Abstract
(See the editorial commentary by Glezen, on pages 1151–3.)
Background. Although pregnancy is a recognized risk factor for severe influenza infection, the effect of influenza on miscarriages and births remains unclear. We examined the relationship between influenza and birth rates during the 1918 pandemic in the United States, Denmark, Sweden, and Norway.
Methods. We compiled monthly birth rates from 1911 through 1930 in 3 Scandinavian countries and the United States, identified periods of unusually low or high birth rates, and quantified births as “missing” or “in excess” of the normal expectation. Using monthly influenza data, we correlated the timing of peak pandemic exposure and depressions in birth rates, and identified pregnancy stages at risk of influenza-related miscarriage.
Results. Birth rates declined in all study populations in spring 1919 by a mean of 2.2 births per 1000 persons, representing a 5%–15% drop below baseline levels (P < .05). The 1919 natality depression reached its trough 6.1–6.8 months after the autumn pandemic peak, suggesting that missing births were attributable to excess first trimester miscarriages in ∼1 in 10 women who were pregnant during the peak of the pandemic. Pandemic-related mortality was insufficient to explain observed patterns.
Conclusions. The observed birth depressions were consistent with pandemic influenza causing first trimester miscarriages in ∼1 in 10 pregnant women. Causality is suggested by temporal synchrony across geographical areas.
Pregnancy has been identified as a risk factor for severe illness and death during all the influenza pandemics of the 20th century and in the 2009–2010 influenza A(H1N1) pandemic. In the 1957 influenza pandemic, ∼50% of the deaths in women of childbearing age occurred in pregnant women and resulted from primary viral pneumonia following influenza infection [1–3]. Similarly, although pregnant women comprise ∼1% of the US population today, they accounted for 5% of influenza-related deaths during the 2009 pandemic; furthermore, pregnant women were at elevated risk for admission to intensive care units and preterm delivery [4–6]. In contrast to the risk of maternal illness during pregnancy, the impact of influenza on the fetus and likelihood of miscarriage has been less studied [7]. Although studies have shown that maternal influenza vaccination improves fetal and infant outcomes [8, 9], little is known about whether maternal influenza infection has the potential to terminate pregnancies, and comprehensive data are lacking on the association between influenza and birth outcomes. This issue could have important implications for public health strategies and clinical care for pandemics as well as seasonal epidemics.
Investigating the impact of influenza on pregnancy is a challenge, because miscarriages may not be directly linked to influenza when patients are referred to clinics, especially if miscarriage occurs several weeks after primary viral infection. In addition, early pregnancy loss may occur unbeknownst to women who have not yet discovered that they are pregnant. Clinical reports from the 1918 pandemic period support a link between influenza and elevated maternal morbidity, mortality, pregnancy loss, and preterm labor [10–12]. In a 1919 report of 1350 pregnant women with influenza, 26% of case subjects miscarried (the largest proportion in the first trimester), whereas 52% of pneumonia-complicated case subjects miscarried [13]. This phenomenon associated with the 1918 pandemic appears to have been geographically widespread; a preponderance of miscarriages following pandemic peak activity was described in the Philippines [14], and an increase in miscarriages in women 5–9 months pregnant followed by a baby boom was reported in Norway [15]. In more recent literature, a possible association was noted between seasonal influenza A(H3N2) and a cluster of miscarriages [16]. Small observational studies of pregnant women infected with the 2009 pandemic influenza A(H1N1) virus reported preterm deliveries and fetal deaths [17, 18]. Despite these geographically limited reports and case series, the association between maternal influenza and pregnancy loss has not been quantified.
In this study, we utilized historical epidemiological records from the 1918 influenza pandemic in 3 Scandinavian countries and the United States to examine the relationship between influenza and pregnancy outcomes at the population level. We hypothesized that if influenza infection in pregnant women can cause miscarriages on a large scale, then a decline in births would occur within 9 months of the peak pandemic activity. Data from contemporaneous national surveillance systems were acquired from library archives to elucidate the potential temporal associations between influenza activity and birth patterns, and to quantify the risk of miscarriage associated with influenza infection.
