Abstract
AIM: To determine the seroprevalence of anti-hepatitis A virus (HAV) antibodies in patients with chronic liver disease (CLD) and to justify the need for hepatitis A vaccination.
METHODS: Patients (n = 119) were enrolled between July and September 2009. The diagnosis of CLD was based on the presence of viral markers for more than 6 mo. The diagnosis of liver cirrhosis was based on clinical, biochemical and radiological profiles. Patient serum was tested for anti-HAV IgG.
RESULTS: The overall anti-HAV seroprevalence was 88.2%. The aetiology of CLD was hepatitis B in 96 patients (80.7%) and hepatitis C in 23 patients (19.3%). Mean age was 44.4 ± 14 years. Patients were grouped according to age as follows: 24 (20.2%) patients in the 21-30 years age group, 22 (18.5%) in the 31-40 years age group, 31 (26.1%) in the 41-50 years age group, 23 (19.3%) in the 51-60 years age group and 19 (16.0%) patients aged greater than 60 years, with reported seroprevalences of 66.7%, 95.5%, 93.5%, 91.3% and 94.7%, respectively. There was a marked increase of seroprevalence in subjects older than 30 years (P = 0.001).
CONCLUSION: Our study demonstrated that patients aged greater than 30 years of age were likely to have natural immunity to hepatitis A. Therefore, hepatitis A vaccination may not be routinely required in this age group.
Keywords: Hepatitis A seroprevalence, Chronic viral hepatitis, Malaysia, Hepatitis A vaccination
INTRODUCTION
Hepatitis A remains a significant problem in Malaysia. Malaysia is among those countries reported to be of intermediate endemicity, along with Thailand and Sri Lanka[1-3]. The hepatitis A virus (HAV) has been reported to be the main cause of symptomatic clinical hepatitis (up to 66.4% in 1996) in the Eastern region of Peninsular Malaysia when compared to other causes of viral hepatitis[4]. Kelantan is one of the states situated in the Eastern region of Peninsular Malaysia. However, over the last 20 years, patterns of endemicity in South-East Asia have changed due to improvements in living standards, with some countries shifting from high to intermediate or intermediate to low endemicities[1,3,5-7]. The prevalence of HAV in Malaysia is expected to fall with time; this means, however, that reintroduction of the virus to a non-immune population could produce a community-level outbreak, which may lead to an increase in morbidity and mortality[2,8].
HAV super-infections in patients with underlying chronic liver disease (CLD) may lead to decompensation of the liver. Acute HAV super-infection is associated with higher morbidity and mortality than are isolated cases of acute HAV infection, leading to an increase in the likelihood of developing a fulminant liver failure[9-14]. Based on epidemiological studies of large hepatitis A outbreaks in Shanghai in the late 1980s, acute hepatitis A in patients with chronic hepatitis B has an even more severe clinical course and higher risk of death[15]. The fatality rates for acute hepatitis A were 5.6 times higher among hepatitis B surface antigen (HBsAg) carriers when compared to HBsAg-negative patients[16]. A similar scenario may also be true for super-infection of hepatitis A in chronic hepatitis C patients. An observational study conducted over a 7-year period among 432 Italian patients with chronic hepatitis C reported that amongst the 17 patients (3.9%) with acute hepatitis A super-infection, 41% progressed into fulminant hepatic failure[17]. This emphasises the need for vaccination in CLD patients without natural immunity.
In the West, hepatitis A vaccination has been recommended in all patients with CLD to prevent super-infection with HAV, which may cause high morbidity and mortality in this group of patients[18-20]. However, in countries where hepatitis A is still endemic, such as Malaysia, the utility of this vaccine must be examined, as it is possible that most patients have already acquired natural immunity[21-23].
Therefore, we aimed to determine the seroprevalence of anti-HAV antibodies in patients with CLD in our region.
