Abstract
Hyperthermia, which is heating of the tumors above 43 °C for about 30 min, has been known to modulate vascular permeability for enhanced chemotherapy. However, it is not clear whether similar effects exists when temperature at tumor sites is elevated above 43 °C, such as temperature achieved in laser-induced photothermal ablation (PTA) therapy. Also, the effect of timing of chemotherapeutic drug administration following heating in the efficiency of drug delivery is not established. In this study, we investigated the impact of near infrared (NIR) laser irradiated anti-EGFR monoclonal antibody C225-conjugated hollow gold nanospheres (C225-HAuNS)on vascular permeability and subsequent tumor uptake of a water-soluble polymer using combined MRI, ultrasound and optical imaging approaches. Magnetic temperature imaging showed a maximum temperature of 65.2 ± 0.10 °C in A431 tumor xenograft of mice treated with C225-HAuNS plus laser and 47.0 ± 0.33 °C in tumors of mice treated with saline plus laser at 4W/cm2 for 3 min (control) at 2 mm from the light incident surface. Dynamic contrast enhanced (DCE) MRI demonstrated greater than 2-fold increase of DTPA-Gd in the initial area under the curve (IAUC90) in mice injected with C225-HAuNS and exposed to NIR laser compared with control mice at 3 min after laser treatment. Similarly, Power Doppler (PD) ultrasound revealed a 4- to 6-fold increase in percentage vascularization in mice treated with C225-HAuNS plus NIR laser compared to control mice and confirmed increased vascular perfusion immediately after laser treatment. Twenty-four hours later, the blood perfusion was shut down. On optical imaging, tumor uptake of PG-Gd-NIR813, which is the model polymeric drug used, was significantly higher (p-value < 0.05) in mice injected with PG-Gd-NIR813 at 5 min after laser treatment than in mice injected with PG-Gd-NIR813 at 24 h after laser treatment and the saline-treated mice. In conclusion, laser irradiation of tumors after intravenous injection of C255-HAuNS induces a thermally mediated modulation of the vascular perfusion, which enhances the delivery of polymeric drugs to the tumors at the time phototherapy is initiated.
Keywords: targeted hollow gold nanoshells, magnetic resonance temperature imaging, ultrasonography, near-infrared optical imaging, molecular imaging
1. Introduction
Conjugation of small-molecular-weight drugs to water-soluble polymers is a promising strategy for improving the therapeutic window of anticancer agents [1-3]. The hypothesis is that the pharmacokinetics can be modulated by the attachment of the water-soluble polymer [4]. Active targeting of these conjugates is achievable with the introduction of a homing moiety to the polymeric carrier.
To date, a dozen polymer-drug conjugates are being studied in clinical trials of various phases. One such conjugate, poly(L-glutamic acid) (PG)-based-paclitaxel conjugate (PG-TXL), a water-soluble drug—has advanced to phase III clinical trials (www.cticseattle.com) [5]. PG-TXL has demonstrated several advantages over its parent drug, including reduced systemic toxicity, enhanced delivery to tumors, and increased therapeutic efficacy [6].
Hyperthermia has been known to modulate vascular permeability for enhanced chemotherapy [7-9]. Hyperthermia is a cancer therapy that relies on the localized heating of tumors above 43 °C for about 30 min [10]. Recently, near infrared (NIR)-activated nanoparticle-induced hyperthermia has been shown to enhance the efficiency of drug delivery and potency to solid tumors [11-13]. However, it is not clear whether similar effect exists when temperature at tumor sites is elevated above 43°C, such as temperature achieved in laser-induced photothermal ablation (PTA) therapy. PTA has been used successfully for ablation of primary and metastatic tumors throughout the body, including liver lesions [14, 15]. To improve the conformality and selectivity of PTA therapy, various gold nanostructures, including silicon-cored gold nanoshells, nanorods, nanocages, and hollow gold nanospheres (HAuNS) have been used as photothermal conducting agents [16-22]. HAuNS is a class of effective photothermal coupling agents characterized with a strong and tunable plasmon absorption at the NIR region (wavelengths 700-850 nm), small size (~40 nm in diameter), and capability of loading chemotherapeutic agents in the hollow interior of the nanoparticles [16, 17, 23]. NIR wavelengths optimally penetrate tissues and the strong absorption of the nanoparticles means that low power densities can be used which do not result in significant heating of normal tissue [24, 25]. Selective ablation of tumor cells has been demonstrated in vitro with HAuNS conjugated to C225, an antibody directed against epidermal growth factor receptor (EGFR) (C225-HAuNS) [17].
In this study, we investigated the impact of NIR laser irradiated C225-HAuNS on vascular permeability and subsequent tumor uptake of a model polymer-drug conjugate. After intravenous administration of C225-HAuNS into tumor-bearing mice followed by laser irradiation, magnetic resonance temperature imaging (MRTI) was used to estimate the induced heating in the tumors due to irradiation of the C225-HAuNS; dynamic contrast-enhanced magnetic resonance imaging (DCE MRI) and Power Doppler (PD) ultrasound were used to longitudinally assess the effect of heating on tumor vascular perfusion; and NIR fluorescence optical imaging was used to estimate relative drug uptake in tumors using PG-NIR dye conjugate as a model polymeric drug.