METHODS
Data
For each population in the Scandinavian countries and the United States, we collected monthly rates of births and population data (Table 1), as well as data on morbidity or mortality due to respiratory diseases (as available), for the period before, during, and after the 1918 pandemic [19–24]. The period 1911–1930 was included in the analysis for Denmark, Norway, and Sweden. We used aggregated respiratory mortality and birth rate data for the 10 states of the 1915 US Birth Registration area (Connecticut; Pennsylvania; Maine; Massachusetts; Michigan; Minnesota; New Hampshire; New York; Vermont; and Washington, DC) for the period 1915–1924. We collected additional detailed data from Denmark on regional differences in pandemic activity and birth rates, as well as stillbirths.
Table 1.
Baseline Demographic Characteristics of the Studied Populations
| Country, region | Population size in 1918, millions of persons | Mean annual birth rate in 1911–1930, births per 1000 persons |
| Denmark, all | 3.03 | 22.8 |
| Copenhagen | 0.66 | 19.3 |
| Rural | 1.72 | 24.2 |
| Norway, all | 2.59 | 22.3 |
| Sweden, all | 5.82 | 27.8 |
| United States, 10 states | 31.20 | 22.4a |
Average for 1915–1924. The 10 states included were Connecticut; Pennsylvania; Maine; Massachusetts; Michigan; Minnesota; New Hampshire; New York; Vermont; and Washington, D.C.
Statistical Analysis
Expected Birth Rates and Confidence Intervals.
First, we used the respiratory mortality data (US states) and influenza morbidity data (Scandinavian countries) to deduce the timing of peak pandemic activity associated with the lethal 1918 autumn wave in each location. We developed a seasonal model to estimate a baseline for birth rates derived from surrounding years, after excluding birth data from the extended pandemic period of January 1918 through December 1920. Because birth rates are known to vary seasonally and over time due to socioeconomic changes unrelated to influenza activity, we subtracted from the monthly birth rates the moving average of the surrounding 73 months according to the following equation:
where Mt is the moving average and Bt the birth rate in month t starting in January 1911 and ending in December 1930. We then computed the seasonal component Sm for each month m of the year (m = 1, 2, …, 12) as the average deviation between the moving average and the observed birth rate over the 20-year period 1911–1930 according to the following equation:
where y is an index for the year.
Thus, in the absence of influenza activity, our expected birth rate Et for every month t in the studied period is E(y×12)+m = M(y×12)+m + S. We also analyzed the data with a multiplicative version of this model, which yielded nearly identical results (data not shown).
The 95% confidence interval around the birth rate baseline was determined for each study population assuming a normal distribution, where the variance was determined as the square of the residuals summed over all months excluding the pandemic period. This estimate of the variance is about twice as large as what would be obtained under the assumption of a Poisson error, and hence is conservative. For each study population, we identified consecutive months in which the observed birth rate was “in excess,” that is, above or below the 95% confidence interval on the baseline for at least 2 months.
Quantifying the Timing of Birth Rate Excesses.
For each study population and periods associated with significant excess birth rates above or below normal levels, we calculated the center of gravity of the excess period. The center of gravity has been used in past research to identify the timing of epidemics [25] and can be considered as the median month of positive or negative excess births. It is calculated as the weighted average of the months belonging to an excess period, where the weights are the monthly residuals (observed birth rate minus baseline birth rate).
Quantitative Assessment of Alternative Hypotheses.
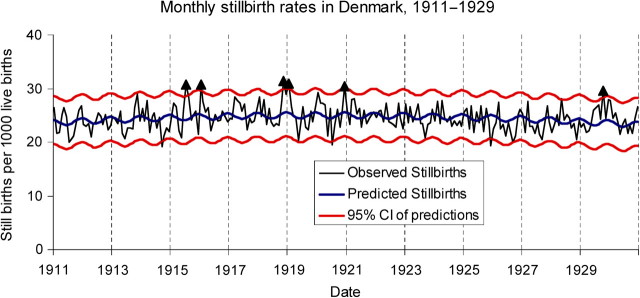
We also explored the potential impact of influenza-related deaths in women of childbearing age on the birth rate patterns observed in our data sets. We compiled data on deaths among women aged 20–49 years from Copenhagen [26]. Finally, we examined evidence of a third trimester effect of influenza on the fetus by exploring time trends in stillbirth data, which were available from Denmark for the period 1911–1930. Using a seasonal modeling approach similar to that used for birth rates, we analyzed stillbirth data from Denmark for evidence of an increase in stillbirths during and after the autumn 1918 influenza peak.
