Abstract
Lower extremity fat mass (LEFM) has been shown to be favorably associated with glucose metabolism. However, it is not clear whether this relationship is similar across varying levels of obesity. We hypothesized that lower amounts of LEFM is associated with higher insulin resistance (IR) and this association may vary according to weight status. Participants with available measures were examined from the Coronary Artery Risk Development in Young Adults study (CARDIA), a multi-center longitudinal study of the etiology of atherosclerosis in black and white men and women aged 38–50 years old in 2005–2006 (n = 1,579). The homeostasis model assessment of IR (HOMAIR) was calculated to estimate IR, regional adiposity was measured using dual energy X-ray absorptiometry (DXA), and weight status was defined according to BMI categories. Obese and overweight participants exhibited higher IR, total fat mass (FM), trunk FM (TFM), and LEFM compared to normal weight participants. After controlling for age, height, race, study center, education, smoking, and cardiorespiratory fitness (CRF), greater LEFM was significantly associated with higher IR only in normal weight men and women. Further adjustment for TFM revealed that lower LEFM was significantly associated with higher IR in overweight and obese men and women and the positive association in normal weight individuals was attenuated. These results suggest that excess adiposity in the lower extremities may attenuate the metabolic risk observed at a given level of abdominal adiposity in overweight and obese individuals. Weight status presents additional complexity since the metabolic influence of adipose tissue may not be homogenous across anatomic regions or level of obesity.
INTRODUCTION
Substantial evidence confirms that accumulation of adipose tissue in specific anatomical compartments, namely the abdomen, confers excess risk for insulin resistance (IR). Although lower extremity fat mass (LEFM) has generally not been considered a correlate of metabolic risk, data from prospective cohort studies indicate that having a larger hip circumference is favorably associated with cardiovascular disease and diabetes risk (1–3). Investigations which use dual energy X-ray absorptiometry (DXA) to measure regional adiposity have provided further evidence that lower amounts of adiposity in the lower extremities is associated with higher IR and unfavorable glucose levels (4–8), yet, the majority of investigations using this technique were conducted in small, single-sex samples (i.e., only women), and/or focused on overweight or obese participants (4,9,10). The association between LEFM and IR has also been reported to vary according to weight status (11), therefore, thorough investigation of this association across a wide range of obesity is warranted.
Our objective was to investigate the associations between IR and DXA-assessed regional adiposity distribution in middle-aged (38–50 years) men and women from The Coronary Artery Risk Development in Young Adults (CARDIA) study. Since having lower amounts of adiposity in the lower extremities has been associated with unfavorable metabolic profiles, we hypothesize that lower amounts of LEFM is associated with higher levels of IR and this association is independent of abdominal adiposity. We further hypothesize that this association varies according to weight status. While exploring the LEFM/IR relationship, we account for additional plausible confounders, i.e., age, sex, race, height, smoking status, education, and cardiorespiratory fitness (CRF).
METHODS AND PROCEDURES
The CARDIA study is a longitudinal investigation of the etiology of cardiovascular disease in a biracial cohort of 5,115 adults aged 18–30 years in 1985–1986. Participants were recruited from Birmingham, AL, Chicago, IL, Minneapolis, MN, and Oakland, CA. Details of study recruitment and design have been previously reported (12). Participants were re-examined 2, 5, 7, 10, 15 and 20 years after baseline. Retention rates ranged from 72% to 91% across examinations; 72% of participants returned for the 20-year examination (n = 3,683). Age, race, education (years), and cigarette smoking status were ascertained by interview at all examinations. The present analyses are based on data collected at the 20-year examination (2005–2006) and as part of the ancillary CARDIA Fitness study in participants with valid anthropometric, glucose, and insulin measures and those without diabetes (n = 1,579). Diabetes was defined as a fasting blood glucose ≥126 mg/dl, postload blood glucose ≥200 mg/dl, or use of antidiabetic medication. Written informed consent was obtained by all participants and the study design, data collection, and analyses were performed in accordance with ethical standards of supervising institutional review boards of all the centers involved.
