Abstract
In order to describe the prevalence of hypercholesterolemia and hypertriglyceridemia in a cohort of HIV-infected children and adolescents in Latin America and to determine associations with highly active antiretroviral therapy (HAART), we performed this cross-sectional analysis within the NICHD International Site Development Initiative pediatric cohort study. Eligible children had to be at least 2 years of age and be on HAART. Among the 477 eligible HIV-infected youth, 98 (20.5%) had hypercholesterolemia and 140 (29.4%) had hypertriglyceridemia. In multivariable analyses, children receiving protease inhibitor (PI)-containing HAART were at increased risk for hypercholesterolemia [adjusted odds ratio (AOR) = 2.7, 95% confidence interval (CI) 1.3–5.6] and hypertriglyceridemia (AOR = 3.5, 95% CI 1.9–6.4) compared with children receiving non-nucleoside reverse transcriptase inhibitor (NNRTI)-containing HAART. In conclusion, HIV-infected youth receiving PI-containing HAART in this Latin American cohort were at increased risk for hypercholesterolemia and hypertriglyceridemia compared with those receiving NNRTI-containing HAART.
Keywords: HIV, cholesterol, triglycerides, pediatric
Introduction
In the USA and Europe great success has been achieved in initiating, maintaining and monitoring HIV positive children and adolescents on antiretroviral therapy (ART), however; concerns about a range of adverse drug effects have also emerged. Numerous studies have found ART to be associated with various metabolic complications [1–10]. Hyperlipidemia is particularly concerning in the pediatric context as children must be on ART for extended, even life-long, periods of time, resulting in long-term exposure to the potentially harmful cardiovascular consequences of elevated lipid levels.
In resource-constrained countries, limited access to ART is still a major obstacle. In 2008, worldwide, only 38% of HIV-infected children in need of ART living in low- and middle-income countries were receiving it [11]. However, improved access to ART has increased the estimated total number of children receiving ART from about 75 000 in 2005, to 198 000 in 2007 and 275 700 in 2008 [11, 12]. New World Health Organization recommendations that all HIV infected infants and children ≤2 years of age start life-long ART, regardless of clinical or immunologic stage, and earlier initiation criteria for those ≥2 years of age will lead to further dramatic increases in the number of infants and children receiving treatment around the world [13, 14]. As this progress is made and more children begin to receive life-saving ART, understanding the adverse drug effects associated with exposure to ART will become increasingly important for clinicians and public-health officials worldwide. Studies of treated HIV-infected children and adolescents in resource-constrained settings are needed in order to document the frequency of metabolic complications among pediatric cohorts in these settings where environmental and other factors that impact on these complications (e.g. diet, co-morbid conditions, race and ethnicity) differ from those in resource-rich settings.
In Latin America, especially in Brazil, pediatric access to ART has been better than the global average. According to the 2009 UNAIDS Universal Access Report, as of December 2008, 76% of the HIV positive children in Latin America and the Caribbean in need of ART were receiving it [11]. The objectives of the present analysis were to describe the prevalence of hypercholesterolemia and hypertriglyceridemia in a cohort of Latin American HIV-infected children and to determine the associations between highly active ART (HAART) and hyperlipidemia, using data collected as part of an ongoing observational cohort study being conducted at multiple Latin American sites [15].
Materials and Methods
NISDI pediatric cohort
In 2002, The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) began enrollment to the NICHD International Site Development Initiative (NISDI) pediatric protocol at 15 sites in Brazil, Mexico and Argentina. In 2006, two additional sites were added, one in Peru and one in Jamaica. A description of this protocol and the cohort has been published [15]. The NICHD Institutional Review Board (IRB), the data management and statistical center IRB, separate in-country ethics committees and national review boards (where required, i.e., Brazil) reviewed and approved the protocol. Informed consent was obtained from either parents or guardians or from subjects who were able to provide consent based upon local laws. Assent was obtained from subjects >8 years of age when developmentally appropriate.
An objective of the NISDI pediatric protocol is to describe early and late outcomes related to HIV disease and ART in HIV-infected infants, children and adolescents. To this end, the following were performed at 6-month intervals: medical history, physical examination, hematology, flow cytometry and standard biochemical assays including total cholesterol and triglycerides. The protocol did not require samples for cholesterol and triglycerides to be collected in the fasting state, but it was the practice at most of the sites to perform these tests when subjects were fasting. Cholesterol and triglycerides were measured by routine methods at each site’s local laboratory. The 2000 CDC growth charts were used to characterize growth parameters [16]. HIV disease was classified according to the CDC Classification System [17].
Study population
To be eligible for this cross-sectional analysis, HIV-infected children in the NISDI pediatric cohort had to be at least 2 years of age at the time of their enrollment study visit or at the time of the first on study cholesterol and triglyceride testing and be on HAART, defined below, on the day the laboratory tests were performed. We excluded children who were not on ART or who were on single or dual ART because selection of therapy was not randomized and thus these groups likely differed from those on HAART in substantial ways. Children with diabetes, nephrotic or nephritic syndrome, uremia or hypothyroidism were also excluded. All study visits for this analysis occurred between September 2002 and September 2006.
