Abstract
The proportion of patients undergoing liver transplantation (LT) with concomitant renal dysfunction markedly increased after allocation by the Model for End-stage Liver Disease (MELD) score was introduced. We examined the incidence of subsequent post-LT end-stage renal disease (ESRD) before and after the policy was implemented. Data on all adult deceased-donor LT recipients between 4/27/95 and 12/31/08 (n=59,242) from the Scientific Registry of Transplant Recipients were linked with Centers for Medicare & Medicaid Services ESRD data. Cox regression was used to (i) compare pre-MELD and MELD eras with respect to post-LT ESRD incidence (ii) determine the risk factors for post-LT ESRD (iii) quantify the association between ESRD incidence and mortality. Crude rates of post-LT ESRD were 12.8 and 14.5 per 1,000 patient-years in the pre-MELD and MELD eras, respectively. Covariate-adjusted post-LT ESRD risk was higher in the MELD era (hazard ratio [HR] =1.15; p=0.0049). African-American race, hepatitis C, pre-LT diabetes, higher creatinine, lower albumin, lower bilirubin and sodium>141 mMol/L at LT were also significant predictors of post-LT ESRD. Post-LT ESRD was associated with higher post-LT mortality (HR=3.32; p<0.0001). The risk of post-LT ESRD, a strong predictor of post-LT mortality, is 15% higher in the MELD era. This study identified potentially modifiable risk factors of post-LT ESRD. Early intervention and modification of these risk factors may reduce the burden of post-LT ESRD.
Keywords: End-stage renal disease, Liver transplant, Model for end-stage renal disease, Mortality, Scientific Registry of Transplant Recipients
Introduction
Post-transplant chronic renal failure is a major post-transplant co-morbidity among non-renal solid organ transplant recipients and is associated with high mortality1. Among non-renal solid organ transplant recipients in the allocation era prior to the use of the Model for End-Stage Liver Disease (MELD), liver transplant (LT) recipients had the second highest incidence of post-transplant chronic renal failure, despite generally lower level of immunosuppression with calcineurin inhibitors compared to heart and lung transplant recipients1.
The MELD score, a measure of waiting list mortality risk, has been used as the basis of deceased-donor liver allocation since February 2002, and serum creatinine is one of the key components of the MELD score. Our previous research showed that serum creatinine is given excess weight in the current MELD formula2. Not surprisingly, the proportion of patients undergoing LT with pre-transplant renal dysfunction significantly increased in the MELD era compared to the pre-MELD era3. Moreover, MELD is unable to distinguish between renal dysfunction due to acute and potentially reversible kidney injury secondary to hepatorenal syndrome or due to chronic kidney disease secondary to hypertension or diabetes.
We previously conducted a single-center MELD-era cohort study that estimated the 5-year cumulative incidence of post-LT chronic renal failure to be 22%4. However, that study did not focus on end stage renal disease (ESRD), and also did not have a pre-MELD comparison group. In the current study, we linked national data from the Scientific Registry of Transplant Recipients (SRTR) and the Centers for Medicare & Medicaid Services (CMS) ESRD program to determine the impact of MELD-based allocation on the incidence of new-onset ESRD among LT recipients. We also sought to determine donor and recipient risk factors associated with new-onset post-LT ESRD as well as evaluate the relationship between new-onset ESRD and post-LT mortality.
Methods
Data Sources and Study Population
Our study was based on data obtained from the SRTR, the CMS ESRD program, and the Social Security Death Master File5. The SRTR maintains a database of all candidates for and recipients of solid-organ transplants in the United States. Patients on waiting lists for organ transplantation and those who receive organ transplants are followed on a periodic basis with the use of data-collection forms completed by organ-transplantation programs and submitted to the Organ Procurement and Transplantation Network. These follow-up data, in addition to data from the network regarding patients on waiting lists and the allocation of organs, are included in the SRTR database. The SRTR supplements information on vital status with data on deaths from the Social Security Death Master File and the Medicare Beneficiary Database maintained by CMS. Data collection by the SRTR is exempt from oversight under the “public benefit or service program” provisions of the Code of Federal Regulations (45 CFR 46.101[b][5]), as approved by the institutional review board of the Health Resources and Services Administration of the Department of Health and Human Services.