Anthropometric assessment
Height, weight, and waist circumference (WC) were measured with participants in light examination clothes and no shoes. WC was measured at the waist girth midway between the xiphoid process and iliac crest in duplicate and averaged. BMI was calculated as the ratio of weight to standing height squared (kg/m2) and participants were categorized according to one of three clinical BMI categories: normal weight (BMI <25.0), overweight (BMI 25.0–29.9), obese (BMI ≥30.0). Adiposity distribution was measured in all study sites by DXA (Hologic QDR 4500W, Delphi 11.2, Discovery XP 12.1, Discovery XP 2002; Hologic, Bedford, MA) for quantification of adiposity in the following regions: lower extremities (i.e., hips, thighs, legs and feet) (LEFM), trunk (TFM), and upper extremities (i.e. arms, forearms and hands) (UEFM). Total body FM (kg and %) and bone-free fat-free mass was also quantified. The separation between the trunk and lower extremities was made by two oblique lines passing through the femoral necks and separation between the trunk and upper extremity regions was made by two oblique lines passing through the humeral heads. TFM included both subcutaneous and visceral fat of this anatomical region. LEFM and UEFM was calculated as the total fat in both corresponding limbs. Since previous reports indicate the presence of artifacts influence DXA adiposity assessment (13,14), participants were excluded from analyses if any foreign objects were present (i.e., jewelry or hairpins (n = 233) or joint replacements (n = 148)). Participants were also excluded if feet (n = 176) or upper extremities (n = 324) were outside of the scanning region.
CRF assessment
CRF was estimated based on the duration that participants were able to walk or run during a standardized symptom-limited graded exercise test using a modified Balke treadmill protocol (15). CRF was estimated by treadmill duration which is strongly correlated with directly measured maximal aerobic capacity (16).
Biochemical analyses
Participants were asked to fast for 12 h and avoid smoking and heavy physical activity for ≥2 h prior to examination. Blood was drawn by venipuncture according to standard procedures across all field centers. Serum was separated by centrifugation, transferred into airtight vials and stored at −70 °C until shipped on dry ice to a central laboratory for processing. Glucose and insulin measurements were performed at Linco Research (now Millipore, Billerica, MA). The concentration of glucose in the stored samples was determined with a Cobas Mira Plus chemistry analyzer (Roche Diagnostics, Indianapolis, IN) using the hexokinase ultraviolet method. Insulin measurements were performed by using a radioimmunoassay with overnight, equilibrium-incubation format. The homeostasis model assessment of IR (HOMAIR) was calculated to estimate IR using the following formula: fasting plasma insulin (µIU/l) × fasting plasma glucose (mmol/l)/22.5 as described by Matthews et al. (17).
Statistical analyses
Univariate differences by sex and BMI categories were evaluated using a Student’s t-test, Mann–Whitney U test, or χ2 test as appropriate. General linear models were used to examine differences between BMI categories adjusting for measures known to influence adiposity distribution (e.g., age, race, study center, height and/or FM as appropriate). Pearson correlation coefficients were used to examine associations between adiposity measures. Variables with skewed distributions (i.e., HOMAIR, and fasting insulin) were transformed by the natural log to approximate normality. Multivariable linear regression was used to examine the association between LEFM and ln(HOMAIR) with adjustment for covariates. In all models, LEFM was standardized per one sex-specific standard deviation. Variance inflation factor was calculated as a collinearity diagnostic for all regression models and the standard cut point of ≥5.0 was used to determine collinearity. Two-way interactions of LEFM with BMI, sex, and race were examined and considered meaningful if P < 0.10 while P <0.05 was considered statistically significant for all other analyses. Analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC).
To assess the influence of participant exclusions resulting from the presence of DXA artifacts or loss to follow-up, a sensitivity analysis using multiple imputation was performed. Datasets were generated based on the procedure described by Raghunathan et al. (18) using IVEware software (19). Values were imputed for all DXA measures in participants with DXA artifacts while other participant data was retained. Five imputed datasets were generated, coefficients and standard errors were pooled using the SAS MINANALYZE procedure, and valid confidence intervals and standard errors adjusted for imputation were estimated.
RESULTS
Participant characteristics for clinical, metabolic, and body composition measures are presented in Table 1. Overall, women exhibited more favorable glucose metabolism (i.e., lower HOMAIR, fasting insulin, blood glucose, and glycosylated hemoglobin (HbA1c)) compared with men. Compared with normal weight participants, overweight and obese men and women also exhibited less favorable glucose metabolism (i.e., higher HOMAIR, fasting insulin, blood glucose, and HbA1c). With respect to body composition, women had similar BMI compared to men, yet lower WC and fat-free mass (kg), and higher total FM, LEFM, UEFM, and TFM. Compared to normal weight participants, overweight and obese participants had higher fat-free mass (kg), FM (kg), UEFM, LEFM, and TFM. To display the interrelatedness between individual adiposity measures and the association with HOMAIR, a correlation matrix of clinical and DXA measures of total and regional adiposity measures adjusted for age, sex, race, and field center is presented in Table 2. Although positive correlations were observed between ln(HOMAIR) and all measures of adiposity, the strongest correlations were observed between ln(HOMAIR) and BMI (r = 0.54), WC (r = 0.58), total FM (kg) (r = 0.53), and TFM (r = 0.56). Positive correlations were also observed between all measures of adiposity with the strongest associations observed between BMI and TFM (r = 0.86), WC and TFM (r = 0.91), and WC and BMI (r = 0.89).