RESULTS
The birth rate model including long-term time trends and seasonal effects explained 93%–95% of the variation in the monthly data outside the pandemic period in all study populations (Supplementary Table 1). The difference between the observed and expected monthly birth rates (residuals) was plotted for each population, together with the morbidity or mortality rate indicators of pandemic influenza activity (Figure 1). The monthly indicators of influenza activity peaked in October–November 1918 in all populations studied except for rural Denmark, where it peaked during late November to early December 1918 (Table 2).
Figure 1.
Timing of influenza pandemic and birth rate reduction in Denmark, Norway, Sweden, and the United States. A, Time series of monthly natality rates in Denmark during several decades surrounding the 1918 pandemic. It can be seen that an unusual and unseasonal birth rate depression occurred in early 1919, followed by an equally unusual increase (compensation) from late 1919 through early 1920. The natality patterns are typical for natality data from each of the populations studied. B, Time series of residual natality rates after subtracting out the expected (modeled) baseline, contrasted with the peak influenza (flu) periods, for Denmark (DK), Norway (NO), Sweden (SE), and the United States (US). For each study population, the severe autumn 1918 peak was followed by a temporary period of compensation 6–9 months later. This decline is attributed to first trimester miscarriages among a subset of all pregnant women who had pandemic influenza. The shaded areas represent the 95% confidence intervals P&I deaths indicate respiratory mortality due to pneumonia and influenza.
Table 2.
Temporal Coincidence in the Mean Peak Influenza Month and Birth Depression Peak Month in Each Country Under Study, Using Center of Gravity Statistics
| Country, region | Month no. of peak influenza in autumn 1918 | Month no. of birth depression center of gravity in 1919 | Lag from peak influenza to center of birth depression, monthsa | Mean pregnancy stage at flu peak, months | Cumulative decrease in birth rate in early spring 1919, rate per 1000 (%)b | Cumulative increase in birth rate in late 1919 to early 1920, rate per 1000 (%)c |
| Denmark, all | 11 | 5.1 | 6.1 | 2.9 | −1.8 (−7.3) | 2.0 (+8.4) |
| Copenhagen | 10 | 4.5 | 6.5 | 2.5 | −1.9 (−9.1) | 0.8 (+3.8) |
| Rural | 11–12 | 6.1 | 6.6 | 2.4 | −1.3 (−5.3) | 1.7 (+6.8) |
| Norway, all | 10 | 4.8 | 6.8 | 2.2 | −2.4 (−9.8) | 2.1 (+8.9) |
| Sweden, all | 10 | 5.0 | 6.5 | 2.5 | −4.0 (−13.5) | 5.8 (+19.9) |
| United States, 10 states d | 10 | 4.6 | 6.6 | 2.4 | −1.8 (−7.6) | 0.3 (+1.3) |
The lag period (in months) was calculated as the difference between the month of peak influenza and that of the center of gravity of the birth depression.
The dip percentage was calculated as the sum of the negative excess birth rates during the dip period divided by the expected annual birth rate.
The percentage of compensation was calculated as the sum of the positive excess birth rates during the compensatory period divided by the expected annual birth rate.
The 10 states included were Connecticut; Pennsylvania; Maine; Massachusetts; Michigan; Minnesota; New Hampshire; New York; Vermont; and Washington, D.C.
A statistically significant period of birth rate depression was identified in all study populations, reaching a remarkably consistent trough 6.1–6.8 months after the peak of the influenza pandemic, based on the center of gravity analysis (Figure 1; Table 2). Birth rates declined by 2.2 births per 1000 persons, on average, in each of the study populations (range, 1.3–4.0 births per 1000 persons), which amounts to a 5%–15% reduction in births relative to annual baseline expectations. This annual reduction corresponds to an excess of ∼1 in 10 pregnant women infected with influenza during their first trimester having miscarried in autumn 1918.
In Scandinavia, the natality decline began in January–February 1919, with the “missing” births concentrated in April and May 1919, 6–7 months after peak pandemic activity. Following this temporal natality depression, a compensatory surge of births was noted 7.5–10 months later in these countries, beginning in October 1919 and peaking in winter 1920. This surge represents an excess of 0.8–5.8 births per 1000 persons—a 4%–20% increase in births relative to annual baseline expectations (Table 2). These patterns were the only sustained deviations from the expected number of births throughout the 30-year study period in these populations. The United States experienced a decrease in births culminating 6.6 months after peak pandemic activity, remarkably consistent with the Scandinavian experience. The compensatory increase in birth rates was not as pronounced in the United States as in the other locations at 0.3 births per 1000 persons (1.3% in excess of annual expectation). No other period of sustained deviations from the expected number of births was observed in the United States throughout the 10 years studied.