The Social Security Death Master File includes updated information on all participants in the Social Security system. Information on deaths reported to the Social Security system for the administration of the death, disability, and retirement benefit programs is kept in the Death Master File database.
CMS maintains a database of all patients treated for ESRD in the United States, which includes information about demographics, treatment, hospitalization, and costs for Medicare beneficiaries and other patients with ESRD who have received maintenance renal replacement therapy. This database also includes records of any changes in vital status or method of renal replacement, including kidney transplantation6. Our study population included candidates 18 years of age and older who received LT between April 27, 1995 and December 31, 2008 (n=59,242). Living donor and multi-organ transplants were excluded. The time period was divided into pre-MELD and MELD eras. Patients who received LT before February 28, 2002 were assigned to the pre-MELD era, and those who received LT on or after February 28, 2002 were assigned to the MELD era.
We constructed an analysis file containing information on the baseline demographic and clinical characteristics of the LT recipients who met inclusion criteria. The analysis file was linked to the CMS ESRD database to identify patients who received renal replacement therapy after transplantation of the liver. The linkage between SRTR data and CMS data was established using matching across patient-level sources, finding similarities in patient identifiers such as social security numbers, health insurance claim number, names and nicknames, gender, and date of birth. More information on the nature of such linkages is described by Dickinson et al.6
Analytical Approach
In the descriptive analysis, continuous variables were expressed as mean ± standard deviation and categorical variables were expressed as proportions. The primary outcome was new-onset post-LT ESRD, defined as the earliest of initiation of chronic dialysis, wait listing for kidney transplantation, and receipt of a kidney transplant ascertained by the CMS 2728 medical evidence form. This form is completed by the patient’s dialysis center within 45 days of initiation of chronic dialysis or receipt of kidney transplantation. The listing for renal transplantation was ascertained from the SRTR data. These are objective and well-defined outcomes captured in the CMS ESRD and SRTR databases. Note that the CMS 2728 form is not completed if the patient is in acute renal failure or is expected to recover renal function. Therefore, a patient who requires dialysis for some period of time after transplantation but recovers their renal function would not be included in the CMS ESRD database. Similarly, patients who receive continuous veno-venous hemodialysis or a few course of RRT in the immediate post-transplant phase would not be included in the CMS ESRD database. Patients in the acute phase of renal failure are not listed for kidney transplantation and, therefore, would not be in the data set either. Since we are looking at post-LT ESRD as a primary outcome, and since listing of kidney transplant is a surrogate for renal replacement therapy, we included both initiation of chronic dialysis and listing for kidney transplantation as our outcome. The date of placement on the waiting list for kidney transplantation was tracked for patients with liver transplants in whom ESRD subsequently developed. Renal replacement modality changes from dialysis to renal transplantation were also recorded in order to identify patients who received a renal transplant from either a living or deceased donor.
Patients were followed from the date of LT to the earliest of new onset ESRD, death or the end of observation period. The incidence rate was calculated as the number of ESRD events divided by total patient time expressed as patient-years.
Given the available data structure and our objectives, death (prior to ESRD onset) was treated as a competing risk; i.e., since post-LT death precludes post-LT new-onset ESRD. Therefore, we modeled the cause-specific hazard of ESRD7, which can be thought of as the rate of ESRD incidence among patients alive and ESRD-free. An alternative approach would be to model the cumulative incidence of ESRD; which can be thought of as the follow-up time-specific probability of ESRD (acknowledging that ESRD onset cannot occur following death. The cumulative incidence of ESRD essentially averages over deceased and surviving patients and, hence, would be most useful for descriptive purposes.
In the first part of the analysis, Cox regression was used to contrast the pre-MELD and MELD eras with respect to ESRD incidence, adjusting for recipient age, gender, race, diagnosis, recipient’s height, weight, status, pre-LT hypertension, diabetes, hospital status at LT, previous LT, history of transjugular intrahepatic portosystemic shunt (TIPS), donor age, donor gender, cold ischemia time, local versus (regionally, nationally) shared organ, and donation after cardiac death (DCD) and the type of immunosuppression. We fitted one model that contrasted covariate-adjusted ESRD incidence rates between the two eras. We then fitted a second model that coded the year-of-transplant as a continuous predictor (to test for a trend over calendar time), but with a change-point at year 2002 (to test for a change in the trend beginning in 2002, the start of MELD-based allocation).
