Abstract
Cortical development involves synaptic formation and elimination. While synaptogenesis predominates earlier and pruning later, the two processes are thought to happen concurrently. Since in adults synaptic strength is modulated by behavioral state, we asked if synaptic remodeling may be affected by sleep and wake. Using two-photon microscopy in adolescent mice, we found that wake results in a net increase in cortical spines, whereas sleep is associated with net spine loss.
Keywords: sleep, cortex, synapse, adolescence, pruning
The maturation of cortical circuits in many species including man is characterized by an initial overproduction of synapses, followed during adolescence by elimination mainly of excitatory synapses1. The phase of massive remodeling of neural circuits during adolescence is thought to be a sensitive period for the pathophysiology of mental disorders2. The genetic, epigenetic, and environmental factors that may lead to structural, functional and ultimately behavioral abnormalities during adolescence are being actively investigated. Among such factors, the sleep/wake cycle has been overlooked but is potentially important, because recent evidence shows that behavioral states have a profound effect on synapses, at least in the adult brain. For instance, in adult flies and rodents, synapses become larger, contain more synaptic proteins and AMPA receptors, and generate stronger excitatory currents after wake, and return to baseline values after sleep3–5. It has been suggested that net synaptic potentiation is a necessary consequence of adapting to a changing environment during wake, and that sleep may be necessary to reestablish synaptic homeostasis6. Therefore, although during development synaptogenesis and pruning can occur concurrently7, they may nevertheless be modulated by sleep and wake. Specifically, given that in the adult brain sleep favors synaptic renormalization by decreasing synaptic size and/or strength, we hypothesized that during adolescence sleep may favor synaptic pruning. In this way, sleep would help maintaining synaptic homeostasis during a period of massive synaptic remodeling.
To test this possibility, we performed two-photon repeated in vivo imaging of yellow fluorescent protein (YFP)-H-expressing mice, where one can follow the growth and retractions of cortical spines within the same neuron across hours. Adolescent mice were tested between postnatal day (P)23 and P44, an age spanning most of the adolescence period (mean ±SD, 29 days ±4, n=35). At this age YFP-H mice have already consolidated sleep/wake patterns and homeostatic sleep regulation (Fig.1a; Supplementary Methods). After skull thinning, branches of apical dendrites of layer V pyramidal neurons were identified and dendritic spines were counted manually. Animals were imaged in sensorimotor cortex twice within ~12–16hrs. We selected only animals that showed strictly comparable image quality between the sessions and consolidated sleep/wake patterns during the previous 8–12hrs (Supplementary Methods). Sleep time in mice used for imaging was estimated using video recordings of locomotor activity combined with direct visual observation. Although this method could not distinguish NREM sleep from REM sleep, it consistently estimated total sleep time with ≥90% accuracy (Fig.1a, Supplementary Fig.1) and avoided the risk of inflammation due to implant of EEG electrodes.