2. Materials and methods
2.1. Materials
Monoclonal anti-EGFR antibody C225 was obtained from ImClone Systems (New York, NY). C225 is a chimeric human-mouse IgG1 that binds EGFR with high affinity [26, 27]. Methoxy-polyethylene glycol-SH (PEG-SH, MW 5000) was obtained from Nektar (Huntsville, AL). PD-10 columns and Spectra/Pro 7 dialysis tubing with molecular weight cut-off of 10,000 were purchased from Amersham-Pharmacia Biotech (Piscataway, NJ). Trisodium citrate dihydrate (>99%), cobalt chloride hexahydrate (99.99%), sodium borohydride (99%), and chloroauric acid trihydrate (ACS reagent grade) were purchased from Fisher Scientific (Pittsburg, PA) and used as received.
PG, 1,3-diisopropylcarbodiimide, gadolinium (III) chloride hexahydrate, 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC), 2-(morpholino)ethanesulfonic acid buffer (MES), N-succinimidyl S-acetylthioacetate (SATA), and phosphate-buffered saline (PBS) were purchased from Sigma-Aldrich (St. Louis, MO). Trifluoroacetic acid was obtained from Chem-Impex International, Inc. (Wood Dale, IL). All solvents were purchased from VWR (San Dimas, CA).
2.2. Synthesis of C225-HAuNS
C225-HAuNS were synthesized according to our previously reported procedures [17, 28]. Briefly, an aqueous solution of C225 (2.5 mg, 0.017 μmol; 5 mg/mL) was first allowed to react with SATA (0.077 mg, 0.332 μmol) at room temperature for 1 h. The resulting conjugate, C225-acetylthioacetate (C225-ATA), was purified by passing it through a gel filtration PD-10 column, using protein dye color (BioRad, Hercules, CA) as an indicator to guide the collection of antibody-containing fractions. C225-ATA was treated with hydroxylamine (50 mM, 50 μL) at room temperature for 2 h to expose free SH. After C225-SH was passed through the PD-10 column, the resulting C225-SH was added to an aqueous solution of HAuNS (1.4 1011 particles/mL) to achieve a final antibody concentration of 5 μg/mL. The suspension was stirred at room temperature for 1 h. Thereafter, PEG-SH was added to the antibody-coated HAuNS to achieve a final concentration of 0.2 mg/mL, and the mixture was reacted for an additional 1 h to ensure that the gold surface was completely covered. C225-HAuNS were centrifuged at 8,000 rpm for 5 min, and the resulting pellet was washed twice with deionized water. The presence of C225 in the supernatant liquid was tested with the BioRad protein dye. After the second wash, no C225 was detected in the supernatant. The purified products were resuspended in 0.1 mM PBS and stored at 4°C until further use. Antibody-conjugated HAuNS were stable in physiological buffers for at least 3 weeks at 4°C without aggregation.
2.3. Synthesis of PG-Gd-NIR813
We created PG-Gd-NIR813, a polymeric dual-modality MRI/optical imaging agent, to model the PG-drug conjugates including PG-paclitaxel. PG-Gd-NIR813 was synthesized according to our previously published method [29]. Briefly, p-aminobenzyl-DTPA(t-butyl ester) (2.1 g, 2.79 mmol) was conjugated to PG (Mn 41,400, 1 g, 7.75 mmol of carboxylic unit) in dimethyl formamide using 1,3-diisopropylcarbodiimide (403 mg, 3.1 mmol) as a coupling agent, as described by Wen et al. [30]. The t-butyl protecting groups were removed by treatment with trifluoroacetic acid at 4°C overnight to yield PG-DTPA. To chelate PG-DTPA with Gd3+, a solution of GdCl3•6H2O in 0.1 M sodium acetate was added to a solution of PG-DTPA in 0.1 M sodium acetate (pH 5.5) in small fractions. The solution was then dialyzed extensively against water (molecular weight cut-off, 10,000) until no free Gd3+ was detected, and lyophilized. NIR813 (4.17 mg, 0.0045 mmol) dissolved in 200 μL of dimethyl formamide was added to a solution of PG-DTPA-Gd (90 mg, 0.698 mmol Glu) in 0.1 M MES buffer (2 mL) in the presence of EDC (10 mg, 0.005 mmol). The reaction mixture was stirred at 4°C overnight while protected from light, filtered through a 0.2-μm filter, dialyzed against PBS and water sequentially, and lyophilized. The yield was 64.6 mg (72%). The conjugate contained about 10% Gd (w/w) and 4.4% (w/w) or 1% (mole dye/mole repeating unit) NIR813.
2.4. Characterization of C225-HAuNS and PG-Gd-NIR813
The particle size of C225-HAuNS was determined using dynamic light scattering at a scatter angle of 90° on a ZetaPLUS particle size analyzer (Brookhaven Instruments Corp., Holtsville, NY). The absorbance of the nanoshells was recorded on a Beckman Coulter DU-800 UV-Vis spectrometer (Beckman Coulter, Fullerton, CA) with a 1.0-cm-optical-path-length quartz cuvette. The concentration of gold atoms of a C225-HAuNS solution was analyzed by inductively coupled plasma mass spectroscopy (Galbraith, Knoxville, TN).
