Abstract
Arteriosclerosis is characterized by the local activation of effector T cells leading to production of pro-inflammatory cytokines, such as IFN (interferon)-γ and IL-17, within the vessel wall. Conversely, the production of anti-inflammatory cytokines, e.g. TGF-β, by regulatory lymphocytes is known to inhibit both the differentiation of naïve T cells into effector T cells and the development of arteriosclerosis in murine models. We investigated the role of TGF-β on the alloreactivity of human effector memory T cells (Tem). Quiescent vascular cells, but not Tem, expressed TGF-β. Blockade of TGF-β activity in co-cultures of CD4+ Tem with allogeneic endothelial cells significantly increased IFN-γ, but not IL-17, secretion. Additionally, serologic neutralization of TGF-β in immunodeficient mouse hosts of human coronary artery grafts into which allogeneic human T cells were adoptively transferred resulted in heavier medial infiltration by Tem, greater loss of medial smooth muscle cells, and increased IFN-γ production within the grafts without significantly reducing either intimal injury or IL-17 production. Protective effects of TGF-β may be limited by fewer TGF-β-expressing vascular cells within the intimal compartment, by a reduction in the expression of TGF-β by vascular cells in rejecting grafts, or possibly to less effective suppression of Tem than naïve T cells.
Keywords: arteriosclerosis, endothelial cells, smooth muscle cells, T cells, interferon-γ
INTRODUCTION
Coronary arteriosclerosis of native and transplanted hearts is characterized by leukocytic infiltrates within the intima and adventitia, activation of immune responses, and the production of IFN (interferon)-γ (1, 2). The vessel media, comprised of densely arranged smooth muscle cells (SMC), is generally resistant to T cell infiltration. In addition to activating and recruiting more leukocytes, in part by pro-immunogenic actions on endothelial cells (EC), IFN-γ also has direct pro-arteriosclerotic effects on SMC by inducing their proliferation, most evident within the intima (3). IFN-γ is largely produced by Th1 cells, a subgroup of CD4+ lymphocytes that differentiate from naïve precursors under control of the transcription factor, T-bet (4). Other effector T cell populations develop under the control of different transcription factors, such as GATA-3-dependent Th2 cell differentiation that produce IL-4 and IL-5 or RORC-dependent Th17 cell differentiation that produce IL-17. These alternative effector cytokines may also exacerbate the arteriosclerotic process (5, 6). In contrast, a subset of regulatory T cells, marked by expression of the Foxp3 transcription factor, may produce anti-inflammatory cytokines, e.g. TGF-β and IL-10, which inhibit the development of arteriosclerosis in murine models (7-9).
TGF-β has long been known to inhibit the proliferation and activation of T cells (10) and more recently has been recognized as playing an essential role, together with IL-6 or certain other pro-inflammatory cytokines, in the differentiation of Th17 cells (11). However, the latter effect may be an indirect phenomenon as TGF-β primarily inhibits the differentiation of naïve CD4+ T cells into Th1 cells and consequently reduces the production of IFN-γ, an anti-Th17 factor (12, 13). While most rodent models of graft rejection involve the activation and differentiation of naïve T cells, human graft rejection may be dominated by the response of memory T cells. This is because in adult humans, at least half of the T cells in the circulation have previously undergone activation, differentiation, and commitment to polarized cytokine production, most likely in response to infections, and are long-lived T memory cells (14). Furthermore, memory cells, like naïve cells that are specific for microbial antigens, have a high frequency of cross reaction to allogeneic cells (15). A subset of these circulating memory T cells, termed effector memory T cells (Tem), lack the CCR7 chemokine receptor required for recirculation to lymph nodes and instead express alternative receptors that allow efficient homing to inflamed peripheral tissues where Tem rapidly activate their effector functions. Tem are likely the predominant source of effector T cells present within arteriosclerotic vessels. To date, direct effects of TGF-β on activation of Tem have not been well studied.
A challenge to investigations of TGF-β function is that its production and processing are complex. There are three types of TGF-β, viz. -β1, -β2, and -β3, that are produced by and act on a broad range of cell lineages, including leukocytes and vascular cells (16, 17). TGF-β, synthesized as a biologically inactive precursor, is processed into a smaller C-terminal peptide (constituting mature or active TGF-β) that is non-covalently bound to the larger N-terminal precursor remnant (referred to as latency-associated peptide or LAP). Binding to LAP masks the exposure of receptor-interacting determinants of the mature protein and thus maintains TGF-β in a latent form (16). The shielding of active TGF-β epitopes by LAP also prevents many antibodies raised against the mature cytokine from binding to latent TGF-β. In contrast, antibodies to LAP are generally reactive with the latent TGF-β complex. Activation of latent cytokine requires conformational changes or proteolysis of LAP induced by TGF-β activators to release the mature protein (16). Active TGF-β binds to type I and II TGF-β receptors and elicits diverse responses, typically with cell type- and context-dependent effects, that play important roles in physiological and pathological processes (17, 18). However, the effects of TGF-β on human immune-mediated vascular remodeling have not been previously described. We tested the hypothesis that TGF-β expression within the vessel wall serves to inhibit arteriosclerosis mediated by alloreactive Tem.