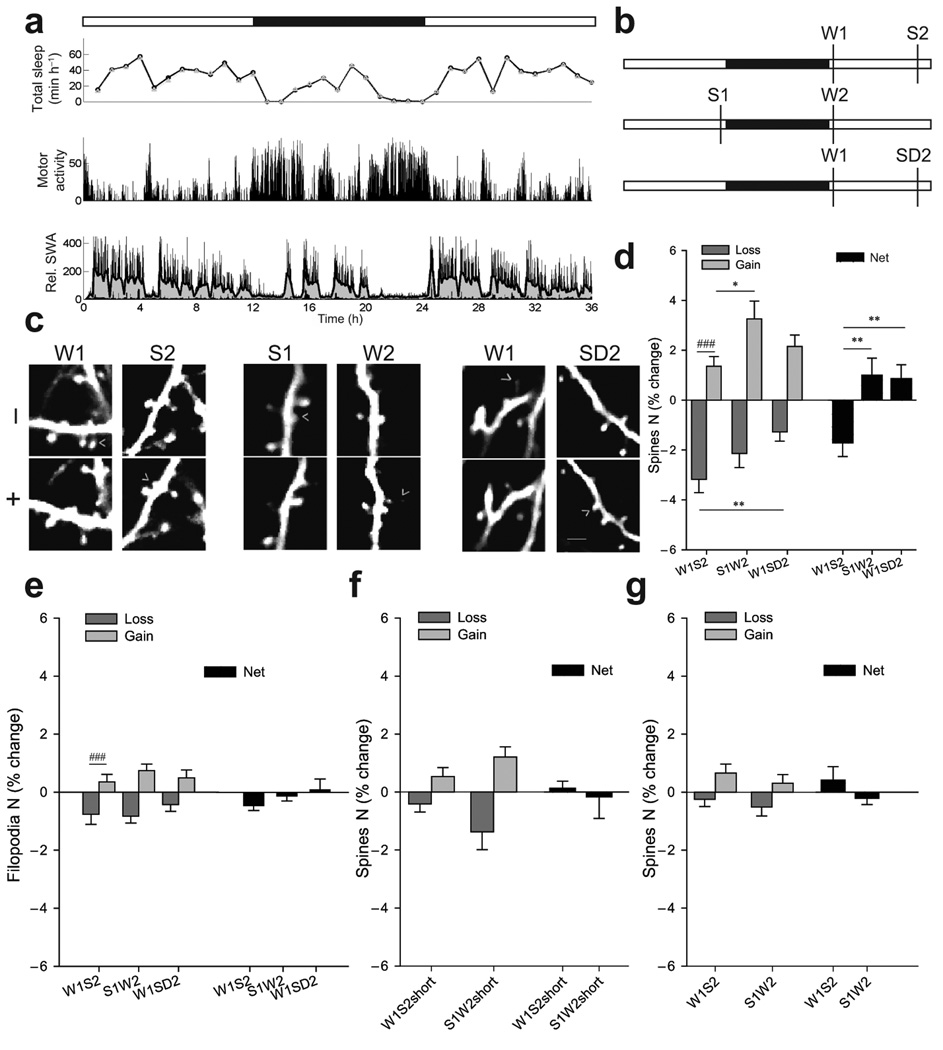
Figure 1.
(a) Sleep/wake pattern in one P30 mouse. White and black bars indicate light and dark phase, respectively. Upper panel, total sleep time based on EEG recordings (circles) or video recording of motor activity (triangles). Middle panel, motor activity detected by video recording. Lower panel, slow wave activity (SWA, 0.5–4Hz EEG power in NREM sleep, % of 24hrs), an index of NREM sleep intensity3. Gray area shows the moving average of ten 4-sec epochs. In ~P30 mice SWA is homeostatically regulated, being high at the beginning of the light phase and declining during sleep. (b) Experimental groups. (c) Repeated imaging of the same dendrite with examples of spine formation and loss (arrowheads; 1 filopodium is shown in W1). Scale bar, 6µm. (d) Spines formed and lost (% of all spines in first session) and net change (mean±SEM; intergroup, *p<0.05, **p<0.01, Kruskal-Wallis; intragroup, ### p<0.001, Wilcoxon). (e) Changes in filopodia (% of all protrusions in first session; mean±SEM; ### p<0.001, Wilcoxon). (f–g) Spine changes after short sleep/wake and in adults.
W1S2 mice (W1, wake first, followed by sleep, S2; n=11) were spontaneously awake for ≥75% of the previous night, imaged shortly after light onset, allowed to sleep for 6–8hrs during the day, and then imaged again (Fig.1b, Supplementary Methods). The duration of each behavioral state (6–8hrs) was chosen to mimic the physiological sleep/wake cycle, since mice were asleep for most of the 12-hour light phase, and awake for most of the night (Fig.1a). S1W2 mice (S1, sleep first, followed by wake, W2; n=11) were imaged after sleep during the day and then after mainly wake at night (Fig.1b). The number of imaged spines/mouse was 237±124 (mean±SD). Overall spine turnover (spines lost + gained, as % of all spines in first session) was similar in the two groups (W1S2=3.5%±1.9, mean±SD; S1W2=4.1%±2.4; p=0.5, Mann-Whitney) and consistent with published values of 5–10% across 48hrs8.