The fluorescence of PG-Gd-NIR813 was measured at an excitation wavelength of 765 nm using a Spex Fluorolog-3 spectrofluorimeter (Jobin Yvon Inc., Edison, NJ). The PG-Gd-NIR813 solution (1 μM equivalent NIR813) in water was placed in a 1-cm quartz cuvette.
2.5. Establishment of tumors in nude mice
All animal work was carried out in the Small Animal Imaging Facility at The University of Texas MD Anderson Cancer Center in accordance with institutional guidelines. Solid A431 tumors overexpressing EGFR were grown subcutaneously in both thighs of nude mice (20-25 g; Harlan Sprague Dawley, Indianapolis, IN) by injecting 1 × 106 viable tumor cells suspended in PBS. When tumors had grown to 0.8-1.3 cm in average diameter (2-3 weeks), the mice were randomly allocated into 2 groups. The first group (n = 10) was imaged using MRTI and DCE MRI as described in Section 2.6. The second group (n = 15) was imaged using PD ultrasound and optical imaging as described in Section 2.7.
2.6. In vivo MRTI and DCE MRI
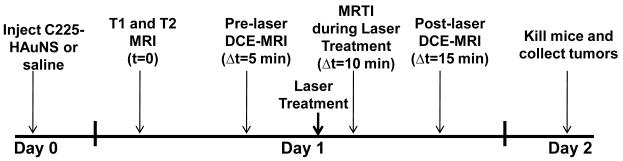
To determine the difference in temperature increase between C225-HAuNS-treated and control mice (injected with saline + laser treatment) the 10 mice randomly allocated for MRTI and DCE MRI were divided into 2 groups of 5 mice each. Mice in group I were injected intravenously with C225-HAuNS (200 μL, 1.4 × 1011 particles/mL), and the mice in group II were injected with saline as a control (200 μL). Scheme 1 shows the schedule for MRI and MRTI in relation to various treatments. Twenty-four hours after the injection of C225-HAuNS or saline, mice were brought to the MRI suite for MRTI and MRI experiments. All MRTI and MRI experiments were performed using a 1.5-T superconductive magnet (Signa Excite HD, GE Healthcare, Waukesha, WI) equipped with high-performance gradients (23 mT/m maximum amplitude and 120 T/m/sec maximum slew rate) and fast receiver hardware (bandwidth, ± 500 MHz). The mouse was positioned so that the tumor was at the center of a 3″ diameter, receive-only surface coil. Core body temperature was measured using a rectal thermometer (RET-3, Braintree Scientific, Inc., Braintree, MA). In addition, a catheter was placed in the tail vein of the mouse to permit intravenous injection of agents.
Scheme 1.
Dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) and magnetic resonance temperature imaging (MRTI) schedule during NIR laser treatment.
Once the mouse was positioned and anesthetized, pre-treatment MRI was performed. Both T1-weighted images (TR/TE 600/10.9 ms, bandwidth ± 25 kHz, 6 excitations, field of view 5 × 2.5 cm, matrix 256 × 192 pixels) and T2-weighted images (TR/TE 4500/15.7 ms, bandwidth ± 25 KHz, 6 excitations, field of view 5 × 2.5 cm, matrix 256 × 192 pixels) were obtained. A gadolinium based contrast agent (Gd-DTPA, Magnevist™) was then injected via tail-vein catheter (0.1 mmol/mL, 1 μL/g body weight), and DCE MRI was performed using a 2D fast spoiled gradient-recalled sequence (TR/TE 8.8/2.2 ms, bandwidth ±19.2 KHz, number of excitation 6, field of view 6 × 6 cm, matrix 256 × 128 pixels, slice thickness 3 mm). The initial area under the curve at 90 sec after contrast injection (IAUC90) was obtained and used as a semi-quantitative metric for tumor vascular perfusion.
A 10 min delay was inserted between DCE MRI and treatment delivery to allow reasonable clearance of contrast agent (DTPA-Gd or Magnevist™). Following this, laser irradiation was performed under real-time MRTI guidance. Baseline images were acquired for 30-s prior to laser exposure, during laser exposure (Diomed 15-plus, Diomed, Inc., Cambridge, UK; centered at 808 nm at 4 W/cm2 for 3 min), and continued for 90 s post-treatment for a total of 5 minutes of imaging. The MRTI acquisition utilized a fast 2D radiofrequency-spoiled gradient-recalled echo sequence (TR/TE 52/8.4 ms, flip angle 30°, bandwidth ± 8.1 kHz, 1 excitation, field of view 6 × 6 cm, matrix 256 × 128 pixels, slice thickness 3 mm). Estimates of tissue temperature changes were generated by performing a complex phase-subtraction of the MR data and assuming changes in phase were a result of the temperature dependent water proton resonance frequency ( −0.01 ppm/°C) [31]. Maps and graphs of temperature and contrast enhancement were generated and analyzed off-line using in-house code written in MATLAB (MathWorks, Natick, MA).