MATERIALS AND METHODS
Sample population
Patients with CLD (n = 119) attending the Gastroenterology Clinic of Universiti Sains Malaysia, Kelantan between July and September 2009 were enrolled after having signed written informed consents. Diagnosis of CLD was based on the presence of HBsAg or anti-hepatitis C virus antibody (anti-HCV) in serum for a period of at least 6 mo. The underlying liver diseases were classified into either liver cirrhosis (LC) or non-cirrhotic CLD. LC was evidenced by previous ultrasonography (i.e., coarse liver architecture, nodular liver surface and blunt liver edges) as well as confirmation of hypersplenism (i.e., splenomegaly on ultrasonography with a platelet count < 100 000/mm3)[3]. Patients with underlying CLD of non-viral origin were excluded.
The patients were classified into the following five groups according to age: (1) Group A: 21 to 30 years; (2) Group B: 31 to 40 years; (3) Group C: 41 to 50 years; (4) Group D: 51 to 60 years; and (5) Group E: greater than 60 years of age. The study was approved by the local university’s Research and Ethics Committee and complied with the Declaration of Helsinki.
Detection of HAV IgG, HBV and HCV infections
Immunity towards hepatitis A was established by detection of anti-HAV IgG using commercially available immunoassay kits for anti-HAV IgG (Abbot Laboratories, Chicago, Illinois, United States) that rely on microparticle enzyme immunoassay methods. Presence of hepatitis B virus (HBV) and hepatitis C virus infection was determined by detection of HBsAg and anti-HCV antibodies, respectively.
Statistical analysis
All data analyses were carried out using SPSS statistical software (Version 12.0.1). Continuous variables were expressed as mean and standard deviation for normally distributed data while categorical variables were expressed as frequency and percentage. A chi-square (χ2) test was used to determine whether significant differences exist between two categorical variables. Results were reported as significant when P < 0.05. For multivariate analyses, a stepwise multivariate logistic regression model was employed to assess the relative importance of variables showing a significant association (P < 0.05) or any other clinically important variables in univariate analysis (P < 0.10). Results of all multivariable analyses were reported as adjusted odds ratio, 95% CI and exact P value.
RESULTS
The mean age at presentation was 44.4 ± 14 years (range 21-76 years). Males comprised the majority of the study population (62.2%). The Malay constituted the highest proportion of the study subjects (80.7%). This distribution reflects the current ethnic diversity in our population.
The aetiology of the underlying liver disease was chronic HBV infection in 96 (80.7%) patients, while chronic HCV infection was present in the remainder of the population (19.3%). The distribution of disease status was LC (14.3%), while others were in the non-cirrhotic group (Table 1).
Table 1.
Patient demographic and clinical data
| Characteristics | Values (%) |
| Mean age (yr) | 44.4 ± 14.0 |
| Male | 74 (62.2) |
| Aetiology of liver disease | |
| HBV | 96 (80.7) |
| HCV | 23 (19.3) |
| Status of liver disease | |
| Non-cirrhotic | 102 (85.7) |
| Liver cirrhosis | 17 (14.3) |
| Prevalence of IgG HAV | 105/119 (88.2) |
HBV: Hepatitis B virus; HCV: Hepatitis C virus; HAV: Hepatitis A virus.
The overall prevalence of anti-HAV was 88.2% (105/119), while seroprevalence differed greatly based on age group: 66.7% in Group A, 95.5% in Group B, 93.5% in Group C, 91.3% in Group D and 94.7% in Group E (Figure 1). The anti-HAV prevalence was significantly lower in patients younger than 30 years of age when compared to those who were in the older age groups (Table 2). Multivariate analysis of age category variables also showed a significant difference between patients younger than 30 years when compared to those who were in the older age groups, with the exception of group D (P = 0.053) (Table 3).
Figure 1.