Most animals showed both spine gain and spine loss in the second session compared to the first (Fig.1c, left and middle). However, intragroup analysis of spine dynamics showed that, when sleep followed wake (W1S2), loss was larger than gain, and overall spine density showed a net decrease (Fig.1d). By contrast, when wake followed sleep (S1W2), spine density showed a net increase, significantly different from the net decrease observed when sleep followed wake (Fig.1d, right). Thus, although spines form and retract at all times, wake appears to favor synaptogenesis whereas sleep favors pruning. Of note, the absolute value of net spine loss in W1S2 mice did not differ from the absolute value of net spine gain in S1W2 (p=1.00, Kruskal-Wallis), consistent with an overall balance of spine density over 24hrs6.
Since spontaneous wake occurs at night and sleep occurs during the day, time of day and/or light exposure could have affected the results. Moreover, it is important to establish if a net decrease in spine density requires sleep, rather than merely the passage of time. Therefore, a third group of mice (W1SD2; wake first, followed by sleep deprivation, SD2; n=13) was imaged after wake at night and then after 6–7hrs of sleep deprivation during the day (Fig.1b). In W1SD2 mice the second imaging session occurred at the same time of day as in W1S2 mice, but after prolonged wake rather than sleep. Overall spine turnover in W1SD2 mice was 2.6%±1.7 (mean±SD), similar to W1S2 (p=0.8) and S1W2 mice (p=0.3; Kruskal-Wallis). Again, both spine gains and losses were observed (Fig. 1c, right), occurring at similar rates (Fig.1d). However, while spine loss was similar in the two wake groups (S1W2, W1SD2), it was higher after sleep than after prolonged wake for the same time of day (W1S2 vs W1SD2, Fig.1d, left). Overall, spine density showed a net increase when sleep deprivation followed wake (W1SD2), significantly different from the net decrease observed when sleep followed wake (W1S2; Fig.1d, right). In all groups spine gains and losses occurred roughly twice as frequently in stubby than in thin spines (60–70% vs 30–40%, ns), while no changes occurred in mushroom spines. Filopodia accounted for 2.6% of all dendritic protrusions, and showed higher turnover than spines (24.3%, ns across groups) but no consistent sleep/wake dependent changes (Fig.1e).
In the current study the age of the mice spanned from P23 to P44, with no difference among groups (p=0.46; Kruskal-Wallis). In mouse cerebral cortex synaptic and spine density increases between P16 and P32, then decreases around the onset of puberty9, 10. Thus, it is likely that in our youngest animals overall synaptic density was growing across days, while the opposite should have occurred in the oldest mice. Nevertheless, by plotting spine changes in each mouse as a function of age we found no indication that the effects of sleep/wake on spine density varied in early relative to late adolescence (Supplementary Figure 2).
In a different experiment the first imaging session occurred, as before, after long wake or long sleep, but mice (mean±SD, 29 days ±5, n=11) were either allowed to sleep or stayed awake for only 2–3hrs before being imaged again (W1S2short, n=6, min of sleep ±SD =206±103; S1W2short, n=5, min of wake =224±112). Both groups showed small spine gains and losses, with no net changes after short sleep or short wake (Fig.1f). Since normally mice sleep for most of the light phase, this suggests that a period of mostly sleep of 7–8hrs, close to the physiological duration of the major inactive phase in mice, may be required to bias spine turnover in favor of pruning.