DCE MRI, as previously described, was repeated post-treatment (10 mins after laser treatment) to assess changes in the IAUC90. Mice were then removed from the scanner and sacrificed 24 h later. Tumors were harvested for histopathological assessment, with half being frozen and half were formalin fixed. Tumor tissue was sliced in 5-μm sections. Frozen slices were fixed and then scanned on the 800-nm channel of a LiCor Odyssey Near-Infrared Imaging System (Lincoln, NE) to assess accumulation of fluorescent NIR dye within tumor tissue. A slice of each formalin-fixed tumor was stained with hematoxylin-eosin (H&E) for microscopic assessment of the extent of damage. Quantification of the extent of necrosis was done by manually contouring the areas of necrosis and whole tumor using Image J software (available at http://rsb.info.nih.gov/ij; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD) in H & E stained slides. The percent necrosis (% necrosis) was calculated by dividing the area of necrosis by the area of the whole tumor multiplied by 100. The difference among the groups was considered statistically significant if p-value < 0.05 using a two-tailed Student’s t test.
2.7. In vivo PD ultrasound and optical imaging
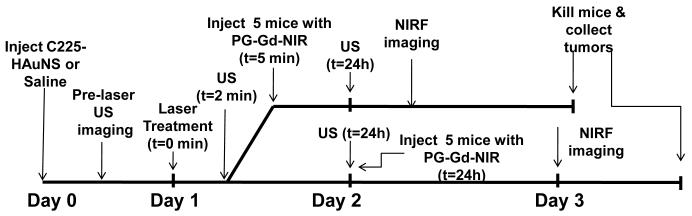
Scheme 2 shows the experimental workflow for PD ultrasound and optical imaging in relation to delivery of various treatments. Mice bearing A431 tumors were injected with C225-HAuNS (200 μL, 1.4 × 1011 particles/mL) via tail vein injection. Twenty-four hours post-injection, mice were randomly allocated into 2 groups of 5 mice each. Mice in group I were injected intravenously with 200 μl of PG-Gd-NIR813 (48 nmol/mouse NIR813 eq or 0.2 mmol Gd/kg) 5 min after laser treatment (3 min after ultrasound imaging); mice in group II were injected intravenously with the same amount of PG-Gd-NIR813 24 h after laser treatment. Twenty-four hours after PG-Gd-NIR813 injection, mice were imaged using an IVIS imaging system (100 series, Xenogen Corp., Alameda, CA) with ICG filter sets (excitation/emission, 710-760/810-875 nm). The field of view was 13.1 cm in diameter. The fluence rate for NIR fluorescence excitation was 2 mW/cm2. The camera settings utilized maximum gain, 2 × 2 binning, 640 × 480 pixel resolution, and an exposure time of 0.8 sec.
Scheme 2.
Ultrasound (US) and near-infrared fluorescence (NIRF) optical imaging schedule during NIR laser treatment.
Ultrasound imaging was performed prior to laser treatment, at 2 min and 24 h after laser treatment. B-mode and PD ultrasound images were captured using a small animal microultrasound (Vevo 770, Visualsonics Inc., Toronto, Canada). B-Mode transmit and receive frequency was centered at 40 MHz with a focal length of 10 mm while PD mode used 30 MHz. Mice were anesthetized using isoflurane (2%) and positioned on the examination table. Tumors were then carefully covered with ultrasound gel (Aquasonic 100, Parker Laboratories, Inc., Fairfield, NJ) for acoustic coupling with the transducer. An ultrasound transducer, fixed on a motor-driven unit above the animal, moved perpendicularly to the beam axis and acquired consecutive slices with a slice thickness of 200 μm. Using the vendor software, regions of interest (ROIs) were drawn around the tumor borders using the B-Mode image containing the largest tumor diameter to estimate percentage of vascularization demonstrating visible flow using PD. After establishing a pre-treatment baseline, one tumor in each mouse was irradiated with laser at 808 nm (4 W/cm2 for 3 min) while the mouse was still affixed to the ultrasound system. Two minutes post-treatment, ultrasound images were acquired again for comparison.
2.8. Ex vivo optical imaging and histological examination
After the in vivo optical imaging, mice were sacrificed by cervical dislocation. Uptake of PG-Gd-NIR813 in the tumors was quantified in terms of relative fluorescence using the Living Image Software v. 3.2. Tissues with p-values <0.05 were considered statistically significant using Student’s T-test. Tumors were then excised, bisected, and processed for histological evaluation as described in Section 2.6.
3. Results
3.1. Synthesis and characterization of C225-HAuNS and PG-Gd-NIR813
The structure of C225-HAuNS is shown in Fig. 1A. The HAuNS were coated with C225 monoclonal antibody and PEG. They had a mean diameter of ~40 nm and a maximum absorbance at 810 nm (Fig. 1C). This absorbance is optimal for NIR laser experiments.
Figure 1.
(A-B) Structures of C225-HAuNS (A) and PG-Gd-NIR813 (B). (C) Absorbance of C225-HAuNS. Note peak at ~810 nm. (D) Fluorescence emission spectrum7 of PG-Gd-NIR813 (excitation wave- length at 776 nm). The maximum fluorescence intensity was at ~813 nm.
To study the delivery of this polymeric drug, we used PG-Gd-NIR813 containing 10% (wt/wt) Gd and 1% (mol/mol) NIR813 as a model drug for PG-drug conjugates. Both Gd and NIR813 reporters were covalently attached to the repeating L-glutamic acid chains. The Gd allowed the agent to be visible on MRI, while the NIR813 emitted fluorescence at 813 nm (Fig. 1D), which rendered it visible on optical imaging.