Definitions
While cut points for abnormal cholesterol and triglycerides may depend upon age, sexual maturation, and other developmental factors, for the purpose of this analysis we used a single definition for each, based upon an American Academy of Pediatrics report that also draws upon recommendations from the American Heart Association [18]. Hypercholesterolemia was defined as a serum cholesterol level of ≥200 mg dl−1, and hypertriglyceridemia was defined at a serum triglyceride level of ≥150 mg dl−1. Subjects were considered to be on HAART if they were taking at least three antiretrovirals that included at least one non-nucleoside reverse transcriptase inhibitor (NNRTI) or protease inhibitor (PI). Subjects were considered to be on: (i) NNRTI-containing HAART if they were receiving an NNRTI along with nucleoside reverse transcriptase inhibitors (NRTIs); (ii) PI-containing HAART if they were receiving at least one PI along with NRTIs; and (iii) PI + NNRTI-containing HAART if they were on a regimen containing at least one PI, an NNRTI and at least one NRTI.
Statistical analysis
The outcomes of interest were hypercholesterolemia and hypertriglyceridemia. Chi-square analysis was used to identify categorical variables associated with the two outcomes, respectively. Variables associated with the outcomes in the bivariate models (at the 20% significance level, p ≤ 0.2) were included in the multivariable logistic regression models. We used stepwise selection and backward elimination methods to determine the most parsimonious model. Only variables with a p-value <0.05 remained in the final model. Statistical analyses were performed using SAS 9.1 software (SAS Institute Inc., Cary, NC, USA).
Results
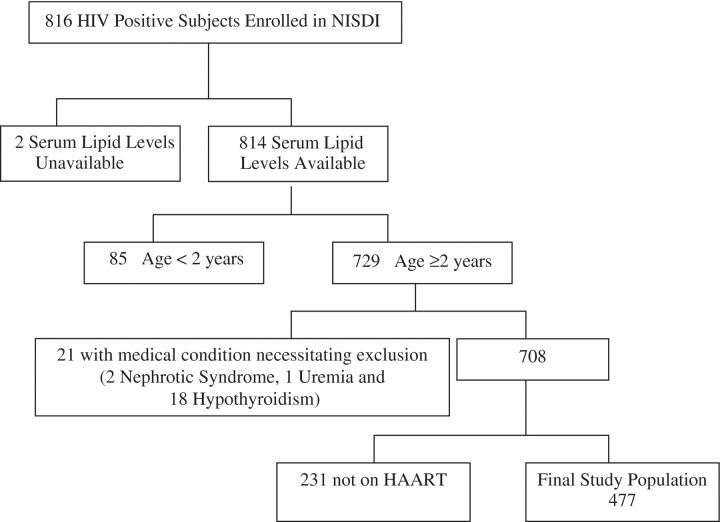
As of October 2007, there were 816 HIV-infected subjects enrolled in the NISDI pediatric cohort. The derivation of the study population for this analysis is shown in Fig. 1. Subjects <2 years of age and all of those with nephrotic syndrome, uremia, and hypothyroidism were excluded from the study. Subjects who were not on ART (n = 109) or who were on single or dual ART (n = 122) at the time of cholesterol and triglyceride testing were excluded. Among the 109 subjects not on ART, only 7 (6.4%) had hypercholesterolemia and 12 (11.0%) had hypertriglyceridemia; among the 122 subjects on single or dual ART only one (0.8%) had hypercholesterolemia and 19 (15.6%) had hypertriglyceridemia. The final study population used in this analysis consisted of 477 subjects on HAART.
Fig. 1.
Derivation of study population.
Characteristics of this study population according to the regimens they were receiving at the time of cholesterol testing are shown in Table 1. Median age overall was 6.8 years (range 2–20.5 years). Most of the 477 children were vertically infected (90.4%) and most had been on their current HAART regimen for at least 6 months (82.9%) at the time of cholesterol and triglyceride testing. The cohort consists of children from Argentina, Mexico, Jamaica, Peru and Brazil with the latter contributing the greatest proportion of children (61.4%). Subjects on NNRTI-containing HAART did not differ significantly from those on PI-containing HAART with respect to the percent who had received a previous HAART regimen or duration of previous HAART, but were significantly more likely to have less severe HIV disease (p < 0.001). A majority of those receiving a PI (58.3%) were on nelfinavir, followed by ritonavir (31.1%).
Table 1.
