Abstract
We detected cross-reactive neutralizing antibodies (NtAb) against hepatitis C virus (HCV) in chimpanzees vaccinated with HCV-1 (genotype 1a) recombinant E1/E2 envelope glycoproteins. Five vaccinated chimpanzees, protected following HCV-1 challenge, were initially studied using the heterologous H77 (genotype 1a) HCVpp assay. All animals had developed NtAb after the second vaccination; 4 animals had reciprocal titers of ≥200 at the time of challenge. Using genotypes 1a–6a HCV pseudoparticles (HCVpp) and cell culture–derived HCV (HCVcc) assays, cross-reactive NtAb were detected against 1a, 4a, 5a, and 6a, with limited reactivity against 2a and 3a. Our study provides encouragement for the development of a recombinant envelope-based vaccine against hepatitis C.
Hepatitis C virus (HCV) infection persists in about 75% of acute cases, and HCV is a major cause of chronic liver disease, including liver cirrhosis and hepatocellular carcinoma. Unfortunately, the development of a hepatitis C vaccine is very challenging. Resolution of HCV infection was associated with robust cellular immunity, but many studies have pointed to an important role also for neutralizing antibodies (NtAb) [1–4]. The vaccine efficacy data emerging from HCV challenge studies in chimpanzees (reviewed in [5, 6]), indicate that it is possible to mount a protective immune response by vaccinating with viral envelope [E] proteins [7].
Choo et al. [1] reported on 7 chimpanzees that were protected against homologous HCV-1 (genotype 1a) challenge after vaccination with recombinant envelope glycoproteins. Additionally, 4 chimpanzees reimmunized with HCV-1 E1/E2 and subsequently challenged with heterologous HCV-H77 (genotype 1a) developed acute resolving (3 animals) or persistent (1 animal) HCV infection [8]. Protection against viral challenge correlated with the vaccine-induced anti-E1/E2 responses (Table 1), but neutralizing antibodies were not measured.
Table 1.
Reciprocal Titers of Hepatitis C Neutralizing Antibodies Against HCVpp/HCVcc of Genotypes 1–6 in Chimpanzees Immunized With Recombinant HCV1 E1/E2 Glycoproteinsa
| Chimpanzee no. | Anti- E1/E2 | NOB (CD-81) | Genotype (strain) | |||||
| 1a (H77) | 2a (J6CF) | 3a (S52) | 4a (ED43) | 5a (SA13) | 6a (HK6a) | |||
| 559 | 37 888 | 3500 | 1600/800 | 50/<100 | <50/<100 | 1600/1600 | 800/6400 | 1600/102 400 |
| 357 | 25 856 | 2500 | 200/<200b | <50/ND | <50/ND | 400/200 | 400/200 | 400/6400 |
| 534 | 22 272 | 600 | 400/<200b | 200/ND | 50/ND | 400/200 | 400/200 | 800/12 800 |
| 653 | 8704 | 1500 | 50/<200 | ND/<200 | ND/<200 | ND/<200 | ND/<200b | ND/12 800 |
| 470 | 5312 | 250 | 800/<200 | <50/ND | <50/ND | 1600/<200 | 1600/<200b | 800/3200 |
Abbreviations: ND, not determined.
Samples analyzed were taken on the day of HCV1 challenge. Anti-E1/E2 titers were determined in enzyme immunoassay with CHO-expressed HCV1 (genotype 1a) E1/E2 glycoproteins. NOB titers reflect that of dilution producing 50% neutralization of binding of recombinant gpE2 subunit. Neutralizing antibody titers were determined by serial 2-fold dilution of chimpanzee serum specimens and subsequent incubation with HCVpp or HCVcc.
Percent neutralization in HCVcc assay approaching 50%.