In the second part of the analysis, Cox regression was used to determine the risk factors for new-onset post-LT ESRD among LT recipients. In this case, only patients transplanted in the MELD era who were not on renal replacement therapy at LT were used since lab measurements (i.e., creatinine, bilirubin, INR, albumin) were generally not available in the pre-MELD era. The model was adjusted for all covariates listed above, plus bilirubin, international normalized ratio (INR), creatinine, change in creatinine pre-LT (slope), sodium and albumin at LT. The slope of creatinine was estimated using least squares regression (i.e., the familiar slope estimator from simple linear regression) based on all available creatinine values from the time of wait listing to the time of LT. The MELD score update is a complex process. Serum bilirubin, creatinine and INR are components of MELD score. For some candidates, it could be one MELD update (creatinine values) between listing and transplant while for others it could be close to 10–15 or greater MELD updates (each including a creatinine value) available to calculate the slope.
As implied previously, we fitted proportional hazards models to the cause-specific hazard of ESRD. This was carried out using PROC PHREG in SAS v9.2 (SAS Institute; Cary, NC, USA). In particular, the input record for each patient was the covariate; time between LT and the earliest of death, ESRD, loss to follow-up and end-of-study (with loss-to-follow-up and end-of-study both treated as independent censoring); and an event indicator taking the value 1 for ESRD and 0 for either censoring or death. Note that the grouping of loss-to-follow-up, end-of-study and death as event=0 should not be mistaken for an assumption that death and ESRD are independent. Coding all these events as event=0 is merely a computational trick to get PHREG to compute the risk sets appropriately. Putter et al. provide an excellent summary of related issues in the competing risks setting8.
For the third component of the analysis, a time-dependent Cox model was used to study the association between post-LT ESRD and mortality; with ESRD (yes/no) coded as a time-dependent binary indicator.
As a sub-analysis, we also modeled the rate of death prior to ESRD onset (i.e., cause-specific hazard of death among patients alive and ESRD-free), again using Cox regression. This model complements the first part of the analysis, in the sense that patients can cease to be alive-and-ESRD-free via two mutually exclusive events: ESRD onset (modeled in the first part of the analysis) and death prior to ESRD (the sub-analysis).
All statistical analyses were conducted using SAS v9.2 (SAS Institute; Cary, NC, USA).
Results
Descriptive Statistics
The study cohort consisted of 59,242 LT recipients (pre-MELD era: n=25,500 and MELD era: n=33,742). Baseline characteristics of LT recipients were similar in both eras (Table 1). On average, LT recipients in the pre-MELD era received younger donor allografts with slightly longer cold ischemia time. Information on serum bilirubin, creatinine, INR, albumin and sodium were not available in the pre-MELD era.
Table 1.
Cohort characteristics at the time of liver transplantation by era.
| Variables | Pre-MELD Era (n=25,500) |
MELD Era (n=33,742) |
|---|---|---|
| Age at LT (years) | 49.9 [10.5] | 52.5 [10.2] |
| % Males | 62 % | 68 % |
| Race/Ethnicity - White - African American - Hispanic - Asian - Other |
78% 7% 11% 4% 1% |
73% 9% 12% 5% 1% |
| Hepatitis C Fulminant Hepatic Failure |
43% 7% |
44% 7% |
| Donor age (years) | 37.6 [17.5] | 41.4 [17.5] |
| Cold Ischemia Time (Hours) | 8.6 [3.9] | 7.5 [3.5] |
Post-LT ESRD Incidence Rates and Era Effect
There were a total of 1,878 and 1,156 ESRD events in the pre-MELD and MELD eras, respectively. The unadjusted incidence rate of post-LT ESRD was 12.8 per 1,000 patient-years in the pre-MELD era and 14.5 per 1,000-patient-years in the MELD era.