Table 1.
Clinical and anthropometric characteristics of participants by sex and weight status: the CARDIA study (N = 1,579)
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Characteristics | Normal weighta | Overweightb | Obesec | Normal weighta | Overweightb | Obesec |
| N | 375 | 270 | 278 | 193 | 315 | 176 |
| Clinical characteristics | ||||||
| Age (years) | 45.2 (3.4) | 45.4 (3.6) | 45.0 (3.9) | 45.1 (3.4) | 45.5 (3.5) | 44.8 (3.6) |
| African American, n (%) | 93 (24.8) | 115 (42.6)e | 191 (68.7)e | 75 (39.1)d | 110 (35.7) | 83 (52.3)d,e |
| Current smokers, n (%) | 43 (11.5) | 48 (17.8)e | 43 (15.1) | 53 (27.6)d | 51 (16.6)e | 20 (12.8)e |
| HbA1c (%) | 5.2 (0.3) | 5.3 (0.3)e | 5.5 (0.5)e | 5.3 (0.4)d | 5.4 (0.5) | 5.5 (0.4)d,e |
| (n = 338) | (n = 243) | (n = 247) | (n = 170) | (n = 284) | (n = 141) | |
| Fasting insulin (µU/ml)f | 9.4 (1.5) | 12.5 (1.5)e | 18.2 (1.6)e | 10.0 (1.5) | 13.2 (1.5)e | 18.9 (1.5)e |
| Fasting glucose (mg/dl) | 88.2 (8.4) | 90.6 (9.1)e | 93.2 (9.3)e | 92.0 (8.8)d | 95.5 (9.2)d,e | 99.3 (8.9)d,e |
| HOMAIRf | 2.0 (1.5) | 2.7 (1.6)e | 4.0 (1.7)e | 2.2 (1.5)d | 3.1 (1.6)d,e | 4.6 (1.6)d,e |
| Anthropometric characteristics | ||||||
| BMI (kg/m2) | 22.2 (1.8) | 27.4 (1.4)e | 34.8 (3.9)e | 22.9 (1.7)d | 27.5 (1.4)e | 33.3 (2.7)d,e |
| WC (cm) | 72.9 (5.2) | 84.3 (6.2)e | 98.0 (9.3)e | 81.7 (6.3)d | 92.7 (5.4)d,e | 105.2 (7.7)d,e |
| FFM (kg) | 41.4 (4.3) | 45.3 (4.8)e | 51.2 (6.1)e | 56.3 (5.7)d | 62.4 (6.3)d,e | 70.5 (6.6)d,e |
| FM (kg) | 17.2 (4.0) | 26.2 (4.2)e | 37.5 (6.9)e | 12.5 (4.4)d | 19.2 (4.0)d,e | 28.2 (6.3)d,e |
| FM (%) | 28.2 (4.9) | 35.5. (4.1)e | 41.1 (4.2)e | 17.4 (5.0)d | 22.7 (3.9)d,e | 27.6 (4.7)d,e |
| LEFM (kg) | 7.4 (1.7) | 10.0 (2.0)e | 13.3 (3.2)e | 4.2 (1.6)d | 6.0 (1.5)d,e | 8.7 (2.2)d,e |
| UEFM (kg) | 2.2 (0.7) | 3.6 (0.9)e | 6.4 (2.7)e | 1.4 (0.6)d | 2.3 (0.6)d,e | 3.7 (1.5)d,e |
| TFM (kg) | 6.8 (2.2) | 11.6 (2.6)e | 16.7 (3.7)e | 5.9 (2.5)d | 9.8 (2.6)d,e | 14.5 (3.8)d,e |
Values are unadjusted means (s.d.) unless otherwise noted; Comparisons adjusted for age, race, study center, height and FM (kg), except weight, BMI, FM (kg), FFM (kg) and FM (%) which were adjusted for age, race, and study center. HOMAIR was calculated as glucose (mmol/l) × insulin (mU/l)/22.5.