We reexamined serum samples from 5 of the chimpanzees from the Choo study [1] to determine if neutralizing antibodies were generated. We compared the capacity of the chimpanzee serum specimens to neutralize genotypes 1–6 HCV pseudoparticles (HCVpp) and cell culture–derived HCV (HCVcc) for each genotype expressing the envelope sequences of the same HCV strain [9, 10]. Our study provides important proof-of-concept evidence that it is possible to induce significant titers of cross-reactive NtAb in chimpanzees vaccinated with recombinant HCV envelope glycoproteins.
METHODS
Chimpanzees had been vaccinated with purified HCV-1 E1/E2 proteins either expressed from a vaccinia vector in Hela cells [1] or from a Chinese hamster ovary (CHO) cell line constitutively expressing HCV-1 E1-E2-p7 [11]. The homologous immunization and challenge procedures were described previously [1]. For the heterologous challenge study, HCV-1 vaccinated and challenged chimpanzees had been reimmunized with HCV-1 E1/E2 and challenged 2–3 weeks later with the H77 strain [8]. Archived serum specimens frozen at −80°C were used for the current study.
HCVpp and HCVcc harboring HCV envelope glycoproteins of genotype (strain) 1a (H77), 2a (J6), 3a (S52), 4a (ED43), 5a (SA13), and 6a (HK6a) were used [9, 10]. HCVpp expressing green fluorescent protein (GFP) were incubated with or without serum specimens at room temperature for 1 hour, added to Huh-7 cells, and then incubated at 37°C for 8 hours. Supernatants were replaced with Dulbecco’s modified Eagle’s medium (DMEM)/10% fetal calf serum (FCS), and incubation was continued at 37°C for 72 hours. GFP-positive cells were counted by flow cytometry analysis. Significant neutralization was defined as a decrease of 50% or greater compared with a control incubated with serum specimens from a preimmunization serum sample. HCVcc virus stocks described previously [9] were used to test the capacity of the prechallenge serum specimens to neutralize HCVcc. Forty to 60 focus forming units (FFUs) of HCVcc were incubated for 1 hour at 37°C with 2-fold dilutions of heat-inactivated serum or as a control with growth medium in a final volume of 50 μL. The virus/serum mixture was incubated for 6 hours at 37°C with Huh7.5 cells, plated the previous day at 6000 cells/well on poly-D-lysine-coated 96-well plates (Nunc). Cells were washed, supplemented with fresh growth medium, and incubated for 48 hours at 37°C. FFUs were visualized by HCV NS5A immunostaining and automatically counted on an ImmunoSpot series 5 UV analyzer (CTL Europe GmbH) [12]. Reciprocal 50% neutralization titers indicate the highest dilution showing at least a 50% reduction of FFU count compared with virus not incubated with serum.
RESULTS
In a retrospective study, we have determined whether 5 chimpanzees vaccinated with recombinant HCV-1 (genotype 1a) E1/E2 heterodimers developed NtAb. At the time of homologous HCV-1 challenge, 4 vaccinees had a significant NtAb response against the heterologous H77pp (genotype 1a), with reciprocal NtAb titers between 200 and 1600, corresponding to previously reported titers in anti-E1/E2 and neutralization of binding (NOB) assays (Table 1). However, the fifth chimpanzee (ch653) had a low NtAb titer (1:50), although the NOB titer was equivalent to that of the other animals (Table 1).
In analyses of serially collected serum samples from the 4 chimpanzees with reciprocal NtAb titers ≥200 at the time of challenge, significant levels of NtAb were detected immediately after the first (ch534) or second (ch559, ch357, and ch470) vaccine administration (Figure 1A). We observed 2 patterns of NtAb response to homologous challenge. NtAb of ch559 and ch470 remained at significant levels for more than 50 weeks after viral challenge. Surprisingly, Choo et al. [1] had previously shown that the total E1/E2 antibodies in ch559, as measured by enzyme-linked immunosorbent assay (ELISA), had a high titer at the day of challenge that rapidly decreased to low levels after week 10 post challenge. This result suggests that NtAb probably represented only a minor subpopulation of the anti-E1/E2 in this chimpanzee. In contrast, in ch357 and ch534, NtAb levels steadily decreased post challenge and eventually reached insignificant levels (Figure 1A). In these animals, NtAb responses paralleled the total anti-E1/E2 titers described previously [1].