MATERIALS AND METHODS
Arteries and Cells
Human research protocols were approved by the review boards of Yale University and the New England Organ Bank. Coronary arteries were obtained from explanted hearts of transplant recipients and donors. EC were isolated from umbilical cord veins, serially cultured, and used after 2-3 passages. Peripheral blood mononuclear cells (PBMC) were obtained by leukapheresis of healthy adult donors. T cell-EC co-cultures were performed as previously described (19, 20) and methodological details are provided as Supporting Information.
Artery Transplantation
Procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee of Yale University. Size-matched segments of human coronary arteries were interposed into the infrarenal aortae of 8-12 wk, female severe combined immunodeficient (SCID)/beige mice (Taconic, Germantown, NY) using an end-to-end microsurgical anastomotic technique. The recipients of adjacent human artery segments were paired as matched experimental vs. control animals, 4-8 mice were grafted from each donor, and data from multiple donors were pooled to generate sufficient numbers for analysis. Where indicated, the hosts received an adoptive transfer of human PBMC (allogeneic to the artery graft). Certain recipients were treated with neutralizing antibody to all forms of TGF-β (cat. #AB-100-NA, R&D Systems, Minneapolis, MN) or non-immunized rabbit IgG (cat. #AB-105-C, R&D Systems).
Cell and Graft Analyses
Protein and mRNA expression were determined as previously described (20, 21) and details of the assays are provided as Supporting Information.
Statistical Analysis
Data were analyzed using Prism 4 software (GraphPad, San Diego, CA). Comparisons between two groups were by t test and between more than two groups were by ANOVA. All P values were two-tailed and values <0.05 were considered to indicate statistical significance.
RESULTS
TGF-β inhibits IFN-γ production by alloreactive CD4+ Tem in vitro
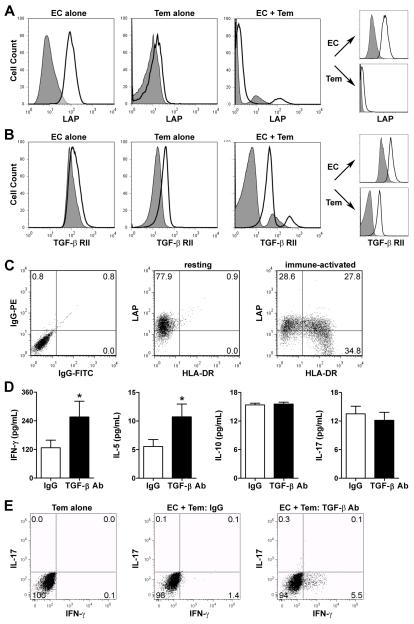
To investigate the effects of TGF-β on the activation of memory T cells, we used a cell co-culture model in which human CD4+ Tem are activated by allogeneic EC that have been pretreated with IFN-γ to re-induce the expression of MHC class II molecules that are down-regulated in vitro (19). The surface expression of TGF-β, as indicated by LAP immunoreactivity, was far greater on EC than on CD4+ Tem when cultured alone and remained unchanged in co-culture at 24 hr (Fig. 1A), a time point prior to EC cytolysis by alloreactive T cells in this system (EC >90% annexin V−/7-AAD−). Similar results were seen after cell permeabilization and with a polyclonal antibody that recognizes both latent and free TGF-β (data not shown). In contrast, the expression of TGF-βRII, necessary for responses to TGF-β, was equally detected on EC and CD4+ Tem when cultured alone and increased on both cell types during co-culture (Fig. 1B).
Figure 1. TGF-β inhibits IFN-γ production by alloreactive CD4+ Tem in vitro.
(A) Flow cytometric analysis of LAP surface expression on IFN-γ-pretreated EC and CD4+ Tem cultured alone or together in serum-free medium for 24 hr. Discrete cell populations from the co-cultures were also individually gated to analyze expression by EC and CD4+ Tem separately (right panels). (B) TGF-β RII surface expression on these cells. (C) LAP and HLA-DR surface expression of untreated EC placed in transwell inserts above co-cultures of CD4+ Tem with either untreated EC (resting environment) or IFN-γ-pretreated EC (immune-activated environment) for 3 d. (D) IFN-γ-pretreated EC were co-cultured with CD4+ Tem in the presence of LAP-β1 and TGF-β1/-β2/-β3 neutralizing antibodies at 10 μg/mL each or irrelevant IgG1 at 20 μg/mL for 24 hr. Cytokine supernatant levels (n=16 from 3 donors) were measured by ELISA. *P<0.05, LAP+TGF-β Ab vs. IgG, paired t test. (E) Alternatively, the co-cultured cells were incubated for a further 30 min with brefeldin A and PMA/ionomycin and then intracellular cytokine staining was performed for IFN-γ and IL-17. Dot plots show % positive cells in each quadrant and flow cytometry data are representative of 3 experiments.
We investigated longer term effects of alloresponses on TGF-β expression by placing untreated EC across a semipermeable membrane from EC/CD4+ Tem co-cultures, a strategy that avoids EC death from contact-dependent cytolytic effector molecules. Some of the EC in the upper chamber show evidence of immune activation as indicated by induction of HLA-DR expression, most likely in response to IFN-γ produced by alloactivated CD4+ Tem in the lower chamber. After 3 d, LAP expression decreased in a subpopulation of these immune-activated EC (Fig. 1C). LAP expression was further diminished on EC within transwell inserts at 7 d, but was not detectable on CD4+ Tem in co-culture at this stage when distinct alloreactive T cell populations have expanded and can be distinguished by proliferation markers (data not shown).