3.2. C225-HAuNS mediate laser-induced thermal effect in tumors in vivo
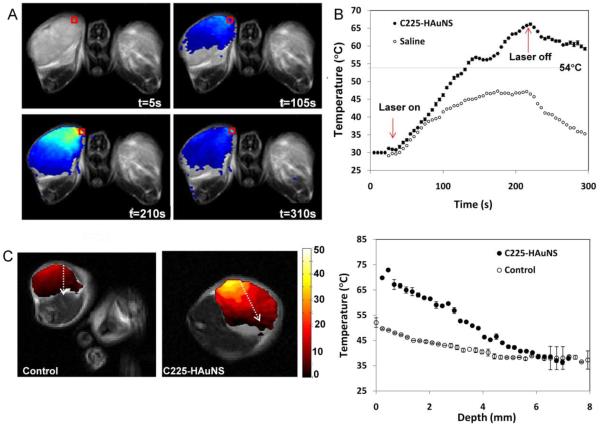
Fig. 2A shows representative MRTI images of tumors at different times after C225-HAuNS injection. At 2 mm depth from the surface, the temperature of the tumor increased during laser treatment, reaching a peak at ~210 s (after ~3 min of laser treatment), and then decreased after the laser was turned off. As shown in Fig. 2B, the mice injected with C225-HAuNS and treated with laser reached an average maximum temperature of 65.2 ± 0.10°C, and the control mice (saline plus laser) had an average maximum temperature of 47.0 ± 0.33°C. This maximum temperature increase was calculated 2 mm from the surface of the tumor. Figure 2C-D shows the plot of temperature as a function of depth. The maximum temperature increase occurs on the surface of the tumor and the temperature decreased going deeper into the tumor. At 6 mm from the surface, there was no difference in temperature between the C225-HAuNS and saline treated mice, and no temperature elevation was detected.
Figure 2.
Impact of C225-HAuNS on laser-induced increase in tumor temperature. C225-HAuNS or saline was injected into mice bearing subcutaneous A431 tumors. At 24 h after injection, tumors were irradiated with laser at 808 nm at 4 w/cm2 for 3 min. (A) Representative MRTI images of C225-HAuNS-treated mice before laser treatment (t = 5 s), during laser treatment (t = 105 s), just before the end of laser treatment (t = 210 s), and after laser treatment (t = 310 s). Red box indicates where the data is taken. (B) Comparison of temperature increase in C225-HAuNS-treated and saline-treated mice at different time points at the region of interest (red box in [A]). (C) Image of tumors at t=210 s (maximal temperature increase). The dashed white arrow from the surface going to the inner part of the tumor indicates where the data was sampled for temperature vs. depth plot (D).
3.3. C225-HAuNS injection followed by laser treatment enhances tumor vascular perfusion in vivo
Fig. 3 shows representative DCE MRI images obtained at 24 h after intravenous injection of C225-HAuNS and before the injection of DTPA-Gd (Fig. 3A), immediately after the injection of DTPA-Gd (Fig. 3B), and at 10 mins after laser treatment and immediately after injection of DTPA-Gd (Fig. 3C). The uptake of Gd-DTPA increased after laser treatment as shown in the quantification in Figure 3D. In the mouse tumor injected with C225-HAuNS, Gd-DTPA uptake approximately doubled 5 min after laser treatment. These results suggested that after laser treatment, tumors had increased vascular perfusion, which is largely attributed to increased perfusion in the area of direct laser irradiation.
Figure 3.
Effect of laser treatment on tumor vascular perfusion with dynamic contrast enhanced magnetic resonance imaging. A group of 5 mice were injected with C225-HAuNS. Twenty four hours after laser treatment, the tumors were treated with NIR laser (~810 nm, power = 4 W/cm2). DCE-MRI images were obtained at pre-contrast before laser (A), post-contrast before laser (B), and post-contrast immediately after laser treatment (C). (D) Quantification of the signal enhancement before and after laser treatment with C225-HAuNS.
3.4. Effect of timing of administration of polymeric drug on efficiency of drug delivery in mice treated with C225-HAuNS plus laser
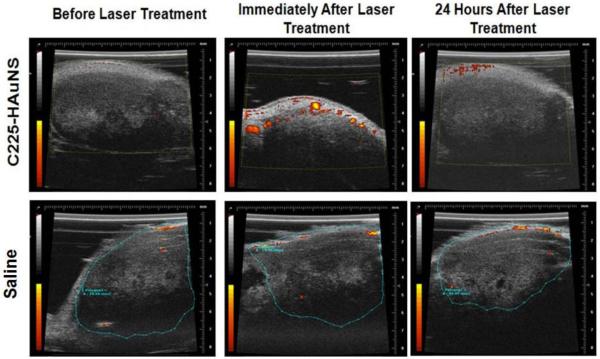
The results observed with DCE MRI were confirmed by the results from PD ultrasound (Fig. 4). The 10 mice injected with C225-HAuNS had a significant increase in tumor blood flow immediately after laser treatment, and blood flow returned to baseline after 24 hours (Fig. 4, top). In contrast, the 5 control mice injected with saline had no changes in tumor blood flow even at 2 mins. after laser treatment (Fig. 4, bottom).
Figure 4.