Figure 1.
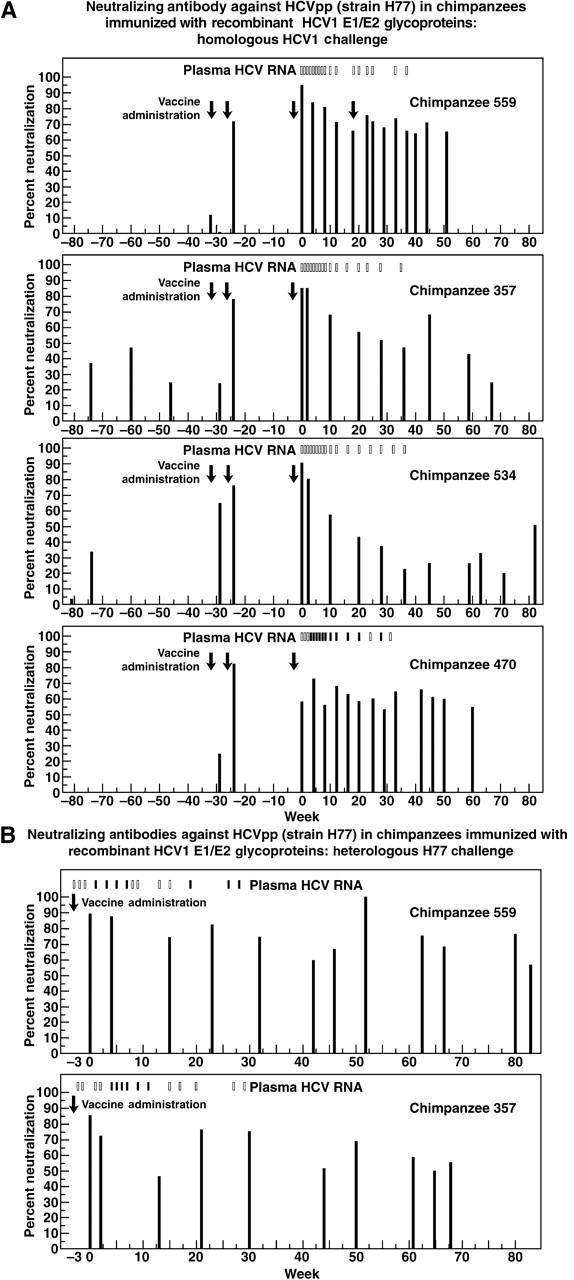
Humoral immune responses in HCV-1 E1/E2-vaccinated chimpanzees. A, Vaccination with HCV-1 E1/E2 and challenge with HCV-1. B, Revaccination with HCV-1 E1/E2 and challenge with H77. The time of vaccinations is indicated by black arrows. The time of challenge is week 0. Qualitative detection of HCV RNA in reverse transcription–nested polymerase chain reaction is indicated (on top) by black (positive) and white (negative) rectangles. Black bars indicate NtAb detected in the HCVpp H77 assay (1:50 dilution tested). Percentage of neutralization was calculated by comparison with serum from a naive animal. Ch653 was not included for analysis of serial samples because it had low HCVpp NtAb titers at the day of challenge.
Two of the protected chimpanzees (ch559 and ch357) were reimmunized with HCV-1 antigen and challenged with a heterologous 1a strain, H77. The repeated vaccination induced a strong NtAb response that persisted over time (Figure 1B). Nevertheless, both chimpanzees became acutely infected after challenge (Figure 1B) [8].