We next investigated if EC synthesis of TGF-β has an effect on Tem activation. To do so, we measured the levels of signature cytokines for several T cell lineages by ELISA at 24 h of EC/Tem co-culture, a time point that precedes detectable T cell proliferation in this system (19). Neutralization of TGF-β activity with blocking antibodies during co-culture increased the secretion of IFN-γ and IL-5, but not IL-10 or IL-17 (Fig. 1D). The increased production of IFN-γ by individual cells was confirmed by intracellular cytokine staining (Fig. 1E). Although human SMC also express LAP, we did not investigate the role of TGF-β in SMC/Tem co-cultures since SMC do not activate T cell alloresponses as they lack essential costimulator molecules required to act as APC (20). These results obtained in vitro indicate that TGF-β inhibits the activation of IFN-γ-producing, but not IL-17-producing, alloreactive CD4+ Tem by EC, a significant extension of its known regulatory role in naïve T cell differentiation. They also predict that this effect may be transient because activated Tem produce soluble mediators that diminish the expression of TGF-β by EC.
Neutralization of TGF-β in humanized mice
To determine if the effects of TGF-β on Tem alloresponses are of relevance in vivo, we used an experimental model of human coronary artery rejection by allogeneic human memory CD45RO+ T cells in immunodeficient mouse hosts (21). Human naïve CD45RA+ T cells do not circulate in these animals after adoptive transfer of PBMC (22). Moreover, these animals lack functional lymph nodes where human naïve or central memory cells can be activated and rejection of allogeneic skin grafts has been shown to be mediated solely by recruitment and activation of Tem (19). We initially established that SCID/beige mice possessed circulating TGF-β (mouse cytokine is cross-reactive with human receptors) whose plasma levels were not modulated by human coronary artery grafts or adoptive transfer of human PBMC (Fig. S1A). Subcutaneous injection of a neutralizing polyclonal antibody to TGF-β at 250 μg, a dose previously shown to be effective for this particular antibody in a murine aortic aneurysm model (23), decreased plasma concentrations of cytokine within 6 hr and this effect persisted up to 96 hr (Fig. S1B). We therefore administered this dose of TGF-β antibody 3x per wk to SCID/beige mouse recipients of human coronary artery grafts starting the day before the adoptive transfer of human PBMC at 1 wk post-op and continuing to 5 wk post-op (Fig. S1C). Treatment with TGF-β antibody vs. IgG administration did not modulate the frequency of circulating human CD3+ T cells to mouse CD45+ leukocytes in SCID/beige mice at 2 wk (5.3±1.9% vs. 4.0±1.2%, respectively; P=0.5580; unpaired t test) and 4 wk (8.0±3.7% vs. 6.4±3.0%, respectively; P=0.7403; unpaired t test) after PBMC inoculation. TGF-β neutralization did result in a trend to higher plasma levels of human IFN-γ and significantly lower plasma levels of TGF-β (Fig. S1D). In the absence of allogeneic PBMC, anti-TGF-β treatment did not detectably alter the morphology or cellular composition of non-rejecting human artery grafts (Fig. S2A-E). These observations confirmed the efficacy and minimized possible confounding effects of TGF-β neutralization in our humanized mouse model.
TGF-β inhibits medial infiltration and SMC loss in rejecting human artery grafts
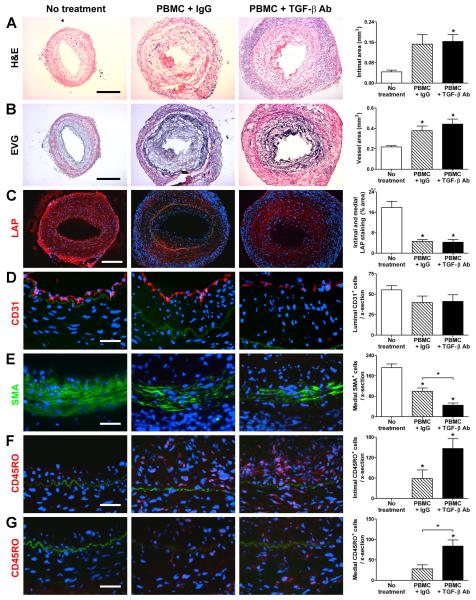
We next examined the effects of neutralizing TGF-β on the allogeneic Tem response to graft arteries. Contrary to expectation, administration of TGF-β antibodies for 4 wk to artery-grafted animals reconstituted with allogeneic human PBMC at 1 wk post-op did not affect intimal expansion or outward remodeling of rejecting coronary arteries (Fig. 2A, B). Further analysis by immunofluorescence microscopy showed decreased LAP expression by vascular cells in rejecting grafts compared to the basal expression in non-rejecting grafts (Fig. 2C). This finding implies that infiltrating T cells reduced endogenous TGF-β production by vascular cells and may explain why neutralizing this cytokine did not augment the intimal response. The number of luminal EC was unaffected by anti-TGF-β treatment (Fig. 2D). However, serological neutralization of TGF-β was not without effect; there were significantly fewer medial SMC in rejecting grafts compared to arteries from mice that did not receive PBMC and the loss of SMC was augmented by anti-TGF-β treatment (Fig. 2E). Medial loss of SMC was associated with significantly increased medial, but not intimal, infiltration by allogeneic CD45RO+ T cells (Fig. 2F, G). The effects of antibody neutralization differed in magnitude among different PBMC donors and did not appear to correlate with the efficiency with which T cells were adoptively transferred to the circulation of the recipient animal in that varying the size of the donor inoculum which altered the number of T cells in the mouse circulation, did not influence the effect of antibody treatment.