Characteristics of the study population
| Characteristics | NNRTI-containing HAART | PI-containing HAART | PI + NNRTI- containing HAART | Total | p-valuea |
|---|---|---|---|---|---|
| (N = 112) | (N = 311) | (N = 54) | (N = 477) | ||
| n (%) | n (%) | n (%) | n (%) | ||
| Age at lab test, years | |||||
| 2–5 | 49 (43.8) | 143 (46.0) | 15 (27.8) | 207 (43.4) | 0.11 |
| 6–11 | 45 (40.2) | 132 (42.4) | 29 (53.7) | 206 (43.2) | |
| ≥12 | 18 (16.1) | 36 (11.6) | 10 (18.5) | 64 (13.4) | |
| Age at time of initiating current HAART regimen, years | |||||
| <2 | 10 (8.9) | 72 (23.2) | 2 (3.7) | 84 (17.6) | <0.001 |
| 2–5 | 57 (50.9) | 140 (45.0) | 24 (44.4) | 221 (46.3) | |
| 6–11 | 33 (29.5) | 81 (26.0) | 21 (38.9) | 135 (28.3) | |
| ≥12 | 12 (10.7) | 18 (5.8) | 7 (13.0) | 37 (7.8) | |
| Gender | |||||
| Female | 59 (52.7) | 167 (53.7) | 25 (46.3) | 251 (52.6) | 0.60 |
| Male | 53 (47.3) | 144 (46.3) | 29 (53.7) | 226 (47.4) | |
| Race | |||||
| White | 41 (50.0) | 104 (53.1) | 10 (41.7) | 155 (51.3) | <0.01 |
| Black/African | 32 (39.0) | 45 (23.0) | 4 (16.7) | 81 (26.8) | |
| Mestizo | 4 (4.9) | 35 (17.9) | 9 (37.5) | 48 (15.9) | |
| Mulato | 5 (6.1) | 12 (6.1) | 1 (4.2) | 18 (6.0) | |
| Missing | 30 | 115 | 30 | 175 | |
| Ethnicity | |||||
| Hispanic/Latino | 59 (84.3) | 199 (98.5) | 40 (100) | 298 (95.5) | <0.0001 |
| Not Hispanic/Latino | 11 (15.7) | 3 (1.5) | 0 (0) | 14 (4.5) | |
| Missing | 42 | 109 | 14 | 165 | |
| BMI category (Z-score percentile) | |||||
| Underweight (≤5th) | 13 (11.9) | 25 (8.1) | 6 (11.1) | 44 (9.3) | 0.72 |
| Normal (5th to 84th) | 79 (72.5) | 233 (75.6) | 43 (79.6) | 355 (75.4) | |
| At risk of overweight (85th to 94th) | 10 (9.2) | 31 (10.1) | 4 (7.4) | 45 (9.6) | |
| Overweight (≥95th) | 7 (6.4) | 19 (6.2) | 1 (1.9) | 27 (5.7) | |
| Missing | 3 | 3 | 0 | 6 | |
| CD4 percent | |||||
| <15 | 12 (13.6) | 35 (12.5) | 9 (17.6) | 56 (13.4) | 0.78 |
| 15–24 | 20 (22.7) | 77 (27.6) | 13 (25.5) | 110 (26.3) | |
| ≥25 | 56 (63.6) | 167 (59.9) | 29 (56.9) | 252 (60.3) | |
| Missing | 24 | 32 | 3 | 59 | |
| CD4 percent, median (range) | 28 (1–56) | 27 (1–54) | 26 (5–60) | 27 (1–60) | 0.34 |
| HIV viral load, copies ml−1 | |||||
| <400 | 39 (35.5) | 103 (33.1) | 26 (48.1) | 168 (35.4) | 0.10 |
| ≥400 | 71 (64.5) | 208 (66.9) | 28 (51.9) | 307 (64.6) | |
| Missing | 2 | 0 | 0 | 2 | |
| HIV viral load, copies ml−1, median (range) | 4045 (25–1 700 000) | 3534 (14–886 000) | 770 (25–291 000) | 3750 (14–1 700 000) | 0.26 |
| HIV class | |||||
| A + N | 47 (42.0) | 71 (22.8) | 8 (14.8) | 126 (26.4) | <0.001 |
| B | 29 (25.9) | 114 (36.7) | 26 (48.1) | 169 (35.4) | |
| C | 36 (32.1) | 126 (40.5) | 20 (37.0) | 182 (38.2) | |
| Country of residence | |||||
| Argentina | 13 (11.6) | 28 (9.9) | 3 (5.6) | 44 (9.2) | <0.0001 |
| Brazil | 83 (74.1) | 195 (62.7) | 15 (27.8) | 293 (61.4) | |
| Jamaica | 12 (10.7) | 1 (0.3) | 0 (0.0) | 13 (2.7) | |
| Mexico | 4 (3.6) | 71 (22.8) | 36 (66.7) | 111 (23.3) | |
| Peru | 0 (0.0) | 16 (5.1) | 0 (0.0) | 16 (3.4) | |
| Duration of time on current HAART regimen, months, median (range) | 16.2 (0–52) | 22.6 (0–76) | 23.2 (3–61) | 21.4 (0–76) | <0.01 |
| Duration of time on current HAART regimen, months | |||||
| <6 | 23 (21.3) | 53 (17.3) | 4 (7.4) | 80 (17.1) | 0.08 |
| ≥6 | 85 (78.7) | 254 (82.7) | 50 (92.6) | 389 (82.9) | |
| Missing | 4 | 4 | 0 | 8 | |
| Previous HAART regimen | |||||
| No | 69 (61.6) | 189 (60.8) | 14 (25.9) | 272 (57.0) | <0.0001 |
| Yes | 43 (38.4) | 122 (39.2) | 40 (74.1) | 205 (43.0) | |
| Duration of time on previous HAART regimen, months, median (range) | 20.8 (0.1–57.4) | 17.0 (0.1–69.0) | 20.1 (3.8–52.8) | 17.8 (0.1–69.0) | 0.20 |
| Mode of transmission | |||||
| Horizontal | 16 (14.3) | 25 (8.0) | 5 (9.3) | 46 (9.6) | 0.16 |
| Vertical | 96 (85.7) | 286 (92.0) | 49 (90.7) | 431 (90.4) |
ap-values are calculated from chi square for categorical variables and Kruskal–Wallis Test for continuous variables.