In order to determine whether the genotype 1a vaccine-induced NtAb could cross-neutralize HCV of other genotypes, the relatively high-titered anti-1a serum specimens collected from ch559, ch357, ch534, and ch470 at the day of HCV-1 challenge were tested against HCVpp of other genotypes (Table 1). All serum specimens efficiently cross-neutralized HCVpp of genotype 4a, 5a, and 6a with reciprocal titers between 400 and 1600 (Table 1). In contrast, the same serum specimens cross-neutralized HCVpp of genotype 2a and 3a only minimally, if at all (Table 1).
Serum specimens collected on the day of HCV-1 challenge were tested also for neutralization of HCVcc in a confirmatory test. Due to the very limited amount of serum specimens remaining, only 200-fold or higher dilutions could be tested against HCVcc of genotypes 1a, 4a, 5a, and 6a, and only ch559 and ch653 serum specimens were tested against genotypes 2a and 3a (Table 1). Prechallenge serum specimens from ch559 had reciprocal NtAb titers of ≥800 against HCVcc of genotype 1a, 4a, 5a, and 6a, but none was detected against 2a and 3a (1:100 dilution tested), mirroring findings in the HCVpp assay. This animal also had the highest total anti-E1/E2 and NOB titers. The remaining 4 animals had reciprocal NtAb titers of 200 or undetectable neutralization titers against all but genotype 6a. Overall however, a correlation between neutralization titers obtained in the HCVpp and HCVcc assay was found for ch559, ch357, and ch534.
DISCUSSION
In a first-generation vaccine study, Choo et al [1] demonstrated excellent efficacy of a recombinant HCV E1/E2 subunit vaccine against challenge with homologous HCV-1 (genotype 1a). This protection correlated with high anti-E1/E2 ELISA and NOB titers. In a subsequent study, the authors indirectly investigated the presence of cross-neutralizing antibodies by measuring anti-E2 NOB antibody titers in immunized guinea pigs; the 1a-derived vaccine induced substantial levels of NOB titers against E2 derived from subtype 1b strains [13]. Since the HCV genome is highly heterogeneous, an efficient HCV vaccine most likely needs to elicit protective, long-lasting, broadly cross-neutralizing antibodies. The recent development of the HCVpp and HCVcc systems has allowed direct investigation of the presence of NtAb in the serum specimens of these immunized chimpanzees, using particles harboring envelope proteins of different genotypes. Using heterologous HCVpp of a closely related genotype 1a isolate (strain H77), we demonstrated that immunization induced robust NtAb production, at least in 4 of the 5 chimpanzees. There was no clear correlation between the NOB titers [8] and the neutralization titers determined here, indicating the importance of using native E1/E2 complexes for assessing neutralizing antibody responses (Table 1). These results are in agreement with a previous study by Stamataki et al. [13] who reported similar results in HCV E1/E2 immunized guinea pigs. In addition, even though a previous study reported that there was a direct correlation between complete protection and total E1/E2 antibody levels [8], we could not demonstrate that the outcome after HCV-1 challenge was directly related to NtAb titers. It should be noted, however, that we did not determine the NtAb titers against the homologous HCV-1 strain. We may also speculate that, as recently described in BALB/c mice by Lin et al. [14], immunization with a purified HCV E1/E2 may also induce a T-cell response that could be involved in the outcome of the infection.
We showed here by using HCVpp of different genotypes that genotype 1a envelope glycoproteins induce significant titers of cross-neutralizing antibodies. Strikingly, the serum specimens from immunized chimpanzees reacted strongly with HCVpp representing genotypes 1a, 4a, 5a, and 6a but weakly with HCVpp bearing envelope proteins of genotype 2a or 3a. These HCVpp data support a serologic classification of HCV in which genotypes 1a, 4a, 5a, and 6a comprise 1 serotype and genotypes 2a and 3a comprise 1 or 2 additional serotypes, as we suggested previously [10]. For future vaccine development, it will be important to determine the nature of cross-neutralizing activity of the vaccine-induced NtAb, including mapping of targeted epitopes and determining at which step of the viral life cycle such NtAb interfere.