To explore the association between influenza and birth rates at a finer geographical scale, we compared the timing of the 1918 influenza pandemic and trends in birth rates in Copenhagen and rural Denmark (Figure 2). Although the timing of the influenza pandemic and natality depression in Copenhagen was consistent with the nationwide patterns described above, the pattern for rural Denmark was shifted by 1–2 months. Influenza morbidity peaked in October 1918 in Copenhagen and November–December 1918 in rural Denmark. Accordingly, the decline in birth rates began in Copenhagen in January 1919, with the most births missing in mid-April 1919; in rural Denmark, the natality dip began in March 1919, with the most births missing in June 1919 (Figure 2). The time elapsed between the peak of the influenza pandemic and center of gravity of missing births was consistent for the 2 regions (6.5 months in Copenhagen; 6.6 months in rural Denmark), suggesting that the same phenomenon occurred in the capital city and rural areas, with a 1–2 month lag between locations.
Figure 2.
Asynchrony in timing of influenza (flu) activity and birth patterns across Denmark. The influenza epidemic struck Copenhagen (Cph) 1–2 months before it hit the rural areas in Denmark. The delayed effect is mirrored by the birth depression, with the birth rate at its lowest in Copenhagen 1–2 months prior to the low point in rural Denmark. The shaded area represents the 95% confidence interval, which happened to be the same for Copenhagen and rural Denmark.
We considered the potential impact of influenza-related deaths in women of childbearing age as a potential explanation for the postpandemic decline in birth rates. In Copenhagen, detailed annual sex-, age-, and cause-specific mortality data were available; a total of 743 women aged 20–49 years were reported to have died from influenza from July 1918 through June 1919, in a population of ∼135 000 women 20–49 years of age [26]. Yet there were ∼1200 births missing in spring 1919 in Copenhagen. Even in the extremely unlikely scenario that all women 20–49 years of age who died in Copenhagen were pregnant when they died, it would explain only 62% of the missing births. Finally, we examined evidence of a third trimester effect on the fetus. If a fetus had been near term at the time of the 1918 pandemic, a miscarriage event would likely have been recorded as a stillbirth at the time of the mother’s pandemic illness in autumn 1918. There were 2 nonconsecutive months of excess stillbirths in Denmark during the pandemic period, in November 1918 and January 1919, representing a total of 11.4 excess stillbirths per 1000 live births for the 2 months, or 64 excess stillbirths. We also observed similar deviations in 4 other months, which were spread out over the study period 1911–1929 (Figure 3).
Figure 3.
Lack of trends in monthly stillbirths in Denmark. Stillbirths are shown as excess numbers per 1000 live births, after normalization by long-term mean. Although there is a significant excess of stillbirths in November 1918 and January 1919, it is not a particularly dramatic or long-lasting signal. The red lines indicate the 95% confidence interval (CI).
Taken together, these findings, of (1) the consistency of the time elapsed between the peak pandemic activity in autumn 1918 and the drop in birth rates 6.1–6.8 months later across 4 study populations in Scandinavia and the Unites States, (2) the lack of a similar drop in birth rates in any other period during 1911–1930, (3) the minor contemporaneous increase in stillbirths, (4) the relatively low number of influenza-related deaths in young adult women associated with the autumn 1918 pandemic, and (5) the compensatory increase in birth rates occurring within 1 year of the pandemic, strongly suggest that the 1918 pandemic was associated with miscarriages in the first trimester of pregnancy.
DISCUSSION
In this study, we explored historical natality, demographics, and health data and documented an unusual 5%–15% decline in natality with a trough 6.1–6.8 months after the peak of the severe autumn 1918 pandemic wave in several Scandinavian countries and the United States. On average, 2.2 births per 1000 persons were missing during spring 1919, corresponding to an excess of ∼1 in 10 pregnant women infected with influenza during their first trimester having miscarried in autumn 1918. We argue that the most parsimonious explanation for this unusual and temporal birth depression is substantial pregnancy losses following influenza infection in autumn 1918 among women who were then in their first trimester of pregnancy (Figure 4). The temporal association observed in all countries was strengthened further by the observation that a 1- to 2-month difference in the timing of pandemic activity between Copenhagen and rural Denmark was mirrored by a similar 1- to 2-month difference in the decline of birth rates in spring of 1919. We also observed that the postpandemic dip in birth rates was followed by a compensatory natality increase in late autumn 1919 and early spring 1920, when natality significantly exceeded the expected rates. This rapid resurgence of births from late autumn 1919 through early spring 1920, especially in Denmark and Sweden, confirmed that fertility was not permanently impacted. We hypothesize that the resurgence represents compensatory pregnancies in women who miscarried due to influenza.