Overall, 98 of the 477 children (20.5%) had hypercholesterolemia and 140 (29.4%) had hypertriglyceridemia. Median cholesterol levels were 150.0 mg dl−1 [inter-quartile range (IQR) 124–170] for the NNRTI-containing HAART group, 170.0 mg dl−1 (IQR 143–196) for the PI-containing HAART group, and 179.5 mg dl−1 (IQR 150–204) for the PI + NNRTI-containing HAART group (Fig. 2a). Median triglyceride levels were 74.5 mg dl−1 (IQR 55–104) for the NNRTI-containing HAART group, 117.0 mg dl−1 (IQR 88–171) for the PI-containing HAART group, and 114.5 mg dl−1 (IQR 91–165) for the PI + NNRTI-containing HAART group (Fig. 2b).
Fig. 2.
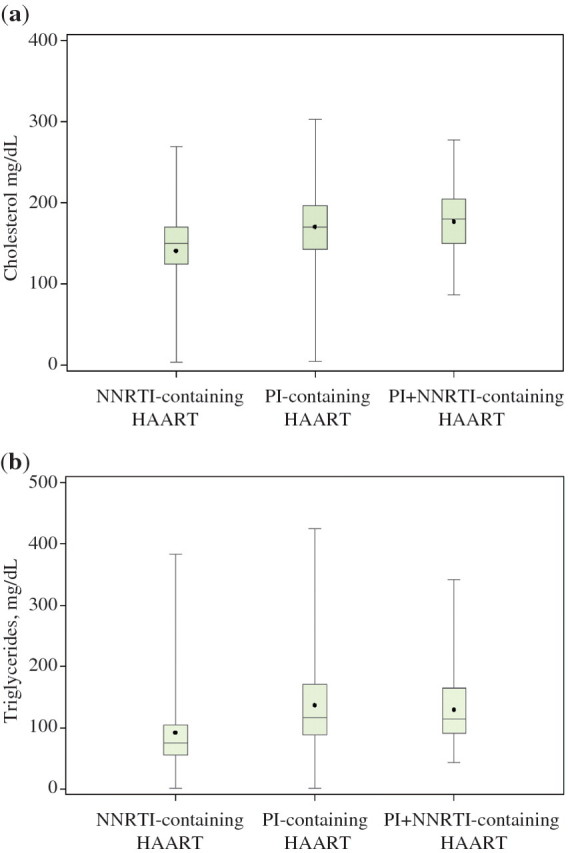
(a) Cholesterol levels according to type of HAART regimen. Comparison of means p < 0.0001. (b) Triglyceride levels according to type of HAART regimen. Comparison of means p < 0.0001. The horizontal line within the box represents the median value and the dot represents the mean value. The top and bottom horizontal lines that make up the box represent the third and first quartiles, respectively. The vertical line extending out of the box represents the overall range (maximum and minimum) of values.
All categorical variables shown in Table 1 were evaluated for their association with hypercholesterolemia and hypertriglyceridemia in bivariate analysis. Race and ethnicity, however, were not considered in the multivariable models due to insufficient data. Although country of residence was associated with hypercholesterolemia and hypertriglyceridemia at the bivariate level (<0.0001 and 0.02, respectively), this variable was not included in the final multivariable models due to insufficient numbers of eligible clients from Jamaica (n = 13) and Peru (n = 16). Although not shown in Table 1, we also evaluated the association between age at the time of initiating first HAART regimen and hypercholesterolemia and hypertriglyceridemia.