Figure 2. TGF-β inhibits medial infiltration and SMC loss in rejecting artery grafts.
Human coronary arteries were interposed into the aorta of SCID-beige mice that received an adoptive transfer of 0.5-3×108 allogeneic human PBMC (from one of 3 PBMC donors) at 1 wk post-op. The animals received no treatment (n=9 from 5 artery donors) or were treated with IgG (n=17 from 9 artery donors) or TGF-β antibody (n=17 from 9 artery donors) at 250 μg s.c., 3x per wk, from 1 to 5 wk post-op. Graft sections were stained with (A) H&E or (B) EVG at 5 wk post-op and representative photomicrographs are shown (bar, 300 μm). Intima and total vessel areas were calculated from EVG-stained graft sections. The artery grafts were also analyzed by immunofluorescence microscopy using (C) PE-labeled anti-LAP (red color), (D) PE-labeled anti-CD31 (red color), (E) FITC-labeled anti-smooth muscle α-actin (SMA) (green color), and (F, G) PE-labeled anti-CD45RO (red color); the individual panels in (F) focus on the intima while those in (G) better illustrate the media. The internal elastic lamina is visible due to auto-fluorescence (green color). Representative photomicrographs are shown at lower magnification for LAP (bar, 200 μm) and at higher magnification (bar, 50 μm) for the cell lineage markers. The images were quantified as % positively-staining intima and media area for LAP or the number of CD31+ luminal cells, SMA+ medial cells, CD45RO+ intimal cells, and CD45RO+ medial cells were counted per cross (x)-section of grafts. *P<0.05, TGF-β Ab or IgG vs. No treatment,P<0.05, TGF-β Ab vs. IgG, one-way ANOVA.
TGF-β inhibits IFN-γ production in rejecting human artery grafts
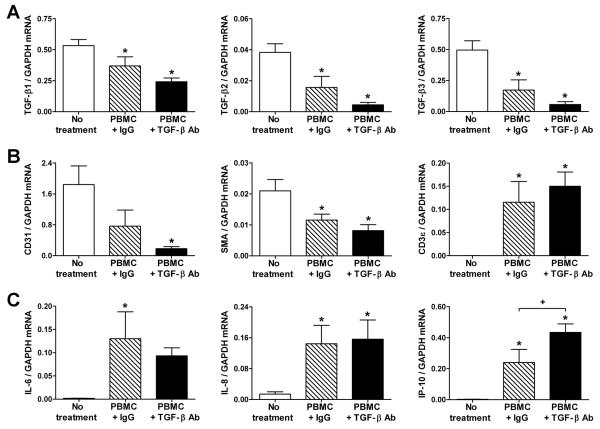
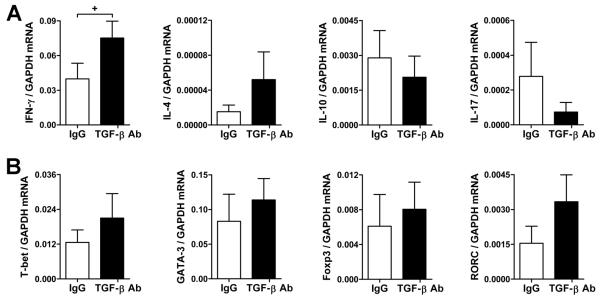
We further analyzed the artery grafts at 5 wk post-op (or 4 wk after adoptive transfer of PBMC) by quantitative RT-PCR. In parallel with changes in LAP protein expression, TGF-β transcripts were decreased in arteries from PBMC-reconstituted mice (Fig. 3A). This decrease was even more profound in animals treated with anti-TGF-β antibody. The expression of CD31, smooth muscle α-actin, and CD3ε cell lineage markers suggested a loss of EC and SMC and an accumulation of T cells in rejecting arteries, respectively (Fig. 3B). The greater attrition of vascular cells correlated with the further diminished expression of TGF-β. Other inflammatory factors produced by vascular cells in response to alloreactive T cells were also examined. The presence of T cells increased IL-6 and IL-8 mRNA in all rejecting arteries unaffected by antibody treatment, but IFN-γ-inducible IP-10 transcripts were significantly higher in grafts from anti-TGF-β-treated subjects (Fig. 3C). Further analysis of T cell cytokines and the transcription factors controlling their production revealed that IFN-γ mRNA expression was also significantly increased in grafts subjected to TGF-β neutralization (Fig. 4A). Changes in the expression of other cytokines and their associated transcription factors did not reach statistical significance, although the abundance of IL-4 and IL-17 mRNA was relatively low and their quantitation was less reliable (Fig. 4A, B). T cell-derived cytokine transcripts were not detected in grafts from hosts that did not receive human T cells (data not shown). Similar findings, including increased IFN-γ transcripts in TGF-β antibody-treated reconstituted hosts, were seen earlier in rejection responses when the artery grafts were analyzed at 3 wk post-op (or 2 wk after PBMC inoculation) (Fig. 5). Additional analysis of the intra-graft cytokine transcript data pooled from all the experiments confirmed that TGF-β neutralization significantly modulated the expression of IFN-γ mRNA, but not that of IL-4, IL-10, or IL-17 (Table S1). Thus, the data from several independent experimental groups based on multiple allogeneic combinations involving 12 artery donors and 4 PBMC donors demonstrate that TGF-β selectively inhibits IFN-γ production by alloreactive memory T cells in vivo.
