and its temporal association with clinical acute GVHD onset supports a pathophysiologic link between the two. Mean albumin levels remained constant in patients with grade II acute GVHD, suggesting that neither the transplant process itself nor upper gut involvement with acute GVHD produces significant protein loss or hypoalbuminemia.
In 1983, Weisdorf et al. documented gut protein loss as an early, often pre-clinical phenomenon in patients with acute GVHD after myeloablative allogeneic HCT [10]. In that study, fecal levels of α1-antitrypsin increased modestly after myeloablative conditioning, but returned to baseline in patients without acute GVHD. In contrast, patients with midgut acute GVHD showed persistent and substantial increases in fecal α1-antitrypsin excretion, suggesting ongoing protein loss. Gut involvement with acute GVHD early after myeloablative HCT is characterized histologically by scattered apoptotic cells in the mucosal crypts, and radiologically by mucosal edema [19,20]. Protein-losing enteropathy does not require ulceration or denudation of intestinal epithelium, but likely occurs through leaky epithelial tight junctions [21]. Data from studies of fecal α1-antitrypsin excretion and from the current study suggest that protein-losing enteropathy in acute GVHD is a reflection of the cytokine milieu in the midgut mucosa. Tight junctions (zonula occludens) in midgut epithelium regulate the transit of lamina propria protein into the lumen and the translocation of luminal bacteria and endotoxin into the lamina propria [21].
While peak acute GVHD grade can be retrospectively correlated with NRM, GVHD grading is only marginally useful as a prospective tool to predict outcome at the time of disease onset [22,23]. Better predictors of clinical outcome with acute GVHD include the area under a disease activity curve [23], failure of initial glucocorticoid treatment, and markers of gut mucosal necrosis after diagnosis [24]. Because these parameters are not available until after the clinical onset of acute GVHD, diagnostic and prognostic tests which can be employed before or at the clinical onset of acute GVHD are needed. Decreasing serum albumin appears to possess the characteristics of such a test. These findings, along with work on other acute GVHD biomarkers, imply that initial therapy for acute GVHD might move from a reactive to a pre-emptive approach, just as therapy for cytomegalovirus reactivation after HCT has moved to a preemptive approach based on detection of CMV DNA. This hypothesis lends itself readily to evaluation in prospective clinical trials.
Pre-emptive approaches to acute GVHD therapy are hampered, however, by the relatively low prevalence of severe acute GVHD [9], which limits the positive predictive value of even highly sensitive and specific diagnostic tests. In this study cohort, 19% of patients requiring systemic therapy for acute GVHD progressed to severe acute GVHD. Given this prevalence, the sensitivity and specificity of serum albumin (69% and 73%, respectively) translate into a positive predictive value of 37% and a negative predictive value of 91% for the development of severe (rather than grade II) acute GVHD. These figures highlight the difficulty of accurately predicting a low-prevalence condition such as severe acute GVHD, a difficulty which is common to all published acute GVHD biomarkers. In this regard, an advantage of serum albumin measurement is that it can be applied at the time of treatment initiation for acute GVHD, rather than in asymptomatic patients without active GVHD. Thus, even “false-positive” patients (those whose acute GVHD is destined to peak at grade II) require some level of acute GVHD therapy, and the hazard of overtreatment can be minimized.
Non-relapse mortality and overall survival are well-defined endpoints with clear clinical relevance, and an ideal biomarker would predict not only peak acute GVHD grade (which correlates only loosely with clinical outcome) but also NRM and OS. In our cohort, albumin decrease predicts a 20% absolute increase in risk of NRM at 6 months from initiation of acute GVHD treatment. The proteomic panel described by Paczesny et al. similarly identifies a high-risk group of patients with a 15% absolute increase in risk of mortality at 3.5 years after HCT [6]. Nishiwaki et al. recently described macrophage infiltration into skin lesions as a predictor of severe acute GVHD and mortality, with reported sensitivities of 25–44% and specificities of 70–87% [25]. A sophisticated proteomic assay developed by Mischak-Weissinger et al. predicts the onset of acute GVHD with a reported sensitivity and specificity of 76% and 85%, respectively, although data on prediction of severe acute GVHD and mortality were not given [5]. Thus, the performance characteristics of serum albumin as an acute GVHD biomarker are similar, and in some cases superior, to those of other recently reported approaches. In contrast to these other approaches, serum albumin measurement has the added advantages of simplicity, low cost, rapid turnaround, and widespread availability.
