Abstract
A major controversy in the study of memory concerns whether there are distinct medial temporal lobe (MTL) substrates of recollection and familiarity. Studies using Received Operating Characteristics (ROC) analyses of recognition memory indicate that the hippocampus is essential to recollection but not familiarity. We report the converse pattern wherein amygdala damage impairs familiarity while sparing recollection. Combined with previous findings, these results dissociate recollection and familiarity by selective MTL damage.
A prevalent view is that the hippocampus supports the recollection of events within the context in which they were experienced whereas the perirhinal cortex supports a sense of familiarity with previously experienced stimuli1. However, others have suggested that the apparent qualitative distinctions between structures supporting recollection and familiarity can be attributed to quantitative differences where strong and weak memories are represented in these areas2. Decisive evidence between these views could be provided by a double dissociation between areas essential to recollection and familiarity, which is not predicted by the memory strength view. Several studies have shown that the hippocampus is critical to recollection, and not familiarity1, and one study reported that unilateral damage to the perirhinal cortex impaired familiarity, but not recollection, in a human3. However, the latter finding is not entirely consistent with the prevalent MTL model in which perirhinal cortex is a key structure of information about events that is essential to the recollection function of the hippocampus.
Here we pursued a novel approach, using ROC analysis of recognition memory in rats. We reasoned that a selective familiarity deficit might be induced by removing modulatory influences that contribute differentially to familiarity processing, while at the same time sparing the flow of item information from perirhinal cortex to the hippocampus. The amygdala modulates memory processing4, 5 and is connected to both the perirhinal cortex and hippocampus6. Also, the amygdala interacts with the hippocampus to support memory in humans and animals7,8. However, the connection to perirhinal cortex is exceptionally robust, and while the amygdala contributes to item memory9 and the `gist' of event memories10, the connection to the hippocampus may contribute most to a subjective `feeling' of remembering but not retrieval of source information that is defining of recollection11. These findings suggest that the amygdala might contribute differentially to perirhinal familiarity representations by supporting an increased attractiveness of the studied stimuli associated with recent `mere exposure'12 and rewards provided during study. Thus, we predicted that bilateral lesions of the amygdala that remove its influence on perirhinal cortex would selectively impair familiarity; notably this prediction stands in contrast to the observation that unilateral amygdala damage does not affect either recollection or familiarity in item recognition in humans3,13.
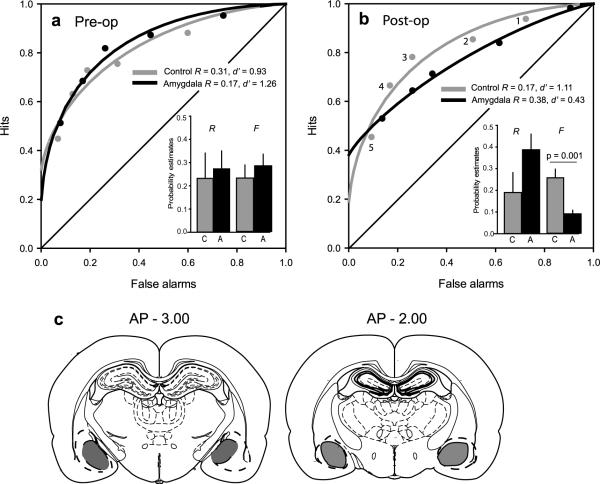
To assess the role of the amygdala in recollection and familiarity, we initially trained rats on an odor recognition task (Fig 1), then divided them into two matched groups based on performance that indicated both a recollection component, reflected in the above-zero Y-intercept of the ROC curve, and a familiarity component, reflected in the `bowing' of the ROC function (Fig 2a, for details, see supplementary information). Post-operatively, controls continued to employ recollection (R = 0.19) and familiarity (d' = 1.11; Fig. 2b). However, amygdala lesioned animals were severely impaired in familiarity while showing intact recollection (group × memory-component interaction F1, 11 = 8.05, P = 0.016) driven by a decrease in the familiarity (d') estimate in lesioned animals (d' = 0.43) compared with controls (t11 = 4.28, P = 0.001). When converted to z-space, the ROC function of lesioned animals was U-shaped (z-ROC quadratic coefficient significantly different from 0; t6 = 4.35, P = 0.005), indicating the absence of a significant contribution of familiarity. In contrast, the ROC curve continued to reflect recollection in lesioned animals (R = 0.38), which appeared quantitatively greater than, but did not significantly differ from that of controls (t11 = − 1.72, P = 0.11). Consistent with these findings, parameter estimates of recollection (R) and familiarity (F, see supplementary information) contributed equally to recognition in controls (t5 = − 0.68, P = 0.53; Fig. 2b), whereas lesioned animals relied largely on recollection and less on familiarity (t6 = 3.54, P = 0.01). There was no significant difference in overall percent correct recognition in rats with amygdala lesions (64%) compared to controls (69%; t11 = 1.58, P = 0.14), and separate ANOVAs did not reveal significant group differences in either the hit rates (F1, 11 = 0.004, P = 0.948; interaction group × bias level: F4, 44 = 1.76, P = 0.154) or the false alarm rates (F1, 11 = 3.08, P = 0.107; interaction of group x bias level: F4, 44 = 1.12, P = 0.359), and there was no shift in the overall bias to respond to target items (see supplementary information).