LT in the MELD-based allocation era was associated with a 15% higher risk of post-LT ESRD compared to the pre-MELD era (hazard ratio [HR]=1.15; 95% confidence interval 1.043–1.268; p=0.0049), based on a Cox regression model adjusted for recipient age, gender, race, diagnosis, recipient’s height, weight, status, pre-LT hypertension, diabetes, hospital status at LT, previous LT, history of TIPS, donor age, donor gender, cold ischemia time, local versus (regionally, nationally) shared organ, and donation after cardiac death (DCD) and the type of immunosuppression.
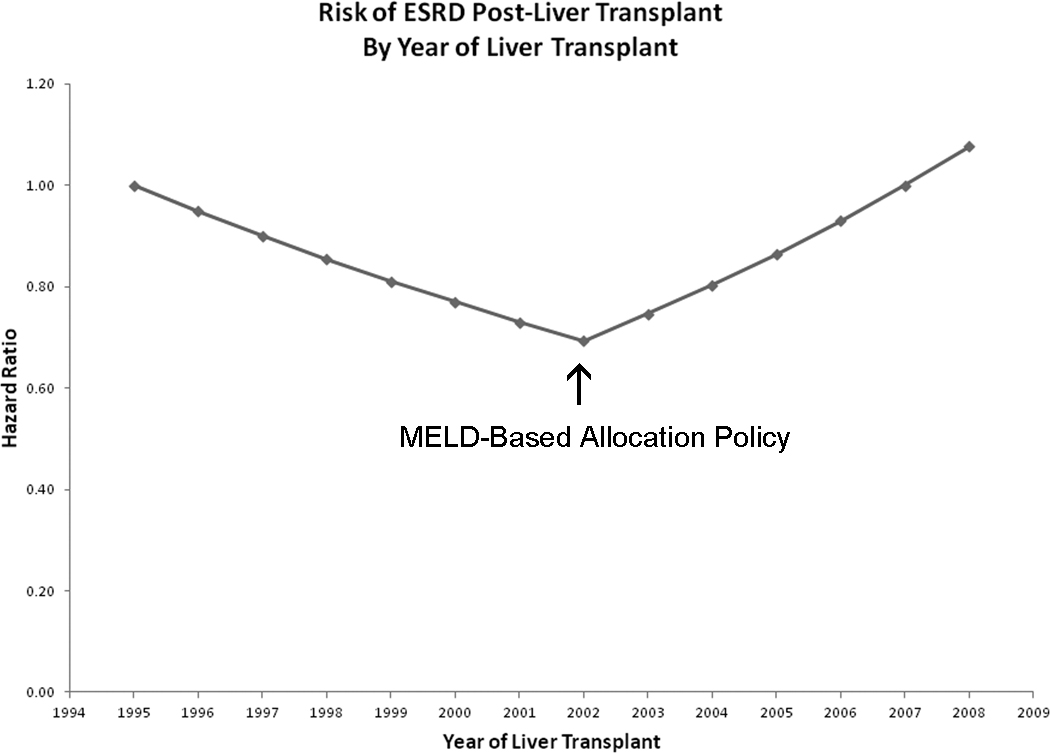
Figure 1 displays trends in covariate-adjusted post-LT ESRD incidence by calendar year of LT. Prior to 2002 (pre-MELD era), rates of new onset post-LT ESRD decreased significantly by 5.1% per year (HR=0.949; 95% confidence interval 0.924–0.975; p<0.0001). However, the trend sharply reversed in 2002 (MELD era), with ESRD incidence increasing by 7.6% per year after year 2002 (p<0.0001).
Figure 1.
The risk of Post-LT ESRD by the year of liver transplant. X-axis shows the year of liver transplantation and Y-axis shows the hazard ratio of post-LT ESRD. The hazard ratio for the year of liver transplantation was obtained from the regression model. Hazard ratio= exp{lin_pred} and lin_pred = ln(0.949)*(YEAR-1995) + ln(1.134)*(YEAR-2002)*I(Year > 2002)
Sub-analysis revealed that post-LT mortality (among patients alive and ESRD-free) was a significant 10% lower in the MELD era (covariate adjusted HR=0.902; 95% confidence interval 0.864–0.941; p<0.0001) relative to the pre-MELD era.