An important limitation is that our findings apply only to patients conditioned with reduced-intensity regimens which do not cause appreciable regimen-related mucosal injury. High-dose chemotherapy or radiation may damage the intestinal mucosa, causing protein loss which overlaps with that caused by acute GVHD and obscuring the value of albumin as a GVHD-specific biomarker. Thus, our current data cannot be extrapolated to support the use of albumin as a biomarker of acute GVHD in patients receiving myeloablative conditioning.
In summary, decreases in serum albumin before acute GVHD onset have substantial predictive value as a biomarker for acute GVHD severity and death in patients undergoing reduced-intensity allogeneic HCT. A simple comparison of serum albumin at the time of acute GVHD treatment initiation to a patient’s baseline value can identify patients with a 20% absolute increase in the risk of death at 6 months. This increased risk appears to be driven entirely by acute GVHD, making this high-risk group a logical target for pre-emptive intensification of acute GVHD therapy. Since many pre-emptive interventions against acute GVHD carry significant risk to the patient, it may be necessary to improve the positive predictive value of decreasing serum albumin by incorporating other biomarkers. While the search for an ideal acute GVHD biomarker remains incomplete, we believe that changes in serum albumin provide a simple, novel, and biologically based addition to the predictive tools available to transplant clinicians.
Acknowledgments
This research was supported by National Institutes of Health grants CA76930, HL36444, CA78902, CA18029, and CA15704.
The authors thank Gresford Thomas and Michelle Bouvier for assistance in data collection and management, and Helen Crawford for assistance with manuscript preparation. We would also like to thank the patients enrolled on these transplant protocols and the staff and physicians who cared for them.
Footnotes
PRESENTED IN PART at the 51st annual meeting of the American Society of Hematology, New Orleans, LA, USA, December 2009.
FINANCIAL DISCLOSURE: The authors have no relevant financial conflicts of interest to disclose.
AUTHORSHIP CONTRIBUTIONS
Study conception and design: G.B.M., A.R.R.
Data collection: P.J.M., R.F.S., B.M.S., D.G.M.
Statistical analysis: B.E.S.
Interpretation and analysis of data: A.R.R., G.B.M., M.M., B.E.S.
Primary authorship of manuscript: A.R.R.
Editing and revising manuscript: All authors.
Final approval of manuscript: All authors
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burroughs L, Mielcarek M, Leisenring W, et al. Extending postgrafting cyclosporine decreases the risk of severe graft-versus-host disease after nonmyeloablative hematopoietic cell transplantation. Transplantation. 2006;81:818–825. doi: 10.1097/01.tp.0000203556.06145.5b. [DOI] [PubMed] [Google Scholar]
- 2.Bacigalupo A, Lamparelli T, Milone G, et al. Pre-emptive treatment of acute GVHD: a randomized multicenter trial of rabbit anti-thymocyte globulin, given on day+7 after alternative donor transplants. Bone Marrow Transplant. 2010;45:385–391. doi: 10.1038/bmt.2009.151. [DOI] [PubMed] [Google Scholar]
- 3.Sormani MP, Oneto R, Bruno B, et al. A revised day +7 predictive score for transplant-related mortality: serum cholinesterase, total protein, blood urea nitrogen, gamma glutamyl transferase, donor type and cell dose. Bone Marrow Transplant. 2003;32:205–211. doi: 10.1038/sj.bmt.1704085. [DOI] [PubMed] [Google Scholar]
- 4.Lunn RA, Sumar N, Bansal AS, Treleaven J. Cytokine profiles in stem cell transplantation: possible use as a predictor of graft-versus-host disease. Hematology. 2005;10:107–114. doi: 10.1080/10245330400001975. [DOI] [PubMed] [Google Scholar]
- 5.Mischak-Weissinger EM, Holler E, Schleuning M, et al. Prospective evaluation of an agvhd-specific proteomic patten in more than 340 patients. Blood. 2009;114:147. #347[abstr.] [Google Scholar]