Figure 3. TGF-β expression is diminished in rejecting artery grafts.
SCID-beige mouse recipients of human coronary artery grafts (n=9 from 5 artery donors) received 1.5×108 allogeneic human PBMC (from one of 2 PBMC donors) at 1 wk post-op and were treated with IgG or TGF-β antibody at 250 μg s.c., 3x per wk, from 1 to 5 wk post-op. The artery grafts were procured at 5 wk post-op and the abundance of transcripts for (A) TGF-β1, TGF-β2, TGF-β3, (B) CD31, SMA, CD3ε, (C) IL-6, IL-8, and IP-10 normalized to GAPDH were measured by quantitative RT-PCR. *P<0.05, TGF-β Ab or IgG vs. No treatment, +P<0.05, TGF-β Ab vs. IgG, one-way ANOVA.
Figure 4. TGF-β inhibits IFN-γ production in rejecting artery grafts.
SCID-beige mouse recipients of human coronary artery grafts (n=17 from 9 artery donors) received 0.5-3×108 allogeneic human PBMC (from one of 3 PBMC donors) at 1 wk post-op and were treated with IgG or TGF-β antibody at 250 μg s.c., 3x per wk, from 1 to5 wk post-op. The artery grafts were procured at 5 wk post-op and the abundance of T cell-associated transcripts for (A) IFN-γ, IL-4, IL-10, IL-17, (B) T-bet, GATA-3, Foxp3, and RORC normalized to GAPDH were measured by quantitative RT-PCR. +P<0.05, TGF-β Ab vs. IgG, paired t test.
Figure 5. TGF-β inhibits IFN-γ production in early rejecting artery grafts.
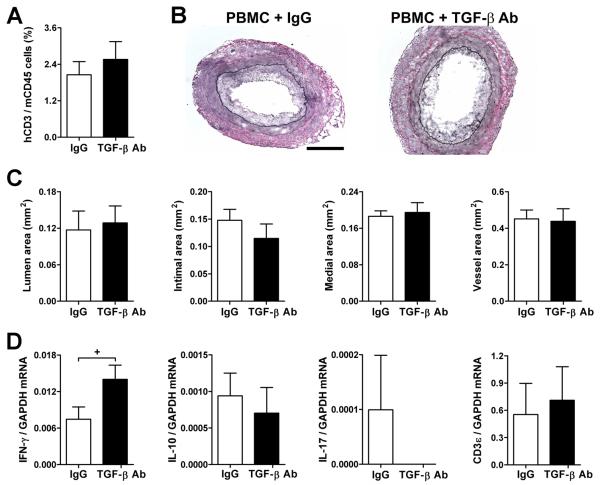
SCID-beige mouse recipients of human coronary artery grafts (n=6 from 3 artery donors) received 3×108 allogeneic human PBMC (from a different PBMC donor to those in Fig. 2) at 1 wk post-op and were treated with IgG or TGF-β antibody at 125 μg s.c., 3x per wk, from 1 to3 wk post-op. (A) Frequency of human CD3+ vs. mouse CD45+ circulating cells at 2 wk post-op (or 1 wk after PBMC inoculation). (B) Representative photomicrographs of EVG-stained graft sections at 3 wk post-op (bar, 300 μm). (C) Lumen, intima, media, and total vessel areas were calculated from EVG-stained graft sections. (D) IFN-γ, IL-10, IL-17, and CD3ε transcripts normalized to GAPDH were measured in grafts at 3 wk post-op by quantitative RT-PCR (IL-4 mRNA was undetectable). *P<0.05, TGF-β Ab vs. IgG, paired t test.
DISCUSSION
The present study, using human cells and tissues, reveals several new findings regarding the role of TGF-β in the pathogenesis of arteriosclerosis. First, we observed that there is basal expression of TGF-β by quiescent vascular cells and that this expression is diminished in artery grafts as a consequence of alloimmune injury. Second, TGF-β prevents medial infiltration and T cell-mediated loss of SMC without significantly affecting inflammation and remodeling of other vascular compartments. Third, TGF-β inhibits IFN-γ production by alloreactive CD4+ Tem cells in vitro and IFN-γ production by alloactivated memory T cells in vivo.