Predictors of Post-LT ESRD in the MELD Era
Table 2 shows recipient and donor risk factors associated with new-onset post-LT ESRD in the MELD era. African-American race (HR=1.57, p<0.0001), history of diabetes (HR=2.20, P<0.0001), hepatitis C as an etiology of liver disease (HR=1.34, p<0.0003), high serum creatinine at the time of LT (HR=4.08, p<0.0001), serum sodium >141 mMol/L (HR=1.42, p<0.0034), low albumin (HR=0.53, p<0.0001), low bilirubin (HR=0.83, p<0.0001), history of TIPS procedure (HR=1.40 p=0.0013), and history of previous LT (HR=1.49, p=0.0012) were the recipient factors associated with higher risk of new onset post-LT ESRD. A larger increase in creatinine slope over time before LT (change in creatinine from listing to LT) was associated with a lower risk of post-LT ESRD compared to those with smaller increase in pre-LT creatinine slope (HR=0.86; p<0.0001). INR, one of the components of MELD score, was not associated with post-LT ESRD in the MELD era (HR=0.86; 95% confidence interval 0.691–1.072; p=0.146).
Table 2.
Recipient and donor risk factors associated with new onset post-LT ESRD in the MELD era.
| Variables | Hazard Ratio (95% CI) | P-value |
|---|---|---|
| Recipient Factors | ||
| Age 18–29 yrs (Ref: 50–59 yrs) | 0.56 (0.32–0.99) | 0.046 |
| African-American (Ref: White) | 1.57 (1.3–1.9) | <0.0001 |
| Cholestatic (Ref: Non-Cholestatic) HCV (Ref: Non-HCV) |
0.64 (0.47–0.88)) 1.34 (1.15––1.58) |
0.006 0.0003 |
| Recipient Diabetes | 2.20 (1.92–2.52) | <0.0001 |
| Ln Creatinine Creatinine slope Ln Bilirubin Ln Albumin Sodium >141 mMol/L/l (Ref: 130–141) |
4.08 (3.6–4.6) 0.86 (0.81–0.91) 0.83 (0.77–0.90) 0.53 (0.42–0.68) 1.42 (1.12–1.79) |
<0.0001 <0.0001 <0.0001 <0.0001 0.0034 |
| TIPS recipients Previous LT |
1.40 (1.14–1.71) 1.49 (1.17–1.89) |
0.0013 0.0012 |
| Donor Factors | ||
| Donor Age (Ref: 30–39) 50–59 yrs 60–69 yrs Donor Female (Ref: Male) Cold ischemia Time (per hour) DCD (Ref: Non-DCD) |
1.27 (1.07–1.52) 1.29 (1.05–1.60) 0.86 (0.76–0.98) 1.03 (1.01–1.04) 1.47 (1.10–1.97) |
0.0074 0.018 0.027 0.0004 0.0098 |
HCV = hepatitis C; LT = liver transplant; DCD = donation after cardiac death.
Donor factors associated with higher risk of new onset post-LT ESRD included age 50–69 years, male gender, and DCD donor. Longer cold ischemia time was associated with higher risk of new onset post-LT ESRD (Table 2). Neither the type of calcineurin inhibitor nor use of antibody induction after LT affected the risk of new onset post-LT ESRD.
Effect of Post-LT ESRD on Patient Survival
Post-LT mortality increased more than threefold upon onset of ESRD, with covariate-adjusted HR=3.32 (p<0.0001), as shown in Table 3. Other recipient and donor factors predicting post-LT mortality are also listed in Table 3.
Table 3.
Predictors of post-LT mortality.