- 6.Paczesny S, Krijanovski OI, Braun TM, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113:273–278. doi: 10.1182/blood-2008-07-167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weissinger EM, Schiffer E, Hertenstein B, et al. Proteomic patterns predict acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood. 2007;109:5511–5519. doi: 10.1182/blood-2007-01-069757. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan R, Daniels J, Fusaro V, et al. Accurate diagnosis of acute graft-versus-host disease using serum proteomic pattern analysis. Exp Hematol. 2006;34:796–801. doi: 10.1016/j.exphem.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weisdorf SA, Salati M, Longsdorf JA, Ramsay NKC, Sharp HL. Graft-versus-host disease of the intestine: a protein losing enteropathy characterized by fecal α-antitrypsin. Gastroenterology. 1983;85:1076–1081. [PubMed] [Google Scholar]
- 11.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 12.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 13.Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–1629. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 14.Sandmaier BM, Maris M, Storer B, et al. A randomized 3-arm phase II study to determine the most promising postgrafting immunosuppression for prevention of acute graft-versus-host disease (GVHD) after unrelated donor hematopoietic cell transplantation (HCT) using nonmyeloablative conditioning for patients with hematologic malignancies: a multi-center trial. Blood. 2009;114:147. #348[abstr.] [Google Scholar]
- 15.Mielcarek M, Storer BE, Boeckh M, et al. Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood. 2009;113:2888–2894. doi: 10.1182/blood-2008-07-168401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 17.Mielcarek M, Burroughs L, Leisenring W, et al. Prognostic relevance of "early-onset" graft-versus-host disease following nonmyeloablative hematopoietic cell transplantation. Br J Haematol. 2005;129:381–391. doi: 10.1111/j.1365-2141.2005.05458.x. [DOI] [PubMed] [Google Scholar]
- 18.Peters T., Jr . All About Albumin: Biochemistry, Genetics, and Medical Applications. San Diego, CA: Academic Press; 1996. [Google Scholar]
- 19.Epstein RJ, McDonald GB, Sale GE, Shulman HM, Thomas ED. The diagnostic accuracy of the rectal biopsy in acute graft-versus-host disease: A prospective study of thirteen patients. Gastroenterology. 1980;78:764–771. [PubMed] [Google Scholar]
- 20.Fisk JD, Shulman HM, Greening RR, McDonald GB, Sale GE, Thomas ED. Gastrointestinal radiographic features of human graft-vs.-host disease. Am J Roentgenol. 1981;136:329–336. doi: 10.2214/ajr.136.2.329. [DOI] [PubMed] [Google Scholar]
- 21.Venkatasubramanian J, Rao MC, Sellin JH. Intestinal electrolyte absorption and secretin. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisinger and Fordtran's Gastroinstestinal and Liver Disease: Pathophysiology, Diagnosis, Management. Philadelphia, PA: Saunders Elsevier; 2010. pp. 1675–1694. [Google Scholar]
- 22.Cahn JY, Klein JP, Lee SJ, et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: a joint Societe Francaise de Greffe de Moelle et Therapie Cellulaire (SFGM-TC), Dana Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood. 2005;106:1495–1500. doi: 10.1182/blood-2004-11-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leisenring WM, Martin PJ, Petersdorf EW, et al. An acute graft-versus-host disease activity index to predict survival after hematopoietic cell transplantation with myeloablative conditioning regimens. Blood. 2006;108:749–755. doi: 10.1182/blood-2006-01-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castilla-Llorente C, Nash RA, McDonald GB, Storer BE, Martin PJ. Prognostic factors and outcomes of severe gastrointestinal graft-vs-host disease (GI GVHD) after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15 (Suppl):S120–S121. doi: 10.1038/bmt.2014.69. [abstr.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishiwaki S, Terakura S, Ito M, et al. Impact of macrophage infiltration of skin lesions on survival after allogeneic stem cell transplantation: a clue to refractory graft-versus-host disease. Blood. 2009;114:3113–3116. doi: 10.1182/blood-2009-03-209635. [DOI] [PubMed] [Google Scholar]