Figure 4.
Delayed effect of 1918 influenza (flu) on births as a result of miscarriage. The depression in birth rates occurring in spring 1919 and lasting several months is consistent with a number of first-trimester miscarriages in women who fell ill with influenza.
This population-level study represented a unique opportunity to investigate the phenomenon of influenza as a risk factor for miscarriage early in pregnancy and to quantify the magnitude of the risk. A first trimester influenza-related miscarriage might often not be noted at the time of the mother’s illness; rather, it would figure as a missing birth event some months later. Interestingly, the birth rate depression period observed in 1919 was relatively protracted, as compared with the short and intense period of influenza mortality in autumn 1918. Variation in the exact stage of pregnancy at which influenza infection occurred (0, 1, 2, or 3 months into pregnancy), and perhaps in the risk of miscarriage at different stages of the first trimester of pregnancy, would result in a protracted period of birth depression lasting 3–6 months, even though autumn pandemic activity was concentrated in 1–2 months. We are not aware of previous population-wide studies examining the timing of influenza activity in relation to natality patterns.
The unusual epidemiology of the 1918 influenza pandemic, wherein deaths were concentrated among young adults, has been well-documented in studies of the United States, United Kingdom, Japan, and Denmark [27–31]. Our study strongly suggests that the number of influenza-related deaths among pregnant women during the autumn 1918 pandemic cannot fully account for the natality patterns we observe. Most importantly, if the deaths of expectant mothers were fully responsible for the dip in births in spring 1919, it would be difficult to explain the compensatory surge in births occurring 7.5–10 months after the dip. Overall, maternal mortality likely played a role in the observed birth depression, as confirmed by high mortality rates in pregnant women in the 1957 and 2009 influenza pandemics [1, 2, 5], but it was not the main factor explaining postpandemic natality trends.
Competing Hypotheses
Below we review competing hypotheses that could potentially have explained the observed trends in birth rates. We considered the possible impact of World War I (WWI), including the absence or death of young men who may have otherwise fathered infants during the summer of 1918. However, in contrast to Sweden and the United States, Denmark and Norway were minimally involved in WWI. Because all 4 countries had the postpandemic birth depression pattern, we conclude that war-related population changes cannot explain the observed patterns. We next explored the potential contribution of nutrition factors to the observed patterns. In Norway, WWI led to food shortages, which have been shown to impact fertility in other populations [32, 33]. In contrast, malnutrition problems decreased in the Danish population after a German blockade in winter 1917 [34]. Although malnutrition may have had an impact on fertility in the years prior to the influenza pandemic, it cannot explain the observed spring 1919 depression in natality, at least not within Denmark. We also considered whether fertility was impacted by pandemic influenza through changes in population behaviors. If many young women and men fell ill or feared infection in the first pandemic wave in the spring and summer of 1918, it might have interfered with reproductive behavior [11]. However, we think this unlikely, as the first pandemic wave was relatively mild with few deaths in both the Unites States and Denmark [28–30]. We also considered the influence of the Great Depression on modeling long-term changes in birth rates; however, our results were robust to exclusion of the years leading to the Great Depression toward the end of our study period.
For pandemic activity to explain the observed birth depressions, one may have expected a signal for each of the 4 pandemic waves during 1918–1920, yet we could only statistically associate the severe autumn 1918 wave (so-called second wave) with such a natality decline in all study populations. The lack of association for the first wave could be explained by the mildness of the infection [28–30], and there may not have been many remaining susceptible pregnant women during the third and fourth waves in the winter of 1918–1919 and 1919–1920. We note that in the Unites States, the birth depression occurred in a “double dip” pattern, beginning as early as November 1918. The first natality dip in the Unites States may have been an effect of the documented spring 1918 wave [28]. If some women in their first trimester had been infected in April 1918 and miscarried, a drop in the birth rate would be expected 6–9 months later, in October–December 1918. The first dip in births in the United States is small, which could be consistent with a minor effect of the spring wave, whereas the second dip later in 1919 is more pronounced and synchronous with those in other countries, and could be explained by the severe influenza burden associated with the autumn 1918 pandemic wave. Overall, we note that the center of gravity index, which measures the month at which 50% of the natality dip had occurred, is highly consistent between the United States and Scandinavia, suggesting a common phenomenon.