CARDIA, Coronary Artery Risk Development in Young Adults; FFM, fat-free mass; FM, fat mass; HbA1c, glycosylated hemoglobin; HOMAIR, homoeostasis model of insulin resistance; LEFM, lower extremity fat mass; TFM, trunk fat mass; UEFM, upper extremity fat mass.
BMI <25.0 kg/m2.
BMI ≥25.0–29.99 kg/m2.
BMI ≥30.0 kg/m2.
Significantly different than women within BMI category, P < 0.05.
Significantly different than normal weight within gender, P < 0.05.
Log transformed before statistical testing and data presented are geometric means (s.d.).
Table 2.
Interrelatedness of anthropometric measures and HOMAIR: the CARDIA study (n = 1,579)
| HOMAIRa | WC (cm) | FFM (kg) | FM (kg) | FM (%) | LEFM (kg) | UEFM (kg) | TFM (kg) | |
|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 0.54 | 0.89 | 0.67 | 0.91 | 0.78 | 0.79 | 0.82 | 0.86 |
| WC (cm) | 0.58 | — | 0.67 | 0.89 | 0.77 | 0.69 | 0.76 | 0.91 |
| FFM (kg) | 0.71 | 0.67 | — | 0.58 | 0.30 | 0.52 | 0.50 | 0.54 |
| FM (kg) | 0.53 | 0.89 | 0.58 | — | 0.92 | 0.90 | 0.85 | 0.95 |
| FM (%) | 0.47 | 0.77 | 0.30 | 0.92 | — | 0.83 | 0.75 | 0.89 |
| LEFM (kg) | 0.39 | 0.69 | 0.52 | 0.90 | 0.83 | — | 0.71 | 0.75 |
| UEFM (kg) | 0.46 | 0.76 | 0.50 | 0.85 | 0.75 | 0.71 | — | 0.73 |
| TFM (kg) | 0.56 | 0.91 | 0.54 | 0.95 | 0.89 | 0.75 | 0.73 | — |
Values are Pearson correlation coefficients adjusted for age, sex, and field center; all correlations are statistically significant, P < 0.0001.
CARDIA, Coronary Artery Risk Development in Young Adults; FFM, fat-free mass; FM, fat mass; HOMAIR, homoeostasis model of insulin resistance; LEFM, lower extremity fat mass; TFM, trunk fat mass; UEFM, upper extremity fat mass; WC, waist circumference.
Log transformed before statistical testing.
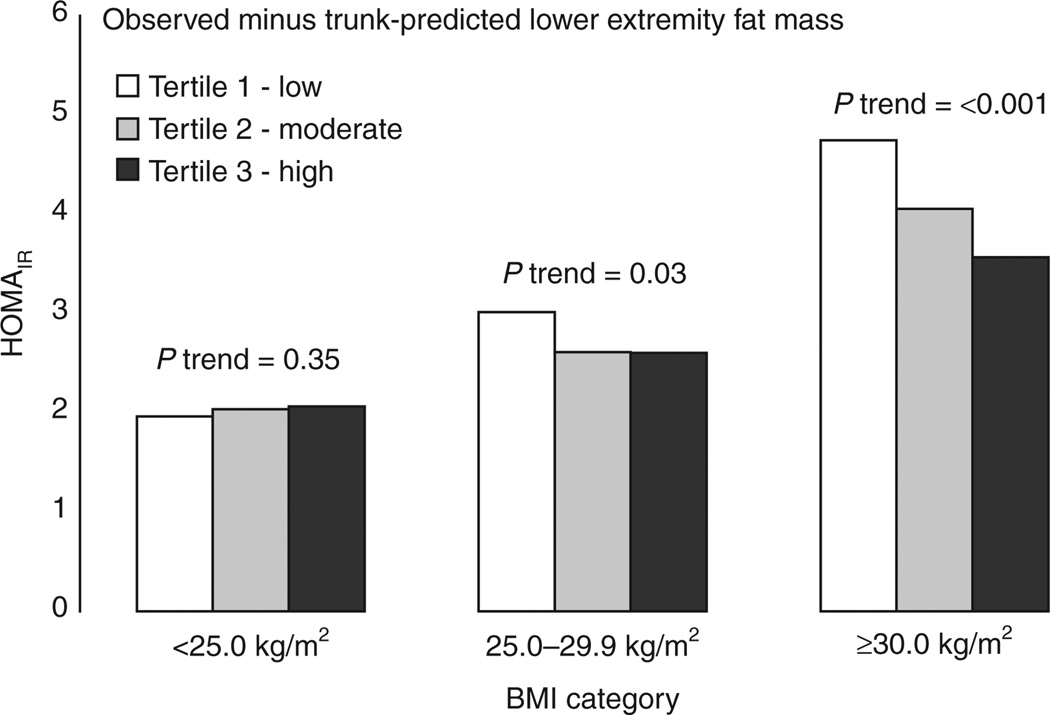
Mean HOMAIR according to differences between observed LEFM and TFM-predicted LEFM are presented by sex and BMI category in Figures 1 and 2. In normal weight participants, having higher observed LEFM than predicted by TFM was associated with higher levels of IR; a trend which was only statistically significant in men. However, in overweight and obese men and women, having higher observed LEFM than was predicted from TFM was associated with lower IR, trends that were statistically significant in all sex and BMI category groups. In multivariable linear regression analyses, several characteristics were independently associated with lower IR including being a current smoker (P < 0.001) and lower CRF (P < 0.001) while male sex was significantly associated with higher IR (P < 0.001) (see Supplementary Table S1 online). When examined with no other adiposity measures in the model (Model 1), higher LEFM was associated with higher IR (P < 0.001). When BMI was added to model (Model 2), lower LEFM