| Variables | Hazard Ratio | 95% CI | P-Value |
|---|---|---|---|
| Post-LT ESRD (Time dependent) | 3.32 | 2.96–3.71 | <.0001 |
| Age 18–29 (Ref: Age 50–59) 30–39 40–49 60–64 65 and above |
0.85 0.80 0.88 1.18 1.40 |
0.72–1.00 0.71–0.91 0.82–0.94 1.09–1.27 1.29–1.52 |
0.0429 0.0009 0.0001 <0.0001 <0.0001 |
| African-American (Ref: White) Hispanic Asian |
1.25 0.86 0.80 |
1.15–1.36 0.79–0.93 0.70–0.92 |
<.0001 0.0003 0.0012 |
| Hepatitis C (Ref: Non-HCV) HCC (Ref: Non-HCC) |
1.39 1.34 |
1.31–1.48 1.25–1.44 |
<.0001 <.0001 |
| Pre-LT DM (Ref: No DM) | 1.18 | 1.11–1.25 | <.0001 |
| Ln Creatinine at LT | 1.13 | 1.07–1.20 | <.0001 |
| Ln Albumin at LT | 0.73 | 0.66–0.81 | <.0001 |
| Hospitalized ICU (Ref: Ambulatory) Hospitalized Non ICU |
1.34 1.16 |
1.20–1.50 1.07–1.25 |
<.0001 0.0001 |
| Life Support at LT (Ref: No life support) | 1.58 | 1.40–1.79 | <.0001 |
| Status 1(Ref: Non-status 1) | 0.74 | 0.64–0.85 | <.0001 |
| Repeat LT (Ref: No) | 1.70 | 1.54–1.87 | <.0001 |
| Portal Vein Thrombosis (Ref: None) | 1.39 | 1.25–1.56 | <.0001 |
| Donor Age 0–17 (Ref: 18–39) 40–49 50–59 60–69 70 and above |
0.90 1.20 1.32 1.58 1.71 |
0.81–1.003 1.12–1.29 1.23–1.42 1.46–1.72 1.53–1.90 |
0.0571 <.0001 <.0001 <.0001 <.0001 |
| Cold Ischemia Time | 1.01 | 1.004–1.02 | 0.0030 |
| DCD (Ref: No) | 1.25 | 1.10–1.43 | 0.0006 |
| Regional (Ref: Local) National |
1.03 1.16 |
0.97–1.10 1.05–1.28 |
0.2893 0.0034 |
Discussion
We are not aware of any prior study that has directly evaluated the impact of MELD-based liver allocation on the risk of new-onset post-LT ESRD. Our results indicated a 15% higher risk of post-LT ESRD in the MELD era compared to the pre-MELD era, independent of the various modifiable and non-modifiable recipient and donor risk factors that were also associated with new-onset post-LT ESRD.
Importantly, post-LT ESRD incidence rates were on the decline until MELD-based allocation took effect in 2002, after which the rates sharply reversed trend and began to increase significantly. The declining rates of ESRD in LT patients in the pre-MELD era could be a combination of centers not transplanting candidates with severe renal dysfunction (known to be associated with worse outcomes), as well as change in immunosuppression practice patterns from cyclosporine, (which is associated with slightly higher risk of post-LT chronic renal failure [HR=1.25,p<0.001]) to tacrolimus after 19941,9.
Compared to the pre-MELD era, post-LT mortality was 10% lower in the MELD era among those who did not develop ESRD. This era effect reflects evolution of the field in terms of better medical and surgical patient management, better diagnostic and interventional techniques and advancement in immunosuppression9. However, once the ESRD developed, the risk of post-LT death increased threefold in our study. Although the post-LT ESRD incidence rates showed an absolute change from 12.8 per 1,000 patient-years to 14.5 per 1,000 patient-years in the pre-MELD era and MELD era, respectively, this 21% relative increase in the post-LT ESRD incidence rates and 15% higher risk of developing post-LT ESRD in the MELD era compared to the pre-MELD era translates to a very high mortality in this group of patients. The higher incidence of new-onset post-LT ESRD in the MELD era may represent the tip of the iceberg. We speculate that the development of post-LT Stage 4 chronic kidney disease has likely also increased in the MELD era. This suggests that many additional cases of post-LT ESRD will be revealed over time and may add to already skyrocketing healthcare cost in terms of additional dialysis cases, increased hospitalization rates secondary to morbidities associated with ESRD.
Pre-LT serum creatinine was one of the strongest predictors of post-LT ESRD in our study. Since creatinine is heavily weighted in the MELD equation currently used for allocation2, the MELD era has been characterized by a significantly higher proportion of candidates undergoing LT with serum creatinine ≥2 mg/dl compared to the pre-MELD era3. This has increased the number of recipients at high risk for new onset post-LT ESRD.