Factors associated with hypercholesterolemia
In bivariate analysis, PI-containing HAART regimen (p < 0.01), high CD4 percent (p < 0.01), low viral load (p < 0.0001), country of residence (p < 0.0001), age at the time of lab test (0.11), and duration of time on HAART ≥6 months (p = 0.18) were associated with high cholesterol at the 20% significance level. These variables, with the exception of country, were considered for multivariable modeling (Table 2), and HAART regimen, CD4 percent, and viral load remained in the final model. Children on PI-containing HAART as well as those on PI + NNRTI-containing HAART were at increased risk for high cholesterol compared to children on NNRTI-containing HAART [adjusted odds ratio (AOR) = 2.7, 95% confidence interval (CI) 1.3–5.6 and AOR = 2.7, 95% CI 1.1–7.0, respectively]. Hypercholesterolemia was also inversely associated with viral load. Children with a detectable viral load were less likely than children with undetectable levels to have high cholesterol (AOR = 0.3, 95% CI 0.2–0.6) and those with a CD4 percent <15 were less likely than those with a CD4 percent >25 to have hypercholesterolemia (AOR = 0.3, 95% CI 0.1–0.9)
Table 2.
Multivariable analysis of factors associated with hypercholesterolemia in HIV-infected children on HAART
| Hypercholesterolemia |
p-valuea | AORb | 95% CI | ||
|---|---|---|---|---|---|
| Yes (N = 98) | No (N = 379) | ||||
| n (%) | n (%) | ||||
| HAART regimen | |||||
| NNRTI-containing | 11 (9.8) | 101 (90.2) | <0.01 | Reference | |
| PI-containing | 73 (23.5) | 238 (76.5) | 2.7 | 1.3–5.6 | |
| PI + NNRTI-containing | 14 (25.9) | 40 (74.1) | 2.7 | 1.1–7.0 | |
| CD4 percent | |||||
| <15 | 4 (7.1) | 52 (92.9) | 0.3 | 0.1–0.9 | |
| 15–24 | 21 (19.1) | 89 (80.9) | <0.01 | 0.7 | 0.4–1.3 |
| ≥25 | 65 (25.8) | 187 (74.2) | Reference | ||
| Missing | 8 | 51 | |||
| Viral load (copies ml−1) | |||||
| <400 | 56 (33.3) | 112 (66.7) | <0.0001 | Reference | |
| ≥400 | 42 (13.7) | 265 (86.3) | 0.3 | 0.2–0.6 | |
| Missing | 0 | 2 | |||
ap-values calculated from chi square for bivariate analysis
bAll variables in Table 1 were considered at bivariate analysis, six variables (HAART regimen, age at lab test, CD4 percent, HIV viral load, duration of time on current regimen, and country of residence) met criteria for the multivariable model (p ≤ 0.2 in bivariate analysis). Country was excluded from the final model due to an insufficient number of clients from Jamaica (0 of 13 with hypercholesterolemia). Only the variables shown in this table remained in the final model.
Factors associated with hypertriglyceridemia
In bivariate analysis, PI-containing HAART regimen (p < 0.001), low body mass index (BMI) (p = 0.10), country of residence (p = 0.02), having received a previous HAART regimen (p = 0.02), age at the time of lab test (p = 0.16), and age at the time of initiating the current HAART regimen (0.03), met the criteria to enter the multivariable hypertriglyceridemia model (Table 3). Only HAART regimen, BMI, age at the time of initiating the current HAART regimen, and having received a previous HAART regimen remained in the final model (Table 3). While the association between age at the time of initiating the first HAART regimen and hypertriglyceridemia met the criteria for inclusion into the multivariable model (p = 0.8), we did not include it due its significant correlation with age at the time of initiating current HAART regimen (r = 0.94). Children on PI-containing HAART were 3.5 times more likely to have high triglycerides than children on NNRTI-containing HAART (AOR = 3.5, 95% CI 1.9–6.4). BMI was also a significant factor with underweight children being at higher risk of high triglycerides than children with a normal BMI (AOR = 2.1, 95% CI 1.1–4.2).
Table 3.