Geographic heterogeneity in pandemic timing may also have affected the US findings [35], because the data available represented an aggregate of 10 states in the Mid-Atlantic, New England, and Midwest regions of the United States. It would be ideal to analyze the US data by individual state, but the data were not publicly available to our knowledge. Other locations would be of particular interest, such as Iceland, where 40% of the population escaped the autumn influenza pandemic of 1918, due to stringently enforced quarantine and travel restrictions [36, 37]. It would be interesting to analyze birth patterns in different parts of Iceland and explore their association with timing of influenza activity.
Biological Mechanisms That May Explain Miscarriage Following Influenza Infection
A handful of studies have explored the effects of viral respiratory disease on pregnancy; most of these have been devoted to explaining severe health outcomes in pregnant women rather than fetuses. However, when pregnant women experience severe systemic reactions, as with influenza infection, it can be harmful to both mother and fetus. Pneumonia can cause high fever and toxicity, which is thought to cause miscarriage in the early stages of pregnancy and premature labor [3]. Hormonal signals lead to mechanical changes that impact respiratory and cardiac function, possibly leading to increased risk of complications when pregnant women contract an infection [7]. Pregnant women undergo immunological adaptations, including systemic suppression of cell-mediated immunity, which may lead to increased susceptibility and/or risk of severe outcomes [38]. During the 2009 influenza A (H1N1) pandemic, severe outcomes were linked to low levels of immunoglobulin G antibodies, which often occurs in pregnancy [39].
The association between the 1918 pandemic and observed birth rates may illustrate a generic association between epidemics of infectious diseases and associated temporal declines in birth rates. Large variations in birth rates have been reported after global epidemics of cholera, dysentery, smallpox, and influenza in France, England, and Wales [40]. In agreement with our epidemiological findings, this suggests a link between pandemics and early pregnancy loss, in which an initial drop in natality is followed by a spike in births [40].
It remains to be seen whether the association between birth rates and the 1918 pandemic can be generalized to seasonal influenza epidemics of lesser severity and to the 2009 influenza A (H1N1) pandemic. A similar phenomenon may have been associated with the 1957 or 1968 pandemics in Denmark or the United States, but it may have been too subtle to measure (data not shown). If influenza-related miscarriages were to blame for the depression and subsequent rise in natality following the 1918 pandemic, perhaps this is more readily observed because of the unusual severity of infection with the 1918 influenza A (H1N1) virus.
Our findings support influenza vaccination recommendations for pregnant women and may have other implications for clinical care of pregnant women during severe influenza pandemics. If our historical results were confirmed by future epidemiological and clinical studies, the risk of influenza-related miscarriage may need to be considered in planned pregnancies. In particular, monitoring cohorts of pregnant women for influenza infection during pandemic and epidemic seasons is essential to fully understand the association between influenza activity and early miscarriages.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/).
Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Acknowledgments
We give many thanks to colleagues at the US National Institutes of Health; Statens Serum Institut, Denmark; and Roskilde University, Denmark. K. B-F. is grateful to Nesli Saglanmak for her helpful feedback on the manuscript and to Katarina Widgren and Sabrina Bacci for their intellectual support.