In HCVcc assays, the 5 vaccinees had relatively high titers of NtAb against genotype 6a, while serum specimens of 3 animals (ch559, ch357, and ch534) neutralized 4a and 5a HCVcc. In contrast, only serum of ch559 neutralized 1a HCVcc (strain H77). However, at the lowest dilution used (1:200), some 1a HCVcc neutralization was observed in 2 additional animals (ch357 and ch534), indicating that 50% neutralization might be achievable using slightly lower dilutions. This could not, however, be tested due to insufficient sample quantity. It was reported that HCV-1 E1/E2-vaccinated guinea pigs had higher NtAb titers against HCV-H77pp than against HCV-H77cc [13]. Furthermore, a differential sensitivity of HCVcc of different genotypes to neutralizing antibodies following a similar pattern as observed here, with a very high sensitivity of 6a, relatively high sensitivity of 4a and 5a, lower sensitivity of 1a, and very limited sensitivity of 2a and 3a HCVcc, was reported [9].
Several factors might contribute to the different results in the HCVpp and HCVcc systems. Even though HCV envelope proteins were from the same HCV strains, they might differ regarding conformation and glycosylation pattern. Thus, it has been shown that major structural differences exist between envelope proteins found at the surface of HCVpp (noncovalent heterodimers) and HCVcc (large covalent complexes) [15]. Further, 6a HCVcc had 2 cell culture adaptive mutations in E1 and E2, which could influence susceptibility to neutralization. In addition, there might be differences in assay conditions. Also, in the HCVpp assay, neutralization titers were calculated in comparison with results obtained with preimmunization serum specimens. Due to the limited quantity of serum specimens, this was not possible in the HCVcc assay. However, in several cases, preimmunization serum specimens were tested in low dilutions against the different HCVcc, and when neutralization results were compared with these values, no significant difference in neutralization titers was seen. Importantly, the preimmunization serum specimens did not neutralize the various HCVcc.
Vaccine-induced production of NtAb was in some cases rather transient. Interestingly, when chimpanzees 559 and 357 were reimmunized, a strong NtAb response was induced and persisted. Yet, both animals became infected with a heterologous 1a strain following challenge, and 1 animal apparently became persistently infected. In a recent study, it was reported that an optimal induction of cross-NtAb, but also broad CD4+ and CD8+ T-cell responses, were obtained by priming with adjuvanted proteins and then boosting with chimeric, defective alphaviruses expressing some HCV proteins [14]. Thus, even though we showed here that immunization with purified E1/E2 was highly immunogenic, these results emphasize that it will be important to identify the appropriate vaccine formulation and regimen capable of inducing the strongest immune response. An effective HCV vaccine will likely need to induce a neutralizing antibody response in combination with robust cellular immune responses.
In conclusion, using the 2 available in vitro assays, our study showed that it is feasible to induce significant titers of cross-reactive NtAb in chimpanzees, the only HCV challenge model, that were vaccinated with recombinant HCV envelope glycoproteins. Thus, these results provide encouragement for the development of recombinant vaccine candidates against hepatitis C.
Acknowledgments
We thank Christine Dong (Chiron) and Kristina Faulk (NIH) for shipping and handling of samples.