Interestingly, a steeper slope of creatinine during the interval from the time of listing to LT was associated with lower risk of post-LT ESRD. A plausible explanation for this observation is that a rapid rise in creatinine before LT may represent an acute renal injury secondary to hepatorenal syndrome, which would be expected to improve after successful LT. Conversely, candidates with a more gradual rise in creatinine are more likely to have pre-existing structural renal disease, which may progress to post-LT ESRD over time.
In addition to previously identified risk factors for new-onset post-LT ESRD such as African-American race, history of pre-LT diabetes, and high pre-LT creatinine1,10–13, our study also showed that serum sodium >141 mMol/L, lower albumin, lower bilirubin, history of TIPS and previous LT were each significantly and independently associated with new-onset post-LT ESRD. Lower albumin levels and history of TIPS represent an advanced stage of cirrhosis. High serum sodium may represent a volume-depleted state, whereas low serum sodium is often associated with potentially reversible hepatorenal syndrome. Our analysis did not identify gender as a predictor of ESRD risk following LT. The degree to which creatinine understates ESRD risk among females may not have been of sufficient magnitude to produce an independently significant gender effect. Also, the degree to which creatinine understates ESRD risk may be lower in post-LT patients.
Our study also showed that a diagnosis of hepatitis C and non-cholestatic liver disease were associated with a higher risk of post-LT ESRD compared to non-hepatitis C and cholestatic liver disease, respectively. Hepatitis C is a frequent diagnosis among non-cholestatic liver disease patients and a lower bilirubin level is commonly seen in non-cholestatic liver disease. The number of candidates listed and transplanted for hepatitis C has increased over time14,15. Candidates with hepatitis C may have pre-existing intrinsic kidney disease, secondary to membranous or membrano-proliferative glomerulonephritis, which may eventually progress to post-LT ESRD.
Various donor factors such as advanced age (≥ 50 years), male gender, prolonged cold ischemia time, and DCD donor type were also associated with a higher risk of post-LT ESRD. These donor factors have also been shown to be associated with poor graft survival16.
The primary limitation of our study is the retrospective observational study design that may result in the potential for bias due to patient selection and unmeasured patient characteristics. Moreover, this study was unable to evaluate the causes of post-LT ESRD because of the lack of renal biopsy and urinalysis information in SRTR data. An additional limitation is unavailable or missing data on creatinine, bilirubin and INR from the pre-MELD era, which could have been used to determine whether the adverse effects of these covariates on post-LT ESRD varied by allocation era. Despite these limitations, this is the largest cohort study from the MELD era to evaluate the impact of current liver allocation policy on post-LT ESRD. The primary outcome of our study was new-onset post-LT ESRD, as opposed to chronic renal failure which was used in previous studies1,4. Post-LT ESRD is more reliably tracked and ascertained than stage 4 chronic kidney disease.
In conclusion, our study has demonstrated that the risk of new-onset ESRD among LT recipients is markedly higher in the MELD era. The risk of post-LT ESRD, which had been declining prior to the implementation of MELD-based allocation, has increased by 7.6% per year since its introduction in 2002. Since the development of ESRD is associated with very high mortality among LT recipients, risk modification directed toward optimizing renal function may be useful in the pre-, peri-, and post-operative management of liver transplant candidates may prevent or delay the post-LT ESRD. These measures may have a significant downstream effect on decrease in the need for dialysis, post-LT ESRD related mortality, need for renal transplantation and healthcare cost. Finally, re-weighting of creatinine in the MELD formula should be considered2. This modest modification to the allocation system may balance the desire to improve overall post-LT outcomes while reducing the risk of renal failure and reducing the wait-list mortality.
Acknowledgements
The authors would like to thank Ms. Shauna Leighton, Medical Editor, Arbor Research Collaborative for Health, Ann Arbor, Michigan, funded by the Scientific Registry of Transplant Recipients for providing editorial assistance.