was significantly associated with higher IR (P < 0.001). Interactions with sex and BMI were examined in the association between LEFM and IR. Interaction models tested only included the first order variables of interest and the interaction terms. Significant interaction between LEFM with continuous BMI (t-value = −4.83, P < 0.001) and sex (t-value = −5.69, P = <0.001), but not race (t-value = −0.64, P = 0.43) was observed with ln(HOMAIR) (Table 3). Similar interactions were also observed between each category of BMI and LEFM with ln(HOMAIR) (overweight and LBF, t-value = −2.60, P = 0.01; obese and LEFM, t-value = −2.75, P = 0.006) therefore, all subsequent analyses were stratified by sex and BMI category. In the reduced models (Model 1), higher LEFM was independently associated with higher ln(HOMAIR) in normal weight men and women after adjustment for age, race, height (cm), current smoking, education (year), and CRF (min), an association which was stronger in men compared to women. In obese and overweight men and women, lower LEFM was significantly associated with higher ln(HOMAIR), an association which was only statistically significant in obese men and women. When the association between LEFM and ln(HOMAIR) was examined after adjusting for TFM (Model 2), the negative association between LEFM and ln(HOMAIR) in overweight and obese men and women was stronger compared to the reduced model while this association was greatly attenuated in normal weight men and women.
Figure 1.
Geometric mean homoeostasis model of insulin resistance (HOMAIR) by tertile of individual differences in observed minus trunk fat-predicted lower extremity fat mass according to weight status in women.
Figure 2.
Geometric mean homoeostasis model of insulin resistance (HOMAIR) by tertile of individual differences in observed minus trunk fat-predicted lower extremity fat mass according to weight status in men.
Table 3.
Linear regression models for ln(HOMAIR) and lower extremity fat mass (kg) by sex and weight status: the CARDIA study (N = 1,579)
| Model 1a | Model 2b | |||
|---|---|---|---|---|
| B ± s.e. | P | B ± s.e. | P | |
| Women | ||||
| Normal weightc | 0.09 ± 0.05 | 0.055 | −0.02 ± 0.05 | 0.718 |
| Overweightd | −0.10 ± 0.05 | 0.076 | −0.12 ± 0.05 | 0.025 |
| Obesee | −0.07 ± 0.03 | 0.031 | −0.14 ± 0.03 | <0.001 |
| Men | ||||
| Normal weightc | 0.15 ± 0.05 | 0.005 | 0.04 ± 0.07 | 0.619 |
| Overweightd | −0.03 ± 0.05 | 0.455 | −0.15 ± 0.05 | 0.002 |
| Obesee | −0.11 ± 0.05 | 0.036 | −0.23 ± 0.05 | <0.001 |
Data are presented per one sex-specific standard deviation unit change in lower extremity fat mass (women s.d., 3.4 kg; men s.d., 2.4 kg).
CARDIA, Coronary Artery Risk Development in Young Adults; HOMAIR, homoeostasis model of insulin resistance.