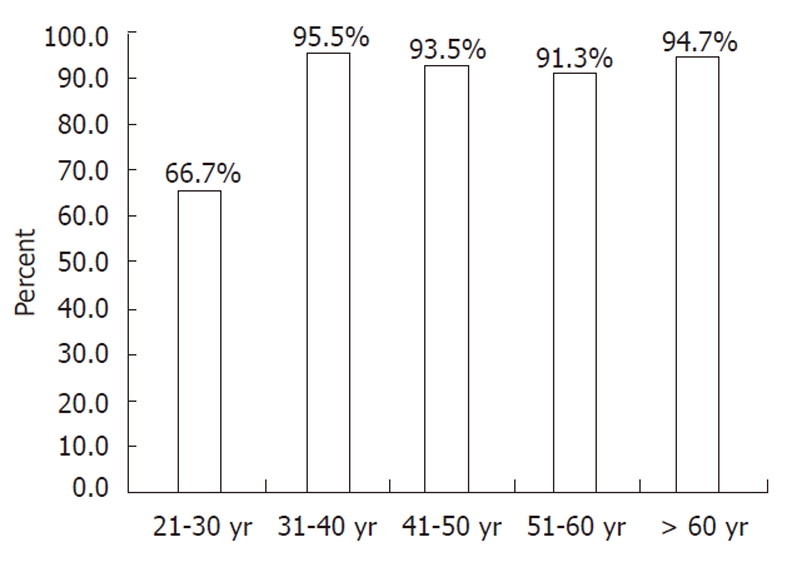
Seropositivity of anti-hepatitis A virus IgG according to age groups.
Table 2.
Univariate analysis of demographic data and anti-hepatitis A virus IgG positivity
| Variables | Anti-HAV IgG n (%) | χ2value | P value | |
| Positive | Negative | |||
| Age (yr), mean ± SD | 45.7 ± 13.4 | 35.1 ± 15.8 | ||
| Age less than 30 yr | 16 (66.7) | 8 (33.3) | 13.473 | 0.0011 |
| Age more than 30 yr | 89 (93.7) | 6 (6.3) | ||
| Race | ||||
| Malay | 84 (87.5) | 12 (12.5) | 0.259 | 0.6111 |
| Non-Malay | 21 (91.3) | 2 (8.7) | ||
| Gender | ||||
| Male | 68 (91.9) | 6 (8.1) | 2.521 | 0.1122 |
| Female | 37 (82.2) | 8 (17.8) | ||
| Aetiology of liver disease | ||||
| Hepatitis B | 83 (86.5) | 13 (13.5) | 1.511 | 0.2191 |
| Hepatitis C | 22 (95.7) | 1 (4.3) | ||
| Status of CLD | ||||
| Non cirrhotic | 88 (86.3) | 14 (13.7) | 2.644 | 0.2161 |
| Liver cirrhosis | 17 (100.0) | 0 (0.0) | ||
| Education level | ||||
| No formal education | 5 (71.4) | 2 (28.6) | 2.024 | 0.1911 |
| Primary | 12 (100.0) | 0 (0.0) | 1.779 | 0.3561 |
| Secondary | 61 (89.7) | 7 (10.3) | 0.331 | 0.5652 |
| Tertiary | 27 (84.4) | 5 (15.6) | 0.628 | 0.5221 |
| Salary category (RM) | ||||
| < 1000 | 48 (84.2) | 9 (15.8) | 1.707 | 0.2572 |
| 1001-2000 | 31 (88.6) | 4 (11.4) | 0.005 | 1.0001 |
| 2001-3000 | 14 (93.3) | 1 (6.7) | 0.43 | 1.0001 |
| > 3000 | 12 (11.4) | 0 (0.0) | 1.779 | 0.3561 |
| Comorbidities | ||||
| Diabetes | 21 (100) | 0 (0.0) | 3.4 | 0.1271 |
| Hypertension | 19 (100.0) | 0 (0.0) | 3.015 | 0.1221 |
| Ischaemic heart | 6 (100.0) | 0 (0.0) | 0.842 | 1.0001 |
Fisher’s exact test;
Pearson χ2 Test. P < 0.05 was considered as significant at the 95% confidence level. HAV: Hepatitis A virus; CLD: Chronic liver disease.