Finally, two groups of adult mice (mean±SD, 90 days ±33, n=10) were selected according to the same stringent S1W2 and W1S2 criteria used for adolescent mice shown in Fig.1d. Overall spine turnover in sensorimotor cortex within ~12–16hrs was similar in the two groups (mean±SD, W1S2=0.9%±0.7, n=6; S1W2=0.8%±0.9, n=4; p=0.8, Mann-Whitney) and included both spine gains and losses. However, all changes were smaller than in adolescent mice (p=0.0002, Mann-Whitney) and were not affected by sleep and wake (Fig.1g). Thus, these results suggest that the effects of behavioral states on spine density are restricted to the developmental periods when significant changes in spine number occur, while the net changes in synaptic strength that accompany sleep and wake in the adult cortex occur without major changes in synaptic number. In adolescent mice most sleep parameters do not differ from those in adults except REM sleep duration, which decreases between P20 and P30 (mean±SD, n≥9/group, % of 24hrs, NREM, P20=31±5; P30=36±3; P41=35±3; P>60 36±3; REM, P20=12±2; P30=7±0.4; P41=7±0.6; P>60=6±1). Higher levels of REM sleep in early adolescence, however, are unlikely to account for the effects of sleep on pruning, because the latter are similar in younger and older adolescent mice (Supplementary Figure 2).
The results presented here show that behavioral state, independent of light, circadian time, and exact developmental stage during adolescence, modulates spine turnover in a manner consistent with the need for synaptic homeostasis6 (see also Supplementary Discussion). It is unknown to which extent these findings can be generalized to other cortical layers/areas. In zebrafish larvae presynaptic terminals of hypocretin neurons undergo circadian and sleep-wake dependent structural changes, the latter consistent with sleep-dependent synaptic renormalization11. Thus, at least during certain phases of development, sleep may facilitate spine elimination. These findings also suggest that sleep deprivation during adolescence may lead to changes in synaptic turnover, since it blocks sleep-related spine pruning. On the other hand, extending wake duration beyond its physiological duration (W1SD2) did not result in a further increase in spine density (Supplementary Figure 3). This is consistent with evidence showing that molecular12, biochemical13 and metabolic14 markers that are higher during spontaneous wake relative to sleep show little further increase after sustained sleep deprivation. One reason may be because after 12–16hrs the pressure to go to sleep is so high that animals cannot stay “fully” awake, and signs of “local” or global sleep become prevalent15.
Supplementary Material
Acknowledgements
The study was supported by NIH Director’s Pioneer award (to GT) and NIMH (1R01MH091326 to GT and CC).
Footnotes
Author contributions. C.C. and G.T. designed the experiments, analyzed data and wrote the paper; S.M. and U.F. performed experiments, analyzed data and contributed to the manuscript; A.B. Nelson gathered EEG pilot data.
Competing financial interests. The authors declare no competing financial interests.
References
- 1.Rakic P, Bourgeois JP, Goldman-Rakic PS. Prog Brain Res. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- 2.Paus T, Keshavan M, Giedd JN. Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 4.Liu ZW, Faraguna U, Cirelli C, Tononi G, Gao XB. J Neurosci. 2010;30:8671–8675. doi: 10.1523/JNEUROSCI.1409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bushey D, Tononi G, Cirelli C. Science. 2011;332:1576–1581. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tononi G, Cirelli C. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Hua JY, Smith SJ. Nat Neurosci. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- 8.Yang G, Pan F, Gan WB. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Felipe J, Marco P, Fairen A, Jones EG. Cereb Cortex. 1997;7:619–634. doi: 10.1093/cercor/7.7.619. [DOI] [PubMed] [Google Scholar]
- 10.Yu X, Zuo Y. Curr Opin Neurobiol. 2011;21:169–174. doi: 10.1016/j.conb.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appelbaum L, et al. Neuron. 2010;68:87–98. doi: 10.1016/j.neuron.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cirelli C, Faraguna U, Tononi G. J Neurochem. 2006;98:1632–1645. doi: 10.1111/j.1471-4159.2006.04058.x. [DOI] [PubMed] [Google Scholar]
- 13.Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G. J Neurosci. 2009;29:620–629. doi: 10.1523/JNEUROSCI.5486-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikonova EV, et al. Sleep. 2010;33:889–900. doi: 10.1093/sleep/33.7.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vyazovskiy VV, et al. Nature. 2011;472:443–447. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.