Model 1: Adjusted for age, race, study center, height (cm), smoking status (current vs. never or former), education (years), and cardiorespiratory fitness (min).
Model 2: Model 1 + trunk fat (kg).
BMI category <25.0 kg/m2.
BMI category 25.0–29.9 kg/m2.
BMI category ≥30.0 kg/m2.
Since LEFM and TFM were moderately correlated in the current sample (r = 0.70, P < 0.01), variance inflation factor was examined for all regression models to check for the influence of collinearity on the variance and P values. In all models, variance inflation factor for all variables was <4.0 which is below the generally accepted cut point of 5.0 indicating that collinearity did not strongly influence the observed standard errors and P values in the linear regression models.
Sensitivity analyses
Since the use of BMI as a measure of obesity is limited by not allowing differentiation between FM and fat-free mass, sensitivity analyses was performed in sex-specific tertiles of total FM (see Supplementary Table S2 online). After similar adjustment, including TFM, lower LEFM was independently associated with higher ln(HOMAIR) in men and women with moderate and high levels of total FM while no significant associations were observed in men and women with low total FM (Model 2).
Over 50% of the participants from the original sample (n = 3,147) were excluded from the current analyses due to missing data as a result of loss to follow up or the presence of DXA artifacts. The analyses based on imputed datasets resulted in consistent point and standard error estimates compared to the estimates from the data set with complete cases (see Supplementary Tables S3–S5 online).
DISCUSSION
In this cross-sectional study of 1,579 middle-aged men and women, lower amounts of DXA-assessed lower extremity adiposity are associated with higher IR in overweight and obese men and women, an association which was most pronounced when examined at a given level of abdominal adiposity. Although overweight and obese participants exhibited more severe IR, LEFM was not independently associated with IR after accounting for amount of fat in the trunk in adults who were normal weight.
In the current investigation, participants who were overweight and obese had levels of HOMAIR that were between 70% and 150% higher, compared to those who were normal weight, a finding which is consistent with other IR studies in overweight and obese participants (20,21). If overweight and obese states are also associated with greater abdominal adiposity, contributing to more severe IR, it is intriguing that higher amounts of LEFM was not favorably associated with IR in normal weight individuals. Individuals with lower levels of adiposity have less variation in adiposity distribution and are more likely to have normal glucose metabolism compared with those who are overweight or obese. We hypothesize that lower amounts of LEFM may only be associated with higher IR under conditions of both excess overall adiposity as well as states of IR, where storage of excess triglycerides in adipose tissue in the lower extremities may offer a less detrimental storage alternative than the abdomen.
If the strongest negative association between LEFM and IR is observed when examined at a given degree of abdominal adiposity, the question still remains as to whether LEFM, per se, exerts beneficial effects on glucose metabolism independent of the mere absence of trunk fat. Several metabolic characteristics of adipocyte size and location offer evidence that LEFM may exert independent metabolic influence. Adipocytes in the lower extremities are larger, less sensitive to lipolytic stimuli, and more sensitive to hormonal influence (i.e., insulin) (22,23) compared with those in the abdomen. Although increases in adipocyte number and size have been reported to occur with increasing obesity (24,25), larger adipocytes are also associated with higher secretion of adipokines which favorably influence IR (26). It is therefore feasible that the large adipocytes in the femoral region experience additional hypertrophy in overweight and obese states, resulting in higher insulin sensitivity and increased secretion of metabolically beneficial adipokines. This combination of factors may partially “offset” the detrimental influence of abdominal adiposity and offer metabolic “defense” against additional glucose dysregulation. Although previous studies have suggested that excess obesity is associated with low levels of circulating adipokines that favorably influence IR (27,28), neither the region of secretion nor the potential for this association to vary in individuals with higher LEFM has been examined.
Many aspects of the composition and biochemistry of adipose and muscle tissue have also been proposed as factors influencing variations in IR, i.e., intramuscular lipid infiltration, substrate utilization capacity, and skeletal muscle mitochondrial density (29–32). DXA assessments are limited to quantification of tissue mass and give no information about the metabolism of muscle or fat. Consequently, distinction between subcutaneous, intramuscular, or visceral fat cannot be made in the current investigation. Future study of these properties as they relate to regional adiposity phenotypes may elucidate the physiologic mechanisms behind the association between leg adiposity and IR. Even though BMI cannot be used to distinguish between adipose and muscle tissue, similar associations were observed between IR and LEFM when weight status was defined by DXA-assessed total FM suggesting the overall findings were not greatly influenced merely by the anthropometric limitations of BMI. Widely accepted cut points for DXA measures of adiposity have not been established, therefore we conclude the use of BMI as a measure of obesity strengthens the clinical relevance of the current findings.