Effect of laser treatment on tumor vasculature with PD ultrasound imaging. A group of 5 mice were injected with C225-HAuNS (top) and another group (n=5) was injected with saline (bottom). Twenty four hours after the injection of C225-HAuNS or saline, pre-laser PD ultrasound imaging was performed. The tumors were then treated with NIR laser (~810 nm, power = 4 W/cm2) for 3 minutes. Immediately and 24 h after laser treatment, the mice were imaged again using PD ultrasound. Notice the increase in vascular perfusion immediately after laser treatment, which died off 24 hours later.
Quantification of NIR fluorescence signals measured ex vivo showed significant increase in the tumor uptake of PG-Gd-NIR813 injected immediately (Fig. 5A) after laser treatment than the tumor uptake of PG-Gd-NIR813 injected at 24 h (Fig. 5B) after laser treatment in mice treated with C225-HAuNS (p value = 0.007). Similarly, there was approximately 2x increase in tumor uptake of PG-Gd-NIR813 in mice treated with C225-HAuNS plus NIR laser than in mice treated with saline plus NIR laser(p value = 0.010) (Fig. 5C). Increased PG-Gd-NIR813 in tumor were confirmed by NIRF imaging of tissue slices using LiCor imaging system, imaged at 800 nm channel (Fig. 5 D-F).
Figure 5.
Efficiency of drug delivery to tumor irradiated with NIR laser. PG-Gd-NIR was used to as the surrogate drug. A total of 10 mice were injected with C225-HAuNS (A-B) and another 5 mice were injected with saline (C). Twenty four hours after injection of the C225-HAuNS, tumors were treated with laser (810 nm, power = 4 W/cm2) for 3 minutes. Five of the 10 mice were immediately injected with PG-Gd-NIR (A) while the rest of the mice were injected the polymer 24 hours later (B). Twenty four hours after the injection of PG-Gd-NIR813, mice were killed and tumors were removed and imaged ex vivo for fluorescence intensity using ICG filter sets in the Xenogen optical imaging system. Control mice (injected with saline, with laser treatment) were also imaged ex vivo (C). Tumors were then mounted on slides and imaged using LiCor fluorescence imaging sytem (D-F). The fluorescence of the ex vivo tumors was quantified and compared (G). Results show that there is a statistically significant increase in the uptake of the mice injected immediately with PG-Gd-NIR as compared to the mice injected 24 hours later (*p-value =0.007, **p-value=0.010).
3.5. Histological evaluation
H&E staining showed that tumors of mice treated with C225-HAuNS and treated with laser showed significantly increased necrosis compared with saline plus laser-treated mice (71.76 ± 22.29 vs. 29.41 ± 12.02; p-value=0.013). These results showed that although treatment with C255-HAuNS and treatment with laser led to temperature increase that caused damage to tumors, uptake of PG-Gd-NIR813, the model polymeric drug, was increased when it was injected immediately after laser treatment.
4. Discussion
Results from our study indicate that changes in tumor vasculature immediately following C225-HAuNS-mediated photothermal therapy could enhance delivery of polymeric drugs to the tumor. Following C225-HAuNS uptake in tumors, treatment of tumors with laser increased their vascular perfusion. Immediate transient increase in vascular perfusion, which we observed on both DCE MRI and PD ultrasound, increased delivery of polymeric drugs to tumors.
Multimodal approaches combining thermal therapy with chemo- or radiotherapy have proven to be effective in the treatment of solid tumors. For example, hyperthermia mediated by polyethylene glycol-coated gold nanoshells (PEG-AuNS) has been shown to modulate the effects of radiation therapy [32]. In our current study, instead of using nontargeted PEG-AuNS, we used targeted C225-HAuNS. Previously, we have shown that C225-HAuNS preferentially accumulate in EGFR-positive tumors and mediated laser-induced increase in tumor temperature to ablative temperatures (>54°C) in vitro [17]. Here, the in vivo MRTI results of mice treated with C225-HAuNS revealed transient temperature elevation of up to 65.2°C on the surface of the tumor where the laser is positioned, but gradually decreased as the heating penetrates deeper into the tumor (Figure 2D). The temperature of the tumors dropped back to body temperature (average body temperature of mice = 31°C) after the laser was shut off (Fig. 2). The heating inside the tumor (65°C-31°C from the surface to the center of the tumor) caused an increase in vascular perfusion as shown on both DCE MRI (Fig. 3) and PD ultrasound (Fig. 4). DCE MRI has the advantage of providing higher resolution than PD ultrasound, but ultrasound does not require injection of contrast agents, is widely available in the clinic, less expensive, and easier to use.
The increase in vascular perfusion can be exploited to enhance the delivery to tumors of polymeric drugs, such as PG-drug conjugates. In this study, we used PG-Gd-NIR813 as a model polymeric drug and evaluated whether there would be a difference in tumor uptake depending on whether PG-Gd-NIR813 was injected immediately or 24 hours after laser treatment. We found that uptake was better with immediate injection because the increase in vascular perfusion after laser treatment was transient. The increase in tumor temperature produced by C225-HAuNS injection followed by laser treatment enhanced the delivery of the PG-Gd-NIR813 injected immediately after laser treatment (Fig. 5). However, after the transient stimulation of perfusion, the vasculature returns to its normal perfusion levels over the next 24 hours (Figure 4), and the tumor uptake of PG-Gd-NIR813 decreased to the same level before NIR laser treatment. Validation with histology also showed that tumors in mice injected with C225-HAuNS and treated with laser (Fig. 6A) induced large necrotic areas, whereas tumors in mice treated with saline did not (Fig. 6B), indicating that i.v. injected C225-HAuNS in combination with NIR laser could mediate effective PTA therapy. Further studies are needed to demonstrate whether PTA ablation in combination with polymeric drug can lead to increased antitumor efficacy.