Financial support. This work was supported by the Danish Medical Research Council (271-07-0555 to V. A.); and the Research and Policy for Infectious Disease Dynamics (RAPIDD) program of the Fogarty International Center and Department of Homeland Security (funding support to L. S. and V. A.) This study was funded in part by the Intramural Influenza Research Program of the Fogarty International Center, National Institutes of Health, which is supported by the International Influenza Unit, Office of Global Affairs, Department of Health and Human Services.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Greenberg M, Jacobziner H, Pakter J, Weisl BA. Maternal mortality in the epidemic of Asian influenza, New York City, 1957. Am J Obstet Gynecol. 1958;76:897–902. doi: 10.1016/0002-9378(58)90027-9. [DOI] [PubMed] [Google Scholar]
- 2.Freeman DW, Barno A. Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol. 1959;78:1172–5. doi: 10.1016/0002-9378(59)90570-8. [DOI] [PubMed] [Google Scholar]
- 3.de Swiet M. Respiratory disease in pregnancy. Postgrad Med J. 1979;55:325–8. doi: 10.1136/pgmj.55.643.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ANZIC Influenza Investigators and Australasian Maternity Outcomes Surveillance System. Critical illness due to 2009 A/H1N1 influenza in pregnant and postpartum women: population based cohort study. BMJ. 2010;340 doi: 10.1136/bmj.c1279. c1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–25. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louie JK, Acosta M, Jamieson DJ, Honein MA. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362:27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic influenza and pregnant women. Emerg Infect Dis. 2008;14:95–100. doi: 10.3201/eid1401.070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinhoff MC, Omer SB, Roy E, Altaye M, Breiman RF, Zaman K. Influenza immunization in pregnancy—antibody responses in mothers and infants. N Engl J Med. 2010;362(17):1644–6. doi: 10.1056/NEJMc0912599. [DOI] [PubMed] [Google Scholar]
- 9.Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vázquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis. 2010;51:1355–61. doi: 10.1086/657309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bland PB. Influenza in its relation to pregancy and labor. Am J Obstetrics. 1919;79:184–97. [Google Scholar]
- 11.Thoroddsen T. Inflúenzan fyrrum og nú. (Influenza, past and present). Laeknabladid. 1919;5:13–23. /74-9. [Google Scholar]
- 12.Woolston WJ, Conley DO. Epidemic pneumonia (Spanish influenza) in pregnancy: effect in one hundred and one cases. J Am Med Assoc. 1918;71:1898–9. [Google Scholar]
- 13.Harris JW. Influenza occurring in pregnant women: a statistical study of 1350 cases. JAMA. 1919;72:978–80. [Google Scholar]
- 14.Calderon F. The influence of influenza on menstruation, pregnancy, and puerperium. JAMA. 1919;73:989–93. [Google Scholar]
- 15.Mamelund SE. Can the Spanish influenza pandemic of 1918 explain the baby-boom of 1920 in neutral Norway? No 01/2003, ed. Oslo: Department of Economics, University of Oslo, 2003. [Google Scholar]
- 16.Stanwell-Smith R, Parker AM, Chakraverty P, Soltanpoor N, Simpson CN. Possible association of influenza A with fetal loss: investigation of a cluster of spontaneous abortions and stillbirths. Commun Dis Rep CDR Rev. 1994;4:R28–32. [PubMed] [Google Scholar]
- 17.Miller AC, Safi F, Hussain S, Subramanian RA, Elamin EM, Sinert R. Novel influenza A(H1N1) virus among gravid admissions. Arch Intern Med. 2010;170:868–73. doi: 10.1001/archinternmed.2010.126. [DOI] [PubMed] [Google Scholar]
- 18.Oluyomi-Obi T, Avery L, Schneider C, et al. Perinatal and maternal outcomes in critically ill obstetrics patients with pandemic H1N1 influenza A. J Obstet Gynaecol Can. 2010;32:443–7. doi: 10.1016/S1701-2163(16)34497-8. 448–52. [DOI] [PubMed] [Google Scholar]
- 19.Danmarks Statistik, (Marriages, Births, and Deaths in the years 1926–30). Statistisk Tabelværk, Rk 5, Litra A, Nr 19. Copenhagen: Danmarks Statistik; 1934. Ægteskaber, Fødte og Døde I Årene 1926–30. [Google Scholar]
- 20.(Population Changes 1911--1926). Det Statistiske Centralbyrå, Norges Offisielle Statistikk. Kristiania/Oslo: I Kommisjob hos H. Aschehough & Co, 1928; Det Statistiske Centralbyrå. Folkemengdens Bevegelse, 1911–1926. [Google Scholar]