Table 3.
Multivariate analysis of age category variables
| Variables (age) | Walds (df) | Adjusted | P value | 95% CI | |
| OR | Lower | Upper | |||
| Group A: 21 to 30 yr | 11.021 (4) | 0.026a | |||
| Group B: 31 to 40 yr | 4.664 (1) | 11 | 0.031a | 1.248 | 96.951 |
| Group C: 41 to 50 yr | 5.236 (1) | 7 | 0.022a | 1.322 | 37.066 |
| Group D: 51 to 60 yr | 3.741 (1) | 5.25 | 0.053 | 0.978 | 28.182 |
| Group E: > 60 yr | 3.884 (1) | 9 | 0.049a | 1.012 | 28.182 |
P < 0.05 was considered as significant at the 95% confidence level. OR: Odds ratio.
The overall prevalence of hepatitis A was 80.5% in the 96 patients with chronic HBV infection and 95.7% in the 23 patients with chronic HCV infection. Anti-HAV was more frequently (100%) detected in LC patients when compared to non-cirrhotic patients (86.3%)
DISCUSSION
A study by Ton et al[24] investigated 100 healthy individuals from Kuala Lumpur in 1983 and reported a seroprevalence of hepatitis A of 78.2%. In 1985, a study reported that 100% of a Malaysian population were anti-HAV positive by 30 years of age[25]. However, in 1992, only 45% of the same age group were antibody-positive, indicating a shifting epidemiology, most probably due to improvements in living standards[25]. In the present study, we found that the overall anti-HAV seropositivity was high, at 88.2%. The higher seroprevalence of hepatitis A in our chronic viral hepatitis patients compared to previously reported seroprevalence for normal individuals was most likely related to the characteristics of the studied population in this region. This study was conducted in Kelantan, which is located in the north-eastern corner of the peninsula facing the South China Sea, with a chiefly agrarian economy. The population has diverse socioeconomic status and large income inequalities. The previous study was conducted in Malaysia’s capital, Kuala Lumpur[24], which has higher living standards, while the other included paediatric age groups with lower expected natural immunity[26]. Therefore, it is possible that the seroprevalence of HAV in other parts of Malaysia might not be similar to our findings, and we recommend further study in each locality to determine this. It is also possible that CLD patients have a higher prevalence of HAV positivity than the normal population. Contradicting this, Joshi and colleagues have revealed similar hepatitis A seroprevalence differences between CLD and normal populations in India[21].
We also show that the main factor that influences the rate of positivity is the age group. The seroprevalence rates in patients in Groups B, C, D and E were greater than 90%, compared to only 66.7% in patients belonging to Group A. These data imply that most patients with chronic viral liver disease who are greater than 30 years of age may have been exposed to HAV infection and have therefore already acquired natural immunity towards the disease. Therefore, routine HAV vaccination cannot be recommended in this age group. However, there remains the need to vaccinate patients aged less than 30 years with chronic liver disease.
Malaysia is a multiracial country consisting of three major ethnic groups: Malay, Chinese and Indian. Because the Indian population is generally very small in Kelantan, we did not manage to enrol any Indian patients, and only the Malay and Chinese races could be compared. There was no significant difference in the seroprevalence rate between these two ethnicities. However, this should be interpreted with caution, as various sociocultural behaviours may also play a role in influencing viral transmission rates.
The anti-HAV seropositive rate was 86.5% in hepatitis B and 95.7% in hepatitis C. Even though chronic hepatitis C is a different disease entity from chronic hepatitis B, there was no significant difference in anti-HAV positivity according to aetiology of underlying chronic liver disease (P > 0.05). This could be due to the small sample size of patients with hepatitis C infection (n = 23) in our study. Notably, the seroprevalence of hepatitis A was higher in hepatitis C patients than in hepatitis B patients, which is consistent with Korean data, where 100% of chronic hepatitis C patients were anti-HAV IgG positive compared to only 86.1% of hepatitis B patients[27]. Similarly, the Korean and Indian studies also failed to demonstrate any significance in hepatitis A seropositivity in relation to chronic liver disease aetiology[21,27].