Multivariable analysis of factors associated with hypertriglyceridemia in HIV-infected children on HAART
| Hypertriglyceridemia |
Adjusted |
||||
|---|---|---|---|---|---|
| Yes (N = 140) | No (N = 337) | p-valuea | AORb | 95% CI | |
| HAART regimen | |||||
| NNRTI-containing | 17 (15.2%) | 95 (84.8%) | <0.001 | Reference | |
| PI-containing | 107 (34.4%) | 204 (65.6%) | 3.5 | 1.9–6.4 | |
| PI + NNRTI-containing | 16 (29.6%) | 38 (70.4%) | 2.1 | 0.9–4.8 | |
| BMI category (Z-score percentile) | |||||
| Normal (5th to 84th) | 98 (27.6%) | 257 (72.4%) | 0.10 | Reference | |
| Underweight (<5th) | 19 (43.2%) | 25 (56.8%) | 2.1 | 1.1–4.2 | |
| Overweight (≥85th) | 21 (29.2%) | 51 (70.8%) | 1.2 | 0.7–2.2 | |
| Missing | 2 | 4 | |||
| Age at time of initiating current HAART regimen, years | |||||
| <2 | 26 (31.0) | 58 (69.0) | 0.03 | 0.4 | 0.2–1.0 |
| 2–5 | 53 (24.0) | 168 (76.0) | 0.3 | 0.1–0.7 | |
| 6–11 | 44 (32.6) | 91 (67.4) | 0.5 | 0.2–1.1 | |
| ≥12 | 17 (45.9) | 20 (54.1) | Reference | ||
| Previous HAART regimen | |||||
| Yes | 72 (35.1) | 133 (64.9) | 0.02 | 1.6 | 1.0–2.5 |
| No | 68 (25.0) | 204 (75.0) | |||
ap-values calculated from chi square for bivariate analysis.
bAll variables in Table 1 were considered at bivariate analysis, six variables (HAART regimen, age at lab test, BMI, country of residence, previous HAART regimen, and age at the time of initiating the current HAART regimen) met criteria for the multivariable model (p ≤ 0.2 in bivariate analysis). Only the variables shown in this table remained in the final model.
Discussion
To our knowledge, the study population presented here is the largest described to date for a Latin American pediatric HIV cohort and demonstrates high rates of hypercholesterolemia and hypertriglyceridemia in HIV-infected children and adolescents. As compared with children on NNRTI-containing HAART, we found that children on PI-containing HAART were approximately three times more likely to have hypercholesterolemia and hypertriglyceridemia, independent of other risk factors. Similar associations between hyperlipidemia and PI-containing HAART have been reported previously in HIV infected pediatric populations, primarily in Europe and the USA [1–3, 5, 7–10]. In a small study, Miller et al. reported a potential protective effect of NNRTI-containing therapy [19], but in a larger study no significant differences were seen between those treated with NNRTI-containing HAART versus PI-containing HAART [9].
Children in our study with a detectable viral load were less likely to have hypercholesterolemia than those with undetectable viral levels. This finding is biologically plausible if detectable viral load is functioning as a proxy for non-adherence to the prescribed regimen, as has been found in similar studies [1, 3, 7]. It is not clear why underweight children were found to have a greater risk of hypertriglyceridemia when other variables were controlled for in the analysis. Of interest, a high BMI was not found to be associated with hypertriglyceridemia as would be expected and has been reported in other studies of both general and HIV infected pediatric populations [1, 20, 21]. However, triglyceride results may have been impacted by the lack of mandatory fasting before the study samples were collected. We did not find children on PI + NNRTI-containing regimens to be at greater risk of hypercholesterolemia or hypertriglyceridemia than those on PI-containing regimens; however, we did not evaluate total-HDL cholesterol ratios which have been shown to be increased in children receiving PI + NNRTI-containing regimens [9].
There are several limitations to our findings. First, data reported are cross sectional in nature. Thus, we have not considered the impact of ART on lipid levels over time. Rather, laboratory results were linked with the antiretrovirals the patient was taking at the time of blood collection and may in reality be affected by previous regimens. However, more than half of the participants were on their first HAART regimen and the vast majority had been on their regimen for at least 6 months at the time of lipid testing. We recognize that children on PI-containing HAART may be those with a longer history of HIV infection and ART and therefore at greater risk for hyperlipidemia, but duration of prior HAART did not differ between these two groups in our study (although those on NNRTI-containing HAART were significantly less likely to have severe HIV disease). Finally, we had hoped to include variables for race, ethnicity, and country in the analysis but were unable to due to inadequate numbers. When we limited the analysis to subjects from Brazil, Argentina and Mexico, we found that subjects from Argentina were at increased risk for hypercholesterolemia as compared to those from Brazil (AOR = 3.3, 95% CI 1.6–6.8). As enrollment continues over time, this information may be available for future analyses.
Though there are no data to suggest that dyslipidemia presents a threat for immediate adverse health effects in the pediatric population, the long term consequences of elevated lipid levels on the cardiovascular system are of great concern. Post-mortem examination of vascular tissue collected through the Pathobiological Determinants of Atherosclerosis in Youth and Bogalusa Heart studies revealed atherosclerotic precursors, such as fatty streaks and fibrous plaques in the arterial lining, present as early as childhood and adolescence [22, 23]. Furthermore, both studies also found a positive association between the presence of such findings and elevated serum cholesterol levels. Perhaps more alarmingly, additional evidence suggests that cardiovascular risk factors experienced during adolescence, including an elevated cholesterol level, may contribute to the development of atherosclerotic changes regardless of whether those risk factors persist in later life [24].