Another limitation of the current findings is the cross-sectional nature of this investigation which restricts causal inference of the observed associations. However, the use of data collected from the CARDIA cohort allowed thorough examination of this research question in a large diverse biracial sample of men and women with great variation in body composition. Similar studies have examined small homogenous samples which limit the scope of investigational findings. HOMAIR may be an indirect measure of IR, however, reasonable correlations between HOMAIR and gold-standard assessments of IR (hyperinsulinemic-euglycemic clamp) have been reported in adult populations with similar levels of IR as those in the current study sample (r = 0.88, P < 0.01 (17), r = 0.85, P < 0.01 (33)). Given the labor-intensive, invasive, and costly nature of employing direct assessment of IR in large epidemiologic studies, we consider the use of HOMAIR appropriate for the purposes of this investigation.
There were a large proportion of participants that were excluded from analyses due to the presence of DXA artifacts or loss to follow-up. It is unclear how the presence of plastic or metal objects or portions of the body out of the scanning region influence the quantification of tissue during a DXA scan. In order to avoid possible measurement error, participants with any such issues were excluded from the main analyses. Since such exclusions could result in a systematic bias of the investigational results, we used multiple imputation to examine how sensitive these findings are to the additional of data imputed from participants with similar characteristics. After imputation of missing data for participants who did not have a DXA scan performed or were excluded due to artifacts, the analyses based on imputed datasets resulted in consistent point and standard error estimates compared to the estimates from complete case data sets. Furthermore, the strengths of the associations were not only similar but also were augmented in this sensitivity analysis suggesting that the associations observed between LEFM and IR in overweight and obese individuals are not strongly influenced by the large amounts of missing data in this sample.
In summary, the association between excess lower extremity adiposity and IR may actually attenuate the metabolic risk associated when examined at a given level of abdominal adiposity in overweight and obese individuals, however, lower leg adiposity may not be unfavorably associated with IR in normal weight individuals who already exhibit low levels of IR. Although obesity remains a risk factor for IR, these results support the hypothesis that the metabolic influence of adipose tissue is not homogenous across regions or by weight status which presents a complex health picture. Regardless of how excess fat is stored in the body, the public health message should remain aimed at the reduction of prevalent obesity and the prevention of excess adiposity.
Supplementary Material
ACKNOWLEDGMENTS
We thank all CARDIA staff and participants at all field centers for their invaluable efforts. This study was supported (or partially supported) by contracts N01-HC-95095, N01-HC-48047, N01-HC-48048, N01-HC-48049, and N01-HC-48050 and by grants RO1-HL-53560, T32-HL-069771-07, and R01-HL-078972. The funders had no role in: the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/oby
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Seidell JC, Pérusse L, Després JP, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. Am J Clin Nutr. 2001;74:315–321. doi: 10.1093/ajcn/74.3.315. [DOI] [PubMed] [Google Scholar]
- 2.Snijder MB, Zimmet PZ, Visser M, et al. Independent and opposite associations of waist and hip circumferences with diabetes, hypertension and dyslipidemia: the AusDiab Study. Int J Obes Relat Metab Disord. 2004;28:402–409. doi: 10.1038/sj.ijo.0802567. [DOI] [PubMed] [Google Scholar]
- 3.Snijder MB, Dekker JM, Visser M, et al. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. Am J Clin Nutr. 2003;77:1192–1197. doi: 10.1093/ajcn/77.5.1192. [DOI] [PubMed] [Google Scholar]
- 4.Aasen G, Fagertun H, Halse J. Regional fat mass by DXA: high leg fat mass attenuates the relative risk of insulin resistance and dyslipidaemia in obese but not in overweight postmenopausal women. Scand J Clin Lab Invest. 2008;68:204–211. doi: 10.1080/00365510701649524. [DOI] [PubMed] [Google Scholar]