All cirrhotic patients were anti-HAV positive, compared to only 86.3% in non-cirrhotic liver disease cases. The higher prevalence in cirrhotics was due to these patients falling into an older age group (mean age 52.4 ± 13.4) than non-cirrhotics (mean age 43.06 ± 13.7), as shown by multiple logistic regression analyses. Various studies, particularly those in highly endemic regions such as India, have demonstrated that the majority of cirrhotic patients of any aetiology are positive for anti-HAV IgG. A study from New Delhi revealed that 97.6% of cirrhotics (248/288) were found to be positive for anti-HAV[28]. Another study from South India demonstrated that 51 out of 52 patients with cirrhosis had antibodies towards HAV[29]. All these studies proposed that the higher seroprevalence of anti-HAV IgG in cirrhotic patients was actually related to increased age. Our findings are in agreement with the results of these studies.
Our study demonstrated that the overall hepatitis A seroprevalence was higher in CLD patients in Kelantan compared to the previously determined prevalence in normal individuals in other parts of Malaysia. Age was the most important factor in determining anti-HAV positivity, and most patients greater than 30 years of age were likely to have natural immunity.
ACKNOWLEDGMENTS
We would like to thank Dr. Habsah Hasan for her skilful help in conducting the anti-HAV IgG tests.
COMMENTS
Background
Populations in developed countries may not have had prior exposure to hepatitis A virus (HAV) and, therefore, no natural immunity; thus, there is a need for hepatitis A vaccination in selected high-risk groups. In view of this, Western guidelines advocate hepatitis A vaccination for those with chronic liver disease (CLD), as infection may lead to further deterioration of liver function, which can then cause significant morbidity and mortality amongst these patients.
Research frontiers
Over the last 20 years, patterns of endemicity in South East Asia have changed due to improvements in living standards, with some countries shifting from high to intermediate or intermediate to low endemicity. The question remains as to the importance of hepatitis A vaccination amongst hepatitis B and C CLD patients in Malaysia, a country of intermediate endemicity for hepatitis A. To answer this, a research team from Universiti Sains Malaysia determined the prevalence and associated factors of natural immunity towards hepatitis A amongst these patients in the eastern region of Peninsular Malaysia.
Innovations and breakthroughs
The study demonstrated that the overall prevalence of natural immunity towards hepatitis A was high (88%). There was a statistically significant difference when the data were broken down according to age. Results revealed that hepatitis B and C CLD patients less than 30 years of age were significantly less likely to have a natural immunity towards hepatitis A. This implies that although hepatitis A vaccination is not needed for the majority of CLD patients, a subset of patients (particularly patients who are younger than 30 years old) will still benefit from being vaccinated.
Applications
Study results show that for north-eastern Peninsular Malaysia, there is currently no need for routine hepatitis A vaccination amongst hepatitis B and C CLD patients. However, CLD patients who are younger than 30 years of age will still benefit from being vaccinated. Patterns of endemicity in South East Asian countries are expected to change; in Malaysia, the prevalence of hepatitis A viral exposure is expected to fall with time. Thus, there is a need to repeat this study in the future, as it is expected that the prevalence of natural immunity towards hepatitis A will fall as the country becomes more developed.
Peer review
The authors investigated the seroprevalence of anti-HAV antibodies in patients with CLD and the need for vaccination in the region of Kelantan, Malaysia.