In the USA, the potential impact of HAART on lipid parameters is recognized, and pediatric ART guidelines recommend regular assessment of a lipid panel [25]. However, recognition is not widespread, as the guidelines for cardiovascular risk reduction in high-risk pediatric populations do not address either HIV or ART [26]. Recognition of and concern for long term cardiovascular risks may be much less evident in resource-limited settings, especially where the capacity for frequent lab monitoring and follow up, availability of resources for diet and exercise related lifestyle modification, access to lipid-lowering agents and a variety of antiretrovirals for regimen customization are not available. Nevertheless, as WHO guidance recommends earlier initiation of life-long treatment in infants and children, the global health community works to scale up universal access to ART for HIV-infected infants and children and while the benefit of ART in saving the lives of children infected with HIV far outweighs the immediate adverse lipid effects of therapy, consideration must ultimately be given to the long-term health outcomes for children on such medications and the impact managing these effects will have on future health care providers and health systems.
Funding
This work was supported by the National Institutes of Health (NICHD Contract # HHSN267200800001C [NICHD Control #: N01-HD-3-3345 and N01-HD-8-0001]).
Appendix 1
NISDI Pediatric Study Group 2010: Principal investigators, co-principal investigators, study coordinators, data management center representatives, and NICHD staff include: Brazil: Belo Horizonte: Jorge Pinto, Flávia Faleiro (Universidade Federal de Minas Gerais); Caxias do Sul: Ricardo da Silva de Souza, Nicole Golin, Sílvia Mariani Costamilan (Universidade de Caxias do Sul/ Serviço Municipal de Infectologia); Nova Iguacu: Jose Pilotto, Beatriz Grinsztejn, Valdilea Veloso, Gisely Falco (Hospital Geral Nova de Iguacu – HIV Family Care Clinic); Porto Alegre: Ricardo da Silva de Souza, Breno Riegel Santos, Rita de Cassia Alves Lira (Universidade de Caxias do Sul/Hospital Conceição); Ricardo da Silva de Souza, Mario Ferreira Peixoto, Elizabete Teles (Universidade de Caxias do Sul/Hospital Fêmina); Ricardo da Silva de Souza, Marcelo Goldani, Margery Bohrer Zanetello (Universidade de Caxias do Sul /Hospital de Clínicas de Porto Alegre); Regis Kreitchmann, Debora Fernandes Coelho (Irmandade da Santa Casa de Misericordia de Porto Alegre); Ribeirão Preto: Marisa M. Mussi-Pinhata, Maria Célia Cervi, Márcia L. Isaac, Bento V. Moura Negrini (Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo); Rio de Janeiro: Ricardo Hugo S. Oliveira, Maria C. Chermont Sapia (Instituto de Puericultura e Pediatria Martagão Gesteira); Esau Custodio Joao, Maria Leticia Cruz, Plinio Tostes Berardo, Ezequias Martins (Hospital dos Servidores do Estado); São Paulo: Regina Celia de Menezes Succi, Daisy Maria Machado (Universidade Federal de São Paulo); Marinella Della Negra, Wladimir Queiroz, Yu Ching Lian (Instituto de Infectologia Emilio Ribas); Mexico: Mexico City: Noris Pavía-Ruz, Patricia Villalobos-Acosta, Dulce Morales-Pérez (Hospital Infantil de México Federico Gómez); Peru: Lima: Jorge Alarcón Villaverde (Instituto de Medicina Tropical ‘Daniel Alcides Carrión’—Sección de Epidemiologia, UNMSM), Maria Castillo Díaz (Instituto Nacional de Salud del Niño), Mary Felissa Reyes Vega (Instituto de Medicina Tropical ‘Daniel Alcides Carrión’—Sección de Epidemiologia, UNMSM); Data Management and Statistical Center: Yolanda Bertucci, Laura Freimanis Hance, René Gonin, D. Robert Harris, Roslyn Hennessey, Margot Krauss, James Korelitz, Sharon Sothern de Sanchez, Sonia K. Stoszek (Westat, Rockville, MD, USA); NICHD: Rohan Hazra, Lynne Mofenson, Jennifer Read, George Siberry, Carol Worrell (Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland).