Figure 6.
Histology. H &E stained tumor slides from mice intravenously injected with C225-HAuNS (A) and saline (B) followed by laser treatment (n=5/group). (C) Quantification of the extent of necrosis 24 hours after laser treatment. Percent necrosis is calculated by dividing the area of necrosis with the whole tumor multiplied by 100. Treatment with C225-HAuNS and NIR laser (A) induced greater degree of necrosis compared with the saline-treated tumors (B). Asterisk (*) indicates p-value=0.013.
Conclusion
In light of these results, we envision a multimodality treatment approach consisting of injection of targeted hollow gold nanospheres followed by local treatment with NIR laser can increase the polymeric drug uptake by administration of a polymeric drug immediately after laser treatment. Because heterogeneous temperature distribution in tumors undergoing photothermal therapy, it is expected that while in areas where temperature reach >54°C, irreversible cellular damage would occur, in areas where the temperature is lower than the thermal death threshold, enhanced tumor uptake of polymeric drugs would ensure sufficient cell killing. We expect that the polymeric drug would be trapped in the tumor after photothermal ablation therapy, thus enabling gradual release of the active drug within the tumor mass. Further work on the antitumor efficacy of combined photothermal ablation therapy and polymer drug conjugates is currently under way in our laboratory.
Acknowledgments
The authors thank Stephanie Deming for editing the manuscript. This work was supported in part by National Institutes of Health grant R01 CA119387, the John S. Dunn Foundation, SPORE Head and Neck Career Development Award P50CA097007 (to M.P.M), and an Odyssey Fellowship (to M.P.M.). The Odyssey Fellowship is supported by the Odyssey Program and the Cockrell Foundation Award for Scientific Achievement at The University of Texas MD Anderson Cancer Center. We also acknowledge the NCI Cancer Center Support Grant CA016672 for the support of MD Anderson’s Small Animal Facility and Small Animal Imaging Facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Li C, Wallace S. Polymer-drug conjugates: recent development in clinical oncology. Adv. Drug Deliv. Rev. 2008;60(8):886–898. doi: 10.1016/j.addr.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Adams ML, Lavasanifar A, Kwon GS. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2003;92(7):1343–1355. doi: 10.1002/jps.10397. [DOI] [PubMed] [Google Scholar]
- [3].Duncan R. Polymer conjugates as anticancer nanomedicines. Nature Rev. Cancer. 2006;6(9):688. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- [4].Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enz. Reg. 2001;41(1):189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- [5].Li C, Wallace S. Polymer-drug conjugates: recent development in clinical oncology. Adv. Drug Deliv. Rev. 2008;60(8):886–898. doi: 10.1016/j.addr.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li C, Yu DF, Newman RA, Cabral F, Stephens LC, Hunter N, Milas L, Wallace S. Complete regression of well-established tumors using a novel water-soluble poly(L-glutamic acid)-paclitaxel conjugate. Cancer Res. 1998;58(11):2404–2409. [PubMed] [Google Scholar]
- [7].Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3(8):487–497. doi: 10.1016/s1470-2045(02)00818-5. [DOI] [PubMed] [Google Scholar]
- [8].Li L, ten Hagen TLM, Schipper D, Wijnberg TM, van Rhoon GC, Eggermont AMM, Lindner LH, Koning GA. Triggered content release from optimized stealth thermosensitive liposomes using mild hyperthermia. J. Control. Release. 2010;143(2):274–279. doi: 10.1016/j.jconrel.2010.01.006. [DOI] [PubMed] [Google Scholar]
- [9].Lammers T, Peschke P, Kuhnlein R, Subr V, Ulbriich K, Debus J, Huber P, Hennink W, Storm G. Effect of radiotherapy and hyperthermia on the tumor accumulation of HPMA copolymer-based drug delivery systems. J. Control. Release. 2007;117(3):333–341. doi: 10.1016/j.jconrel.2006.10.032. [DOI] [PubMed] [Google Scholar]
- [10].Pankhurst Q, Connolly J, Jones S, Dobson J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D: Applied Physics. 2003;36(13):R167–R181. [Google Scholar]
- [11].Hauck TS, Jennings TL, Yatsenko T, Kumaradas JC, Chan WCW. Enhancing the toxicity of cancer chemotherapeutics with gold nanorod hyperthermia. Adv. Mater. 2008;20(20):3832–3838. [Google Scholar]
- [12].Park J-H, von Maltzahn G, Ong LL, Centrone A, Hatton TA, Ruoslahti E, Bhatia SN, Sailor MJ. Cooperative nanoparticles for tumor detection and photothermally triggered drug delivery. Adv. Mater. 22(8):880–885. doi: 10.1002/adma.200902895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang J, Chen HY, Xu L, Gu YQ. The targeted behavior of thermally responsive nanohydrogel evaluated by NIR system in mouse model. J. Control. Release. 2008;131(1):34–40. doi: 10.1016/j.jconrel.2008.07.019. [DOI] [PubMed] [Google Scholar]