- 21.Statistiska Centralbyrån. Historisk statistik för Sverige (Historical Statistics of Sweden) Stockholm: A.B. Allmänna Föelaget; 1969. Del 1. Befolkning (Part 1. Population): 1720–1967. [Google Scholar]
- 22.Statistiska Centrabyrån, Sveriges Officiella Statistik: Folkmängden. och dess förändringar. Stockholm: Kungl. Boktryckeriet. P. A. Norstedt, & Söner; 1939. Statistiska Centralbyrån. Befolkningsrörelsen: Åren 1921–1930 (split into different editions) pp. 54–121. 50–59. [Google Scholar]
- 23.Linder F, Grove R. Vital statistics rates in the United States: 1900–1940. Washington, DC: United States Government Printing Office; 1947. [Google Scholar]
- 24.Bureau of the Census. Mortality Statistics 1915–1924. Washington, DC: Government Printing Office; 1917–1927. [Google Scholar]
- 25.Pitzer VE, Viboud C, Simonsen L, et al. Demographic variability, vaccination, and the spatiotemporal dynamics of rotavirus epidemics. Science. 2009;325:290–4. doi: 10.1126/science.1172330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen S. Stadslægens beretning fra 1918 (The Medical Officer's report for 1918) Copenhagen: City of Copenhagen: 1919:. Influenza epidemien i København: Juli 1918–Juni 1919 (The influenza epidemic in Copenhagen: July 1918–June 1919) pp. 55–62. [Google Scholar]
- 27.Noymer A, Garenne M. The 1918 influenza epidemic's effects on sex differentials in mortality in the United States. Popul Dev Rev. 2000;26:565–81. doi: 10.1111/j.1728-4457.2000.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson DR, Simonsen L, Edelson PJ, Morse SS. Epidemiological evidence of an early wave of the 1918 influenza pandemic in New York City. Proc Natl Acad Sci U S A. 2005;102:11059–63. doi: 10.1073/pnas.0408290102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glezen WP. Emerging infections: pandemic influenza. Epidemiol Rev. 1996;18:64–76. doi: 10.1093/oxfordjournals.epirev.a017917. [DOI] [PubMed] [Google Scholar]
- 30.Andreasen V, Viboud C, Simonsen L. Epidemiologic characterization of the 1918 influenza pandemic summer wave in Copenhagen: implications for pandemic control strategies. J Infect Dis. 2008;197:270–8. doi: 10.1086/524065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richard SA, Sugaya N, Simonsen L, Miller MA, Viboud C. A comparative study of the 1918–1920 influenza pandemic in Japan, USA and UK: mortality impact and implications for pandemic planning. Epidemiol Infect. 2009;137:1062–72. doi: 10.1017/S0950268809002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salmonsen HM, Haffner M. Verdenskrigen (Norge). (The World War [Norway]). Copenahagen: JH Schultz Forlagsboghandel. 1928. Salmonsens Konversationsleksikon. (Haffner M. Salmonsen's Encyclopedia). 2nd ed; pp. 924–6. [Google Scholar]
- 33.Wynn A, Wynn M. The effects of food shortage on human reproduction. Nutr Health. 1993;9:43–52. doi: 10.1177/026010609300900106. [DOI] [PubMed] [Google Scholar]
- 34.Bloch CE. Nutrition classics from: The Journal of Hygiene 19: 283-301, 1921. Clinical investigation of xerophthalmia and dystrophy in infants and young children (xerophthalmia et dystrophia alipogenetica) Nutr Rev. 1974;32:176–9. doi: 10.1111/j.1753-4887.1974.tb06316.x. [DOI] [PubMed] [Google Scholar]
- 35.Pearl R. Influenza studies: further data on the correlation of explosiveness of outbreak of the 1918 epidemic. Public Health Rep. 1921;36:273–98. [Google Scholar]
- 36.Gottfredsson M, Halldorsson BV, Jonsson S, et al. Lessons from the past: familial aggregation analysis of fatal pandemic influenza (Spanish flu) in Iceland in 1918. Proc Natl Acad Sci U S A. 2008;105:1303–8. doi: 10.1073/pnas.0707659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gottfredsson M. The Spanish flu in Iceland 1918: lessons in medicine and history. Laeknabladid. 2008;94:737–45. [PubMed] [Google Scholar]
- 38.Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis. 2006;12:1638–43. doi: 10.3201/eid1211.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon CL, Johnson PD, Permezel M, et al. Association between severe pandemic 2009 influenza A(H1N1) virus infection and immunoglobulin G(2) subclass deficiency. Clin Infect Dis. 2010;50:672–8. doi: 10.1086/650462. [DOI] [PubMed] [Google Scholar]
- 40.Hotelling H, Hotelling F. Causes of birth rate fluctuations. J Am Stat Assoc. 1931;26:135–49. [Google Scholar]