Funding Sources: Dr. Sharma was the recipient of American Society of Transplantation/Roche clinical science faculty development grant for 2008–10. Dr. Sharma is also supported by National Institutes of Health (NIH) grant KO8 DK-088946. The statistical methodology development and analysis for this investigation was supported in part by National Institutes of Health (NIH) grant 2R01 DK-70869 to Dr. Schaubel. Drs. Sharma and Schaubel are also supported by Michigan Institute for Health and Clinical Research NIH-Clinical and Translational Sciences Award UL1RR024986. The Scientific Registry of Transplant Recipients is funded by contract number 231-00-0116 from the Health Resources and Services Administration (HRSA), US Department of Health and Human Services. The views expressed herein are those of the authors and not necessarily those of the US Government. This study was approved by HRSA's SRTR project officer. HRSA has determined that this study satisfies the criteria for the IRB exemption described in the “Public Benefit and Service Program” provisions of 45 CFR 46.101(b)(5) and HRSA Circular 03.
Abbreviations
- CMS
Centers for Medicare & Medicaid Services
- DCD
Donation after Cardiac Death
- ESRD
End-Stage Renal Disease
- HR
Hazard ratio
- LT
Liver Transplantation
- MELD
Model for End-Stage Liver Disease
- RRT
Renal replacement therapy
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
Disclosure:
This research was presented, in part, as a free communication at the American Transplant Congress, 2010, held in San Diego, California.
References
- 1.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Schaubel DE, Sima CS, Merion RM, Lok AS. Re-weighting the model for end-stage liver disease score components. Gastroenterology. 2008;135:1575–1581. doi: 10.1053/j.gastro.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Gonwa TA, McBride MA, Anderson K, Mai ML, Wadei H, Ahsan N. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant. 2006;6:2651–2659. doi: 10.1111/j.1600-6143.2006.01526.x. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, Welch K, Eikstadt R, Marrero JA, Fontana RJ, Lok AS. Renal outcomes after liver transplantation in the model for end-stage liver disease era. Liver Transpl. 2009;15:1142–1148. doi: 10.1002/lt.21821. [DOI] [PubMed] [Google Scholar]
- 5.Levine GN, McCullough KP, Rodgers AM, Dickinson DM, Ashby VB, Schaubel DE. Analytical methods and database design: implications for transplant researchers, 2005. Am J Transplant. 2006;6:1228–1242. doi: 10.1111/j.1600-6143.2006.01277.x. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson DM, Bryant PC, Williams MC, et al. Transplant data: sources, collection, and caveats. Am J Transplant. 2004;4(Suppl 9):13–26. doi: 10.1111/j.1600-6135.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 7.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2nd ed. New York: Wiley; 2002. [Google Scholar]
- 8.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 9.A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. The U.S. Multicenter FK506 Liver Study Group. N Engl J Med. 1994;331:1110–1115. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 10.Gonwa TA, Klintmalm GB, Levy M, Jennings LS, Goldstein RM, Husberg BS. Impact of pretransplant renal function on survival after liver transplantation. Transplantation. 1995;59:361–365. [PubMed] [Google Scholar]
- 11.Nair S, Verma S, Thuluvath PJ. Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology. 2002;35:1179–1185. doi: 10.1053/jhep.2002.33160. [DOI] [PubMed] [Google Scholar]
- 12.Campbell MS, Kotlyar DS, Brensinger CM, et al. Renal function after orthotopic liver transplantation is predicted by duration of pretransplantation creatinine elevation. Liver Transpl. 2005;11:1048–1055. doi: 10.1002/lt.20445. [DOI] [PubMed] [Google Scholar]
- 13.Bahirwani R, Campbell MS, Siropaides T, et al. Transplantation: impact of pretransplant renal insufficiency. Liver Transpl. 2008;14:665–671. doi: 10.1002/lt.21367. [DOI] [PubMed] [Google Scholar]
- 14.Berg CL, Steffick DE, Edwards EB, et al. Liver and intestine transplantation in the United States 1998–2007. Am J Transplant. 2009;9:907–931. doi: 10.1111/j.1600-6143.2009.02567.x. [DOI] [PubMed] [Google Scholar]
- 15.Rakela J, Vargas HE. Hepatitis C: magnitude of the problem. Liver Transpl. 2002;8:S3–S6. doi: 10.1053/jlts.2002.35855. [DOI] [PubMed] [Google Scholar]
- 16.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]