Footnotes
Supported by Short term grant No. 304/PPSP/61310014 from the Universiti Sains Malaysia
Peer reviewer: Yoshiaki Iwasaki, MD, PhD, Associate Professor, Health Service Center, Okayama University, 2-1-1, Tsushima-Naka, Kita-ku, Okayama 700-8530, Japan
S- Editor Sun H L- Editor Logan S E- Editor Li JY
References
- 1.Baaten GG, Sonder GJ, Dukers NH, Coutinho RA, Van den Hoek JA. Population-based study on the seroprevalence of hepatitis A, B, and C virus infection in Amsterdam, 2004. J Med Virol. 2007;79:1802–1810. doi: 10.1002/jmv.21009. [DOI] [PubMed] [Google Scholar]
- 2.Barzaga BN. Hepatitis A shifting epidemiology in South-East Asia and China. Vaccine. 2000;18 Suppl 1:S61–S64. doi: 10.1016/s0264-410x(99)00467-3. [DOI] [PubMed] [Google Scholar]
- 3.Kunasol P, Cooksley G, Chan VF, Isahak I, John J, Loleka S, Villar EP, Poovorawan Y, Seong NH, Sulaiman HA, et al. Hepatitis A virus: declining seroprevalence in children and adolescents in Southeast Asia. Southeast Asian J Trop Med Public Health. 1998;29:255–262. [PubMed] [Google Scholar]
- 4.Saat Z, Sinniah M, Kin TL, Baharuddin R, Krishnasamy M. A four year review of acute viral hepatitis cases in the east coast of peninsular Malaysia (1994-1997) Southeast Asian J Trop Med Public Health. 1999;30:106–109. [PubMed] [Google Scholar]
- 5.Lee SD. Asian perspectives on viral hepatitis A. J Gastroenterol Hepatol. 2000;15 Suppl:G94–G99. doi: 10.1046/j.1440-1746.2000.02239.x. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsen KH, Koopman JS. The effects of socioeconomic development on worldwide hepatitis A virus seroprevalence patterns. Int J Epidemiol. 2005;34:600–609. doi: 10.1093/ije/dyi062. [DOI] [PubMed] [Google Scholar]
- 7.Jacobsen KH, Koopman JS. Declining hepatitis A seroprevalence: a global review and analysis. Epidemiol Infect. 2004;132:1005–1022. doi: 10.1017/s0950268804002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khairullah NS, Merican DI. Hepatitis disease management programs in Malaysia. J Gastroenterol Hepatol. 2004;19 Suppl:S13–S16. doi: 10.1111/j.1440-1746.2003.03393.x. [DOI] [PubMed] [Google Scholar]
- 9.Keeffe EB. Acute hepatitis A and B in patients with chronic liver disease: prevention through vaccination. Am J Med. 2005;118 Suppl 10A:21S–27S. doi: 10.1016/j.amjmed.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Song HJ, Kim TH, Song JH, Oh HJ, Ryu KH, Yeom HJ, Kim SE, Jung HK, Shim KN, Jung SA, et al. Emerging need for vaccination against hepatitis A virus in patients with chronic liver disease in Korea. J Korean Med Sci. 2007;22:218–222. doi: 10.3346/jkms.2007.22.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saab S, Lee C, Shpaner A, Ibrahim AB. Seroepidemiology of hepatitis A in patients with chronic liver disease. J Viral Hepat. 2005;12:101–105. doi: 10.1111/j.1365-2893.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 12.Cooksley G. The importance and benefits of hepatitis A prevention in chronic liver disease patients. J Gastroenterol Hepatol. 2004;19 Suppl:S17–S20. doi: 10.1111/j.1440-1746.2003.03394.x. [DOI] [PubMed] [Google Scholar]
- 13.Keeffe EB. Is hepatitis A more severe in patients with chronic hepatitis B and other chronic liver diseases? Am J Gastroenterol. 1995;90:201–205. [PubMed] [Google Scholar]