The expression of TGF-β has been reported by the majority of previous studies as low in non-diseased arteries and increased in vascular cells and infiltrating leukocytes after arterial injury or inflammation (24-27). These observations supported the interpretation that TGF-β was a pro-arteriosclerotic factor due to its pro-fibrotic properties. An alternative hypothesis was subsequently proposed that TGF-β may play an anti-arteriosclerotic role (28). This latter interpretation has been supported by serologic neutralization of TGF-β in hyperlipidemic mice (29) and by genetic manipulations in mice in which a loss of TGF-β signaling in T cells or a partial deficiency of TGF-β in leukocytes increased the extent of atherosclerosis and graft arteriosclerosis (7-9). Furthermore, a few prior studies have reported a higher expression of TGF-β by vascular cells of non-diseased than of diseased arteries in keeping with a protective function of TGF-β that may diminish with disease progression (30-32). Our results obtained by analyzing human cells and tissues in immunodeficient mouse hosts demonstrate significant TGF-β expression by quiescent vascular cells of human coronary arteries that decreases with arterial inflammation. Parallel changes in all three forms of TGF-β transcripts suggest that the loss of TGF-β protein expression after immune-mediated injury is due to decreased cytokine production rather than primary loss of sequestered cytokine from the extracellular matrix. We also found that the surface expression of TGF-β is decreased on immune-activated EC in vitro. Similarly, we found decreased expression of TGF-β mRNA and protein in clinical specimens of atherosclerotic coronary arteries, in cultured coronary arteries after procurement injury, and in activated SMC (S.K., A.H.L., ms. submitted). The relative absence of TGF-β expression by leukocytes in our models may reflect a bias towards analysis of memory Th1 cells rather than their regulatory counterparts. For example, the adoptive transfer of human PBMC to SCID/beige mice results in exclusive reconstitution by CD45RO+ memory T cells, whereas naïve T cells, B cells, NK cells, and macrophages are not detected in the circulation (22). Suppressive cell-mediated activity in this system has been reported only after reconstitution with large numbers of ex vivo-expanded regulatory T cell populations (33). Even for in vitro settings, TGF-β production in response to antigen recognition is not readily detectable unless particular regulatory T cell populations are selected (34). Our results do not rule out TGF-β expression by rare T cell subtypes or by T cell populations that are not adequately represented in our systems. The source of circulating murine TGF-β1 in unreconstituted SCID/beige mice is independent of T cells in these immunodeficient animals and likely derives from a broad range of non-immune cells, such as vascular cells and hepatocytes (35).
The distribution of resident and infiltrating leukocytes in the arterial wall is uneven. In clinical specimens of atherosclerosis and graft arteriosclerosis, the majority of inflammatory cells accumulate within the intima and adventitia (36, 37). The relative sparing of the media by immune responses is regarded as a sign of immune privilege (38). The mechanism for medial immunoprivilege is speculated to result from mechanical barriers to leukocyte trafficking provided by the elastic laminae delineating this vascular compartment. We have also determined biological processes responsible for medial immunoprivilege, such as the immunosuppressive effects of tryptophan depletion within the microenvironment resulting from the preferential expression of indoleamine 2,3-dioxygenase by SMC in response to T cell-derived IFN-γ (39). Our current data demonstrate that the expression of TGF-β by SMC provides an additional mechanism to suppress T cell responses within the media. Since TGF-β expression by SMC decreases during allograft rejection, this cytokine may serve to maintain medial immunoprivilege against resident leukocytes of quiescent arteries, whereas the IFN-γ-inducible expression of indoleamine 2,3-dioxygenase may play a more prominent role in medial immunoprivilege to infiltrating leukocytes of inflamed arteries. We are unsure by which of multiple possible mechanisms TGF-β acts to reduce the accumulation of alloreactive memory T cells within the arterial media, i.e. reduced recruitment, retention, proliferation, or survival (10, 16). In our model of human artery graft rejection, the expanded neointima is largely populated by infiltrating human T cells and there are few SMC. The limited number and the activated status of SMC within the neointima may account for the lack of significant protective effects of TGF-β within this compartment. Although we show that cultured EC express TGF-β which partially suppress IFN-γ production by alloreactive CD4+ Tem cells, these non-professional antigen presenting cells also express numerous costimulatory molecules which enables activation of memory T cells (19). In contrast, the absence of essential costimulatory molecules by SMC underlies their inability to activate allogeneic T cells (20) and uncovers a dominant role on immune responses by anti-inflammatory molecules expressed by this cell type. An important consideration is that our humanized mouse system of allogeneic arterial injury occurs in the absence of concomitant immunosuppression - a clinically relevant factor, and thus may more mimic acute arteritis rather than chronic graft rejection.