References
- 1.Carter RJ, Wiener J, Abrams EJ, et al. Dyslipidemia among perinatally HIV-infected children enrolled in the PACTS-HOPE cohort, 1999–2004: a longitudinal analysis. J Acquir Immune Defic Syndr. 2006;41:453–60. doi: 10.1097/01.qai.0000218344.88304.db. [DOI] [PubMed] [Google Scholar]
- 2.Cheseaux JJ, Jotterand V, Aebi C, et al. Hyperlipidemia in HIV-infected children treated with protease inhibitors: relevance for cardiovascular diseases. J Acquir Immune Defic Syndr. 2002;30:288–93. doi: 10.1097/00126334-200207010-00004. [DOI] [PubMed] [Google Scholar]
- 3.Farley J, Gona P, Crain M, et al. Prevalence of elevated cholesterol and associated risk factors among perinatally HIV-infected children (4–19 years old) in Pediatric AIDS Clinical Trials Group 219C. J Acquir Immune Defic Syndr. 2005;38:480–7. doi: 10.1097/01.qai.0000139397.30612.96. [DOI] [PubMed] [Google Scholar]
- 4.Gafni RI, Hazra R, Reynolds JC, et al. Tenofovir disoproxil fumarate and an optimized background regimen of antiretroviral agents as salvage therapy: impact on bone mineral density in HIV-infected children. Pediatrics. 2006;118:e711–18. doi: 10.1542/peds.2005-2525. [DOI] [PubMed] [Google Scholar]
- 5.Lainka E, Oezbek S, Falck M, et al. Marked dyslipidemia in human immunodeficiency virus-infected children on protease inhibitor-containing antiretroviral therapy. Pediatrics. 2002;110:e56. doi: 10.1542/peds.110.5.e56. [DOI] [PubMed] [Google Scholar]
- 6.McComsey GA, Leonard E. Metabolic complications of HIV therapy in children. AIDS. 2004;18:1753–68. doi: 10.1097/00002030-200409030-00004. [DOI] [PubMed] [Google Scholar]
- 7.Tassiopoulos K, Williams PL, Seage GR, 3rd, et al. Association of hypercholesterolemia incidence with antiretroviral treatment, including protease inhibitors, among perinatally HIV-infected children. J Acquir Immune Defic Syndr. 2008;47:607–14. doi: 10.1097/QAI.0b013e3181648e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor P, Worrell C, Steinberg SM, et al. Natural history of lipid abnormalities and fat redistribution among human immunodeficiency virus-infected children receiving long-term, protease inhibitor-containing, highly active antiretroviral therapy regimens. Pediatrics. 2004;114:e235–42. doi: 10.1542/peds.114.2.e235. [DOI] [PubMed] [Google Scholar]
- 9.Chantry CJ, Hughes MD, Alvero C, et al. Lipid and glucose alterations in HIV-infected children beginning or changing antiretroviral therapy. Pediatrics. 2008;122:e129–38. doi: 10.1542/peds.2007-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Paediatric Lipodystrophy Group. Antiretroviral therapy, fat redistribution and hyperlipidaemia in HIV-infected children in Europe. AIDS. 2004;18:1443–51. doi: 10.1097/01.aids.0000131334.38172.01. [DOI] [PubMed] [Google Scholar]
- 11.Towards Universal Access: Scaling Up Priority HIV/AIDS Interventions in the Health Sector. Progress Report. Geneva, Switzerland: WHO, UNAIDS, UNICEF; 2009. [Google Scholar]
- 12.Children and AIDS: A Stocktaking Report. New York, NY: Unite for Children, Unite Against AIDS; 2007. [Google Scholar]
- 13.Antiretroviral Therapy for HIV Infection in Infants and Children WHO 2010. [28 September 2010, date last accessed]. http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf. [Google Scholar]
- 14.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hazra R, Stoszek SK, Hance LF, et al. Cohort Profile: NICHD International Site Development Initiative (NISDI): a prospective, observational study of HIV-exposed and HIV-infected children at clinical sites in Latin American and Caribbean countries. Int J Epidemiol. 2009;38:1207–14. doi: 10.1093/ije/dyn239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.A SAS Program for the CDC Growth Charts. [4 December 2008, date last accessed]. http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/sas.htm. [Google Scholar]
- 17.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 18.Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 19.Miller TL, Orav EJ, Lipshultz SE, et al. Risk factors for cardiovascular disease in children infected with human immunodeficiency virus-1. J Pediatr. 2008;153:491–7. doi: 10.1016/j.jpeds.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss R, Caprio S. The metabolic consequences of childhood obesity. Best Pract Res Clin Endocrinol Metab. 2005;19:405–19. doi: 10.1016/j.beem.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 22.Berenson GS, Srinivasan SR, Bao W, et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–6. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 23.McGill HC, Jr, McMahan CA, Malcom GT, et al. Effects of serum lipoproteins and smoking on atherosclerosis in young men and women. The PDAY Research Group. Pathobiological determinants of atherosclerosis in youth. Arterioscler Thromb Vasc Biol. 1997;17:95–106. doi: 10.1161/01.atv.17.1.95. [DOI] [PubMed] [Google Scholar]
- 24.Raitakari OT, Juonala M, Kahonen M, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–83. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 25. Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. 23 February 2009 http://aidsinfo.nih.gov/ContentFiles/PediatricGuidelines.pdf (2 July 2010, date last accessed) [Google Scholar]
- 26.Kavey RE, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710–38. doi: 10.1161/CIRCULATIONAHA.106.179568. [DOI] [PubMed] [Google Scholar]