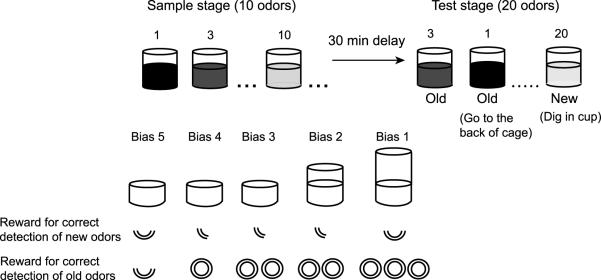
Figure 1.
Recognition memory task. Each day rats studied ten stimuli composed as scented sand in cups with buried rewards, and then recognition is tested on the studied (“old”) odors plus another ten “new” odors. Hits are correctly identified old odors, and false alarms are new odors incorrectly identified as old. Response bias was manipulated by varying the height of the cup and the amount of reward received for correctly digging in new test cups or correctly withholding the response to old test stimuli and receiving reward elsewhere.
Figure 2.
ROC function in recognition performance. A. Pre-surgery ROC for controls and animals later given amygdala lesions. B. Post-operative ROC functions in control animals and amygdala lesioned animals. Flattened ROC curve in amygdala lesioned animals indicates loss of familiarity. Response criteria: 5 = conservative to 1 = liberal. Insets: Parameter estimates (+SEM) of recollection (R) and familiarity (F) for controls and lesioned animals. C. Reconstruction of amygdala lesions at − 3.0 mm (68% damage) and − 2.00 mm (44% damage) posterior to bregma. Grey, average lesion across animals; dotted line, largest lesion.
Our findings indicate that bilateral amygdala damage results in impaired familiarity, sparing recollection, whereas our previous study showed that hippocampal damage impaired recollection, sparing familiarity, in rats performing the identical behavioral test14. This double dissociation accounts for a previous report of recognition deficits following combined but not separate amygdala-hippocampal damage, consistent with the notion that these two areas make independent contributions to recognition15. Furthermore, these findings support a model of recognition memory in which the amygdala contributes to aspects of familiarity processed by the perirhinal cortex as distinct from the information about stimuli it passes on to the hippocampus in support of recollection.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Andrew Yonelinas and Magdalena Sauvage for their thoughtful comments on data analysis. This work was supported by National Institute of Mental Health Project Grant MH52090.
This research was supported by NIMH MH52090.
Footnotes
AUTHOR CONTRIBUTIONS. H.E. and A.F. designed the study and wrote the manuscript. A.F. carried out the surgery and analyzed the data. R.P., D.M., and A.F. performed the experiment, and R.P. and A.F. performed the histological analysis.
References
- 1.Eichenbaum H, Yonelinas AP, Ranganath C. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Squire LR, Wixted JT, Clark RE. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowles B, Crupi C, Mirsattari SM, Pigott SE, Parrent AG, Pruessner JC, Yonelinas AP, Köhler S. Proc Natl Acad Sci U S A. 2007;104:16382–16387. doi: 10.1073/pnas.0705273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGaugh JL. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 5.LaBar KS, Cabeza R. Nat Rev Neurosci. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- 6.Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- 7.Babiloni C, Vecchio F, Mirabella G, Buttiglione M, Sebastiano F, Picardi A, Di Gennaro G, Quarato PP, Grammaldo LG, Buffo P, Esposito V, Manfredi M, Cantore G, Eusebi F. Human Brain Mapping. 2009;30:2077–2089. doi: 10.1002/hbm.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Packard MG, Teather LA. Neurobio Learn Mem. 1998;69:163–203. doi: 10.1006/nlme.1997.3815. [DOI] [PubMed] [Google Scholar]
- 9.Kensinger EA, Schacter DL. J Neurosci. 2006;26:2564–70. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adolphs R, Tranel D, Buchanan TW. Nat Neurosci. 2005;8:512–518. doi: 10.1038/nn1413. [DOI] [PubMed] [Google Scholar]
- 11.Sharot T, Delgado MR, Phelps EA. Nat Neurosci. 2004;7:1376–80. doi: 10.1038/nn1353. [DOI] [PubMed] [Google Scholar]
- 12.Zajonc RB. J Pers Soc Psychol. 1968;9:1–27. [Google Scholar]
- 13.Bowles B, Crupia C, Pigott S, Parrent A, Wiebe S, Janzen L, Köhler S. Neuropsychologia. 2010;48:2640–2647. doi: 10.1016/j.neuropsychologia.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Fortin NJ, Wright SP, Eichenbaum H. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aggleton JP, Blindt HS, Rawlins JNP. Behav Neurosci. 1989;103:962–974. doi: 10.1037//0735-7044.103.5.962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.