- 5.Van Pelt RE, Jankowski CM, Gozansky WS, Schwartz RS, Kohrt WM. Lower-body adiposity and metabolic protection in postmenopausal women. J Clin Endocrinol Metab. 2005;90:4573–4578. doi: 10.1210/jc.2004-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snijder MB, Visser M, Dekker JM, et al. Health ABC Study. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48:301–308. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 7.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 8.Paradisi G, Smith L, Burtner C, et al. Dual energy X-ray absorptiometry assessment of fat mass distribution and its association with the insulin resistance syndrome. Diabetes Care. 1999;22:1310–1317. doi: 10.2337/diacare.22.8.1310. [DOI] [PubMed] [Google Scholar]
- 9.Aasen G, Fagertun H, Halse J. Insulin resistance and dyslipidaemia in obese premenopausal and postmenopausal women matched for leg/trunk fat mass ratio. Scand J Clin Lab Invest. 2009;69:505–511. doi: 10.1080/00365510902778734. [DOI] [PubMed] [Google Scholar]
- 10.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 11.Aasen G, Fagertun H, Halse J. Effect of regional fat loss assessed by DXA on insulin resistance and dyslipidaemia in obese women. Scand J Clin Lab Invest. 2010;70:229–236. doi: 10.3109/00365511003628328. [DOI] [PubMed] [Google Scholar]
- 12.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 13.Ott SM, Ichikawa LE, LaCroix AZ, Scholes D. Navel jewelry artifacts and intravertebral variation in spine bone densitometry in adolescents and young women. J Clin Densitom. 2009;12:84–88. doi: 10.1016/j.jocd.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madsen OR, Egsmose C, Lorentzen JS, Lauridsen UB, Sørensen OH. Influence of orthopaedic metal and high-density detection on body composition as assessed by dual-energy X-ray absorptiometry. Clin Physiol. 1999;19:238–245. doi: 10.1046/j.1365-2281.1999.00168.x. [DOI] [PubMed] [Google Scholar]
- 15.Sidney S, Haskell WL, Crow R, et al. Symptom-limited graded treadmill exercise testing in young adults in the CARDIA study. Med Sci Sports Exerc. 1992;24:177–183. [PubMed] [Google Scholar]
- 16.Sellers DR, Kennealy JA, Kirkland JS, Vittorio N, Oloff CM. Correlates of maximal oxygen consumption during treadmill exercise. Aviat Space Environ Med. 1977;48:111–114. [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Raghunathan TE, Lepkowski JM, Van Hoewyk J. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27:85–95. [Google Scholar]
- 19.Raghunathan TE, Solenberger PW, Van Hoewyk J. IVEware: Imputation And Variance Estimation Software. Ann Arbor, MI: Institute for Social Research, University of Michigan; 1998. [Google Scholar]
- 20.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6 Suppl 1:60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloomgarden ZT. Obesity and diabetes. Diabetes Care. 2000;23:1584–1590. doi: 10.2337/diacare.23.10.1584. [DOI] [PubMed] [Google Scholar]
- 22.Richelsen B, Pedersen SB, Møller-Pedersen T, Bak JF. Regional differences in triglyceride breakdown in human adipose tissue: effects of catecholamines, insulin, and prostaglandin E2. Metab Clin Exp. 1991;40:990–996. doi: 10.1016/0026-0495(91)90078-b. [DOI] [PubMed] [Google Scholar]
- 23.Arner P. Differences in lipolysis between human subcutaneous and omental adipose tissues. Ann Med. 1995;27:435–438. doi: 10.3109/07853899709002451. [DOI] [PubMed] [Google Scholar]
- 24.Lundgren M, Svensson M, Lindmark S, et al. Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia’. Diabetologia. 2007;50:625–633. doi: 10.1007/s00125-006-0572-1. [DOI] [PubMed] [Google Scholar]
- 25.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43:1498–1506. doi: 10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
- 26.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 27.Jürimäe J, Jürimäe T, Ring-Dimitriou S, et al. Plasma adiponectin and insulin sensitivity in overweight and normal-weight middle-aged premenopausal women. Metab Clin Exp. 2009;58:638–643. doi: 10.1016/j.metabol.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14:741–751. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelley DE. Skeletal muscle triglycerides: an aspect of regional adiposity and insulin resistance. Ann N Y Acad Sci. 2002;967:135–145. [PubMed] [Google Scholar]
- 30.Toledo FG, Menshikova EV, Ritov VB, et al. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes. 2007;56:2142–2147. doi: 10.2337/db07-0141. [DOI] [PubMed] [Google Scholar]
- 31.Goodpaster BH, Wolfe RR, Kelley DE. Effects of obesity on substrate utilization during exercise. Obes Res. 2002;10:575–584. doi: 10.1038/oby.2002.78. [DOI] [PubMed] [Google Scholar]
- 32.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277:E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 33.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.