- 14.Hadler SC. Global impact of hepatitis A virus infection changing patterns. In: Hollinger FB, Lemon SM, Margolis H, editors. Viral Hepatitis and Liver Disease. Baltimore: Lippincott Williams and Wilkins; 1990. pp. 14–20. [Google Scholar]
- 15.Reiss G, Keeffe EB. Review article: hepatitis vaccination in patients with chronic liver disease. Aliment Pharmacol Ther. 2004;19:715–727. doi: 10.1111/j.1365-2036.2004.01906.x. [DOI] [PubMed] [Google Scholar]
- 16.Yao G. Clinical spectrum and natural history of viral hepatitis in a 1988 Shanghai epidemic In : Viral Hepatitis and Liver Disease. Baltimore: Lippincott Williams and Wilkins; 1991. [Google Scholar]
- 17.Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G, Ferraro T, Concia E. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:286–290. doi: 10.1056/NEJM199801293380503. [DOI] [PubMed] [Google Scholar]
- 18.Fiore AE, Wasley A, Bell B P. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWRRecomm Rep. 2006;55(RR07):1–23. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Prevention of hepatitis A through active or passive immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1999;48:1–37. [PubMed] [Google Scholar]
- 20.National Institutes of Health. National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: June 10-12, 2002. Hepatology. 2002;36:S3–S20. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed] [Google Scholar]
- 21.Joshi N, Rao S, Kumar A, Patil S, Rani S. Hepatitis A vaccination in chronic liver disease: is it really required in a tropical country like India? Indian J Med Microbiol. 2007;25:137–139. doi: 10.4103/0255-0857.32720. [DOI] [PubMed] [Google Scholar]
- 22.Wasley A, Fiore A, Bell BP. Hepatitis A in the era of vaccination. Epidemiol Rev. 2006;28:101–111. doi: 10.1093/epirev/mxj012. [DOI] [PubMed] [Google Scholar]
- 23.Hollinger FB, Eickhoff T, Gershon A, Jong EC, Koff RS. Discussion: who should receive hepatitis A vaccine? A strategy for controlling hepatitis A in the United States. J Infect Dis. 1995;171 Suppl 1:S73–S77. doi: 10.1093/infdis/171.supplement_1.s73. [DOI] [PubMed] [Google Scholar]
- 24.Ton SH, Thiruselvam A, Lopez CG, Noriah R. Prevalence of hepatitis A virus infection in normal individuals and hospital patients in Kuala Lumpur. Med J Malaysia. 1983;38:279–281. [PubMed] [Google Scholar]
- 25.Malik YA, Baharin R. Changing prevalence of Hepatitis A in Malaysia. Dec 4-7; Manila, Philippines. Singapore. In: Proceedings of the 4th Western Pacific Congress on Chemotherapy and Infectious Diseases;; 1994. p. MediMedia Asia, 1994. [Google Scholar]
- 26.Tan DS, Fang R, Collett D, Ooi BG. A seroepidemiologic study of hepatitis A in Malaysia. Southeast Asian J Trop Med Public Health. 1986;17:201–204. [PubMed] [Google Scholar]
- 27.Kim do Y, Ahn SH, Lee HW, Kim SU, Kim JK, Paik YH, Lee KS, Han KH, Chon CY. Anti-hepatitis A virus seroprevalence among patients with chronic viral liver disease in Korea. Eur J Gastroenterol Hepatol. 2007;19:923–926. doi: 10.1097/MEG.0b013e3282efa432. [DOI] [PubMed] [Google Scholar]
- 28.Acharya SK, Batra Y, Saraya A, Hazari S, Dixit R, Kaur K, Bhatkal B, Ojha B, Panda SK. Vaccination for hepatitis A virus is not required for patients with chronic liver disease in India. Natl Med J India. 2002;15:267–268. [PubMed] [Google Scholar]
- 29.Xavier S, Anish K. Is hepatitis A vaccination necessary in Indian patients with cirrhosis of liver? Indian J Gastroenterol. 2003;22:54–55. [PubMed] [Google Scholar]