We find that TGF-β inhibits the production of IFN-γ by isolated CD4+ Tem in vitro and by CD45RO+ memory T cells in vivo. The levels of IFN-γ production varied between donors and reflected differences in the degree of host reconstitution and graft infiltration by human T cells, but may also be a function of variable degrees of HLA-mismatches between human arteries and allogeneic T cells. It is unlikely that mouse cytokines play a role in our model. Historically, we have repeatedly shown that mouse leukocytes do not infiltrate human arteries in unreconstituted SCID/beige mice and that graft rejection is dependent on the introduction of human T cells (21, 39). Variable numbers of mouse leukocytes may be non-specifically recruited to rejecting human artery grafts in human PBMC-reconstituted hosts, however the vast majority of the infiltrating cells are human CD45RO+ T cells and the probes used to measure transcripts are specific for human sequences and do not detect mouse homologues, including that for cytokines and housekeeping genes (40). Previous studies in murine models of atherosclerosis and graft arteriosclerosis have shown that IFN-γ production is inhibited by loss of TGF-β signaling (7-9), likely due to effects on the differentiation of naïve T cells as laboratory mice, unlike humans, have few circulating memory T cells. IL-17 production was not assessed in those studies as they predated the concept of Th17 immune responses. More recent reports have determined that TGF-β inhibits Th1 development by inhibition of critical transcription factors, such as T-bet, and that loss of the anti-Th17 factor, IFN-γ enhances IL-17 production (12, 13). These observations suggest that TGF-β exerts its primary effect on Th1 cells and is not directly required for the generation of Th17 cells as previously thought (11). Our analyses in humanized mice suggest that TGF-β can also modulate the cytokine polarization of memory T cells without changes in T-bet expression. We find a trend to diminished IL-17 production after TGF-β neutralization in vivo, but these changes were variable, perhaps due to relatively low abundance levels, and did not reach statistical significance. The modest effects of TGF-β on Th17 responses in our model are consistent with indirect regulation of IL-17 production via IFN-γ (12, 13) or possibly suppression of only a subset of TGF-β-dependent, non-pathogenic, IL-17-producing RORC+/T-bet− T cells (41). Interestingly, a loss of TGF-β expression by vascular cells of inflamed arteries may explain our previous finding of the selective expansion of dual IL-17/IFN-γ-producing vs. IL-17-producing CD4+ T cells within atherosclerotic coronary arteries compared to the circulation (6) by a TGF-β-independent pathway required for the generation of this pathogenic Th17 cell subset (41).
In summary, our data support a protective role for TGF-β in human graft arteriosclerosis, especially within the vessel media where SMC are most abundant. Although our serologic neutralization strategy prevents definitive conclusions regarding the cell types producing or responding to TGF-β, the results are strongly suggestive that human vascular cell expression of TGF-β inhibits activation of Tem that are committed to Th1 cell effector functions. Further proof of this hypothesis will require the testing of specific TGF-β antagonists in suitable patient populations, such as those receiving immunotherapy for cancer.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the NIH (PO1 HL70295).
Nonstandard abbreviations
- EC
endothelial cell
- IFN
interferon
- LAP
latency-associated peptide
- PBMC
peripheral blood mononuclear cells
- SCID
severe combined immunodeficient
- SMC
smooth muscle cell
- Tem
effector memory T cell
Footnotes
DISCLOSURES
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Pober JS. Chronic rejection. Immunity. 2001;14:387–397. doi: 10.1016/s1074-7613(01)00119-4. [DOI] [PubMed] [Google Scholar]
- 3.Tellides G, Pober JS. Interferon-gamma axis in graft arteriosclerosis. Circ Res. 2007;100:622–632. doi: 10.1161/01.RES.0000258861.72279.29. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2003;163:1117–1125. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, et al. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson AK, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. Disruption of TGF-beta signaling in T cells accelerates atherosclerosis. J Clin Invest. 2003;112:1342–1350. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gojova A, Brun V, Esposito B, Cottrez F, Gourdy P, Ardouin P, et al. Specific abrogation of transforming growth factor-beta signaling in T cells alters atherosclerotic lesion size and composition in mice. Blood. 2003;102:4052–4058. doi: 10.1182/blood-2003-05-1729. [DOI] [PubMed] [Google Scholar]
- 9.Koglin J, Glysing-Jensen T, Räisänen-Sokolowski A, Russell ME. Immune sources of transforming growth factor-beta1 reduce transplant arteriosclerosis: insight derived from a knockout mouse model. Circ Res. 1998;83:652–660. doi: 10.1161/01.res.83.6.652. [DOI] [PubMed] [Google Scholar]
- 10.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 11.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 12.Santarlasci V, Maggi L, Capone M, Frosali F, Querci V, De Palma R, et al. TGF-beta indirectly favors the development of human Th17 cells by inhibiting Th1 cells. Eur J Immunol. 2009;39:207–215. doi: 10.1002/eji.200838748. [DOI] [PubMed] [Google Scholar]
- 13.Das J, Ren G, Zhang L, Roberts AI, Zhao X, Bothwell AL, et al. Transforming growth factor beta is dispensable for the molecular orchestration of Th17 cell differentiation. J Exp Med. 2009;206:2407–2416. doi: 10.1084/jem.20082286. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 15.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267–2275. [PubMed] [Google Scholar]