Financial support. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). J. B. was the recipient of a professorship at the University of Copenhagen with external funding from the Lundbeck Foundation.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Choo QL, Kuo G, Ralston R, et al. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci USA. 1994;91:1294–8. doi: 10.1073/pnas.91.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farci P, Alter HJ, Wong DC, et al. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc Natl Acad Sci USA. 1994;91:7792–6. doi: 10.1073/pnas.91.16.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feray C, Gigou M, Samuel D, et al. Incidence of hepatitis C in patients receiving different preparations of hepatitis B immunoglobulins after liver transplantation. Ann Intern Med. 1998;128:810–6. doi: 10.7326/0003-4819-128-10-199805150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Knodell RG, Conrad ME, Ginsberg AL, Bell CJ. Efficacy of prophylactic gamma-globulin in preventing non-A, non-B post-transfusion hepatitis. Lancet. 1976;1:557–61. doi: 10.1016/s0140-6736(76)90357-3. [DOI] [PubMed] [Google Scholar]
- 5.Houghton M. Prospects for prophylactic and therapeutic vaccines against the hepatitis C viruses. Immunol Rev. 2011;239:99–108. doi: 10.1111/j.1600-065X.2010.00977.x. [DOI] [PubMed] [Google Scholar]
- 6.Mikkelsen M, Bukh J. Current status of a hepatitis C vaccine: encouraging results but significant challenges ahead. Curr Infect Dis Rep. 2007;9:94–101. doi: 10.1007/s11908-007-0003-6. [DOI] [PubMed] [Google Scholar]
- 7.Dahari H, Feinstone SM, Major ME. Meta-analysis of hepatitis C virus vaccine efficacy in chimpanzees indicates an importance for structural proteins. Gastroenterology. 2010;139:965–74. doi: 10.1053/j.gastro.2010.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coates S, Choo QL, Kuo G, et al. Protection of chimpanzees against heterologous 1a viral challenge using a gpE1/gpE2 heterodimer vaccine. In: Jilbert AR, Grgacic EVL, Vickery K, Burrell CJ, Cossart YE, editors. Proceedings of the 11th International Symposium on Viral Hepatitis and Liver Disease, Sydney 2003. Melbourne, Australia: The Australian Centre for Hepatitis Virology; 2003. pp. 118–23. [Google Scholar]
- 9.Gottwein JM, Scheel TK, Jensen TB, et al. Development and characterization of hepatitis C virus genotype 1-7 cell culture systems: role of CD81 and scavenger receptor class B type I and effect of antiviral drugs. Hepatology. 2009;49:364–77. doi: 10.1002/hep.22673. [DOI] [PubMed] [Google Scholar]
- 10.Meunier JC, Engle RE, Faulk K, et al. Evidence for cross-genotype neutralization of hepatitis C virus pseudo-particles and enhancement of infectivity by apolipoprotein C1. Proc Natl Acad Sci USA. 2005;102:4560–5. doi: 10.1073/pnas.0501275102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spaete RR, Alexander D, Rugroden ME, et al. Characterization of the hepatitis C virus E2/NS1 gene product expressed in mammalian cells. Virology. 1992;188:819–30. doi: 10.1016/0042-6822(92)90537-y. [DOI] [PubMed] [Google Scholar]
- 12.Gottwein JM, Scheel TK, Callendret B, et al. Novel infectious cDNA clones of hepatitis C virus genotype 3a (strain S52) and 4a (strain ED43): genetic analyses and in vivo pathogenesis studies. J Virol. 2010;84:5277–93. doi: 10.1128/JVI.02667-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamataki Z, Coates S, Evans MJ, et al. Hepatitis C virus envelope glycoprotein immunization of rodents elicits cross-reactive neutralizing antibodies. Vaccine. 2007;25:7773–84. doi: 10.1016/j.vaccine.2007.08.053. [DOI] [PubMed] [Google Scholar]
- 14.Lin Y, Kwon T, Polo J, et al. Induction of broad CD4+ and CD8+ T-cell responses and cross-neutralizing antibodies against hepatitis C virus by vaccination with Th1-adjuvanted polypeptides followed by defective alphaviral particles expressing envelope glycoproteins gpE1 and gpE2 and nonstructural proteins 3, 4, and 5. J Virol. 2008;82:7492–503. doi: 10.1128/JVI.02743-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vieyres G, Thomas X, Descamps V, Duverlie G, Patel AH, Dubuisson J. Characterization of the envelope glycoproteins associated with infectious hepatitis C virus. J Virol. 2010;84:10159–68. doi: 10.1128/JVI.01180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