- [14].Gillams A. Tumour ablation: current role in the liver, kidney, lung and bone. Cancer Imaging. 2008;8(Spec No A):S1–5. doi: 10.1102/1470-7330.2008.9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Beland M, Mueller PR, Gervais DA. Thermal ablation in interventional oncology. Semin. Roentgenol. 2007;42(3):175–190. doi: 10.1053/j.ro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- [16].Lu W, Xiong C, Zhang G, Huang Q, Zhang R, Zhang JZ, Li C. Targeted photothermal ablation of murine melanomas with melanocyte-stimulating hormone analog-conjugated hollow gold nanospheres. Clin. Cancer Res. 2009;15(3):876–886. doi: 10.1158/1078-0432.CCR-08-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Melancon MP, Lu W, Yang Z, Zhang R, Cheng Z, Elliot AM, Stafford J, Olson T, Zhang JZ, Li C. In vitro and in vivo targeting of hollow gold nanoshells directed at epidermal growth factor receptor for photothermal ablation therapy. Mol. Cancer Ther. 2008;7(6):1730–1739. doi: 10.1158/1535-7163.MCT-08-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. 2003;100(23):13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Loo C, Lowery A, Halas N, West J, Drezek R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005;5(4):709–711. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- [20].Chen J, Wang D, Xi J, Au L, Siekkinen A, Warsen A, Li Z-Y, Zhang H, Xia Y, Li X. Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells. Nano Lett. 2007;7(5):1318–1322. doi: 10.1021/nl070345g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang C, Chen J, Talavage T, Irudayaraj J. Gold nanorod/Fe3O4 nanoparticle “nano-pearl-necklaces” for simultaneous targeting, dual-mode imaging, and photothermal ablation of cancer cells. Angew. Chem. 2009;121(15):2797–2801. doi: 10.1002/anie.200805282. [DOI] [PubMed] [Google Scholar]
- [22].Dickerson EB, Dreaden EC, Huang X, El-Sayed IH, Chu H, Pushpanketh S, McDonald JF, El-Sayed MA. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008;269(1):57–66. doi: 10.1016/j.canlet.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].You J, Zhang G, Li C. Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS Nano. 4(2):1033–1041. doi: 10.1021/nn901181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19(4):316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- [25].Wang W, Ke S, Wu Q-P, Charnsangavej C, Gurfinkel M, Gelovani JG, Abbruzzese JL, Sevick-Muraca EM, Li C. Near-infrared optical imaging of integrin avb3 in human tumor xenografts. Mol. Imaging. 2004;3:343–351. doi: 10.1162/15353500200404148. [DOI] [PubMed] [Google Scholar]
- [26].Mendelsohn J. Epidermal growth factor receptor inhibition by a monoclonal antibody as anticancer therapy. Clin Cancer Res. 1997;3(12 Pt 2):2703–2707. [PubMed] [Google Scholar]
- [27].Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin. Cancer Res. 1995;1(11):1311–1318. [PubMed] [Google Scholar]
- [28].Schwartzberg AM, Olson TY, Talley CE, Zhang JZ. Synthesis, characterization, and tunable optical properties of hollow gold nanospheres. J. Phys. Chem. B. 2006;110(40):19935–19944. doi: 10.1021/jp062136a. [DOI] [PubMed] [Google Scholar]
- [29].Melancon MP, Wang Y, Wen X, Bankson JA, Stephens LC, Jasser S, Gelovani JG, Myers JN, Li C. Development of a macromolecular dual-modality MR-optical imaging for sentinel lymph node mapping. Invest. Radiol. 2007;42(8):569–578. doi: 10.1097/RLI.0b013e31804f5a79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wen X, Jackson EF, Price RE, Kim EE, Wu Q, Wallace S, Charnsangavej C, Gelovani JG, Li C. Synthesis and characterization of poly(L-glutamic acid) gadolinium chelate: a new biodegradable MRI contrast agent. Bioconjug. Chem. 2004;15(6):1408–1415. doi: 10.1021/bc049910m. [DOI] [PubMed] [Google Scholar]
- [31].Hekmatyar SK, Kerkhoff RM, Pakin SK, Hopewell P, Bansal N. Noninvasive thermometry using hyperfine-shifted MR signals from paramagnetic lanthanide complexes. Int. J. Hyperthermia. 2005;21:561–574. doi: 10.1080/02656730500133801. [DOI] [PubMed] [Google Scholar]
- [32].Diagaradjane P, Shetty A, Wang JC, Elliott AM, Schwartz J, Shentu S, Park HC, Deorukhkar A, Stafford RJ, Cho SH, Tunnell JW, Hazle JD, Krishnan S. Modulation of in Vivo Tumor Radiation Response via Gold Nanoshell-Mediated Vascular-Focused Hyperthermia: Characterizing an Integrated Antihypoxic and Localized Vascular Disrupting Targeting Strategy. Nano Lett. 2008;8(5):1492–1500. doi: 10.1021/nl080496z. [DOI] [PMC free article] [PubMed] [Google Scholar]