- 16.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ten Dijke P, Arthur HM. Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8:857–869. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 18.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 19.Shiao SL, Kirkiles-Smith NC, Shepherd BR, McNiff JM, Carr EJ, Pober JS. Human effector memory CD4+ T cells directly recognize allogeneic endothelial cells in vitro and in vivo. J Immunol. 2007;179:4397–4404. doi: 10.4049/jimmunol.179.7.4397. [DOI] [PubMed] [Google Scholar]
- 20.Zhang P, Manes TD, Pober JS, Tellides G. Human vascular smooth muscle cells lack essential costimulatory molecules to activate allogeneic memory T cells. Arterioscler Thromb Vasc Biol. 2010;30:1795–1801. doi: 10.1161/ATVBAHA.109.200758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Burns WR, Tang PC, Yi T, Schechner JS, Zerwes HG, et al. Interferon-gamma plays a nonredundant role in mediating T cell-dependent outward vascular remodeling of allogeneic human coronary arteries. FASEB J. 2004;18:606–608. doi: 10.1096/fj.03-0840fje. [DOI] [PubMed] [Google Scholar]
- 22.Tereb DA, Kirkiles-Smith NC, Kim RW, Wang Y, Rudic RD, Schechner JS, et al. Human T cells infiltrate and injure pig coronary artery grafts with activated but not quiescent endothelium in immunodeficient mouse hosts. Transplantation. 2001;71:1622–1630. doi: 10.1097/00007890-200106150-00023. [DOI] [PubMed] [Google Scholar]
- 23.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majesky MW, Lindner V, Twardzik DR, Schwartz SM, Reidy MA. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991;88:904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikol S, Isner JM, Pickering JG, Kearney M, Leclerc G, Weir L. Expression of transforming growth factor-beta 1 is increased in human vascular restenosis lesions. J Clin Invest. 1992;90:1582–1592. doi: 10.1172/JCI116027. 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahadori L, Milder J, Gold L, Botney M. Active macrophage-associated TGF-beta co-localizes with type I procollagen gene expression in atherosclerotic human pulmonary arteries. Am J Pathol. 1995;146:1140–1149. [PMC free article] [PubMed] [Google Scholar]
- 27.Bobik A, Agrotis A, Kanellakis P, Dilley R, Krushinsky A, Smirnov V, et al. Distinct patterns of transforming growth factor-beta isoform and receptor expression in human atherosclerotic lesions. Colocalization implicates TGF-beta in fibrofatty lesion development. Circulation. 1999;99:2883–2891. doi: 10.1161/01.cir.99.22.2883. [DOI] [PubMed] [Google Scholar]
- 28.Metcalfe JC, Grainger DJ. Transforming growth factor-beta and the protection from cardiovascular injury hypothesis. Biochem Soc Trans. 1995;23:403–406. doi: 10.1042/bst0230403. [DOI] [PubMed] [Google Scholar]
- 29.Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamaté C, Merval R, et al. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res. 2001;89:930–934. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]
- 30.Grainger DJ, Kemp PR, Liu AC, Lawn RM, Metcalfe JC. Activation of transforming growth factor-beta is inhibited in transgenic apolipoprotein(a) mice. Nature. 1994;370:460–462. doi: 10.1038/370460a0. [DOI] [PubMed] [Google Scholar]
- 31.Borkowski P, Robinson MJ, Kusiak JW, Borkowski A, Brathwaite C, Mergner WJ. Studies on TGF-beta 1 gene expression in the intima of the human aorta in regions with high and low probability of developing atherosclerotic lesions. Mod Pathol. 1995;8:478–482. [PubMed] [Google Scholar]
- 32.Xie JJ, Wang J, Tang TT, Chen J, Gao XL, Yuan J, et al. The Th17/Treg functional imbalance during atherogenesis in ApoE(−/−) mice. Cytokine. 2010;49:185–193. doi: 10.1016/j.cyto.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Nadig SN, Wieckiewicz J, Wu DC, Warnecke G, Zhang W, Luo S, et al. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. 2010;16:809–813. doi: 10.1038/nm.2154. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longenecker G, Thyagarajan T, Nagineni CN, Flanders KC, Factor V, Miller G, et al. Endocrine expression of the active form of TGF-beta1 in the TGF-beta1 null mice fails to ameliorate lethal phenotype. Cytokine. 2002;18:43–50. doi: 10.1006/cyto.2002.1025. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe M, Sangawa A, Sasaki Y, Yamashita M, Tanaka-Shintani M, Shintaku M, et al. Distribution of inflammatory cells in adventitia changed with advancing atherosclerosis of human coronary artery. J Atheroscler Thromb. 2007;14:325–331. doi: 10.5551/jat.e489. [DOI] [PubMed] [Google Scholar]
- 37.van Loosdregt J, van Oosterhout MF, Bruggink AH, van Wichen DF, van Kuik J, de Koning E, et al. The chemokine and chemokine receptor profile of infiltrating cells in the wall of arteries with cardiac allograft vasculopathy is indicative of a memory T-helper 1 response. Circulation. 2006;114:1599–1607. doi: 10.1161/CIRCULATIONAHA.105.597526. [DOI] [PubMed] [Google Scholar]
- 38.Dal Canto AJ, Swanson PE, O’Guin AK, Speck SH, Virgin HW. IFN-gamma action in the media of the great elastic arteries, a novel immunoprivileged site. J Clin Invest. 2001;107:R15–22. doi: 10.1172/JCI11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuffy MC, Silverio AM, Qin L, Wang Y, Eid R, Brandacher G, et al. Induction of indoleamine 2,3-dioxygenase in vascular smooth muscle cells by interferon-gamma contributes to medial immunoprivilege. J Immunol. 2007;179:5246–5254. doi: 10.4049/jimmunol.179.8.5246. [DOI] [PubMed] [Google Scholar]
- 40.Ahmad U, Ali R, Lebastchi AH, Qin L, Lo SF, Yakimov AO, et al. IFN-gamma primes intact human coronary arteries and cultured coronary smooth muscle cells to double-stranded RNA- and self-RNA-induced inflammatory responses by upregulating TLR3 and melanoma differentiation-associated gene 5. J Immunol. 2010;185:1283–1294. doi: 10.4049/jimmunol.0902283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.