Abstract
Introduction:
Cigarillo smoking likely exposes users to many of the same toxicants (e.g., nicotine, carbon monoxide [CO]) as cigarette smoking. Yet, few controlled clinical laboratory studies evaluating of the effects of cigarillos exist. This study evaluated the cardiovascular response, toxicant exposure, subjective effects, and puffing topography of a popular cigarillo brand, Black & Mild (B&M).
Methods:
Sixteen B&M smokers (M ± SD = 1.9 ± 2.5 cigarillos/day for 3.4 ± 3.5 years) participated in 2 counterbalanced conditions: lit (ACTIVE) or unlit (SHAM) B&M in which they completed two 10-puff smoking bouts (30-s interpuff intervals).
Results:
Plasma nicotine concentrations for ACTIVE increased significantly from pre-smoking (M ± SEM = 2.0 ± 0.0 ng/ml) to 5 min after Bouts 1 (5.3 ± 0.8 ng/ml) and 2 (4.9 ± 0.9 ng/ml) but did not increase above 2.0 ± 0.0 ng/ml at any timepoint for SHAM. Heart rate increased significantly from pre- to post-smoking for ACTIVE but not for SHAM. Average expired-air CO levels, collapsed across time, were 14.3 ± 0.8 ppm for ACTIVE and 4.5 ± 0.2 ppm for SHAM. Neither condition reduced symptoms of nicotine/tobacco abstinence reliably, although ratings for some measures were significantly lower for ACTIVE than for SHAM. ACTIVE, but not SHAM, produced a variety of positive effects related to product sensory characteristics (e.g., “satisfying,” “pleasant”). Smoking topography did not differ across the two conditions.
Conclusions:
Ten puffs from a B&M cigarillo deliver active doses of nicotine and considerable amounts of CO but do not suppress abstinence-induced withdrawal symptoms reliably. The nicotine delivery profile suggests that cigarillo smoking may promote nicotine/tobacco dependence and the CO exposure likely poses significant health risks.
Introduction
Tobacco use accounts for more than 400,000 U.S. deaths each year (World Health Organization, 2002) due to cardiovascular disorders, cancers, and other health problems (Kure et al., 1996; Zang & Wynder, 1996). Much of this morbidity and mortality is attributable to the toxicants that smokers of cigarettes inhale, including the dependence-producing drug nicotine, gases like carbon monoxide (CO; Lakier, 1992), and carcinogens like tobacco-specific nitrosamines (Hecht & Hoffmann, 1989). Cigar smoking is also linked to cancer and other health disorders, and cigar smoke contains many of the same toxicants as cigarette smoke (Shanks & Burns, 1998). Despite these health concerns, cigar smoking is often mistakenly believed to be less lethal than cigarette smoking (Baker, Dye, Denniston, & Ainsworth, 2001). Additionally, unlike cigarette smoking, cigar smoking rates have not declined in recent years (U.S. Department of Health and Human Services, 2008). In fact, the use of a certain type of cigar—the cigarillo—has increased substantially during the past 10 years (Maxwell, 2009). Cigarillos are generally 100–110 mm in length and 10–12 mm in diameter, and distinguished from other cigar products based on their tobacco content weight: 3–10 lbs/1,000 for cigarillos versus <3 lbs/1,000 for little cigars and >10 lbs/1,000 for large cigars (Centers for Disease Control and Prevention, 2007). While the prevalence of these products increases, science lags behind: Little is known about cigarillo smoke toxicant exposure and effects. Preliminary findings (Pickworth et al., 2010) suggest that cigarillo smoking is associated with more CO but less nicotine than cigarette smoking. No study, however, has examined the direct product effects or withdrawal suppression ability of cigarillo smoking. It was hypothesized that cigarillo smoking would produce increases in plasma nicotine, heart rate, and CO, as well as a variety of subjective effects, relative to a sham smoking control.
Methods
Participants
Ten men (six non-White) and six women (five non-White) completed this institutional review board–approved study. Participants were recruited from the greater Richmond community by advertisements and word of mouth. Participants were aged 18–55 years (M ± SD = 27.7 ± 10.8) and reported smoking five or more Black & Mild (B&M) cigarillos per month (1.9 ± 2.5 cigarillos/day) for 6 or more months (3.4 ± 3.5 years). Nine participants reported concurrent use of cigarettes, with an average of 11.7 ± 7.9 cigarettes/day for 8.3 ± 8.0 years for this subgroup. Cigarette smokers and nonsmokers did not differ on any demographic characteristic, including race, gender, age, education level, frequency of B&M use, and other drug use (Fs < 3.2 and χ2 < 6.9, ps > .05). Exclusion criteria included chronic health or psychiatric conditions, use of alcohol or marijuana more than 20 days in the past 30 days, use of any other illicit drugs, regular use of medications (except vitamins or birth control), and pregnancy or breast feeding.
Study Design and Procedures
Participants completed two counterbalanced 2-hr sessions: lit (ACTIVE) or unlit (SHAM) B&M, smoked twice per session with 60 min separating the two bouts. Sessions were separated by 48 or more hours and preceded by 12 or more hours of tobacco abstinence. Following verification of overnight abstinence (CO ≤ 10 ppm), a catheter was inserted into a forearm vein and recording of physiological measures commenced. Thirty minutes later, baseline subjective effects were rated, CO was measured, and 7 ml of blood was sampled. Next, participants were administered a condition-assigned B&M of their preferred flavor (9 = regular, 7 = wine) and tip type (9 = wood, 7=plastic). The B&M was smoked over 5 min according to a standardized procedure: 10 puffs, 30-s interpuff intervals (IPIs). At 5, 15, 30, and 45 min post-smoking, subjective measures were assessed and blood and breath sampled. Sixty minutes after the first product use, this same pattern of events was repeated: cigarillo administration with pre- and post-smoking assessment of subjective, CO, and blood measures. At the end of session, the catheter was removed and participants were paid $125.
Physiological Measures
Expired-air CO levels were measured via a BreathCo monitor (Vitalograph, Lenexa, KS). Blood samples were centrifuged and the plasma separated and stored at −70° C. Plasma was analyzed for nicotine using liquid chromatography-tandem mass spectrometry (modified version of Naidong, Shou, Chen, & Jiang, 2001; see Breland, Kleykamp, & Eissenberg, 2006, for details). HR was measured every 20 s via noninvasive computerized equipment (Model 507E; Criticare Systems).
Subjective Measures
Items for all subjective measures are presented in Table 1. Three questionnaires consisted of Visual Analog Scale (VAS) items: Hughes and Hatsukami (1986); Direct Effects of Nicotine Scale (DENS) assesses incidence of nicotine-related side effects: Evans et al. (2006); and Direct Effects of Tobacco Scale (DETS) assesses cigarette smoking effects: Foulds et al. (1992) word “cigarette” replaced with “B&M”). VAS items are presented as a word or phrase centered above a horizontal line that ranges from 0 (not at all) to 100 (extremely). Participants’ score is the distance of the vertical mark placed on the line from the left anchor, expressed as a percentage of total line length. The Tiffany–Drobes Questionnaire of Smoking Urges: Brief Form (Cox, Tiffany, & Christen, 2001) consists of 10 items rated on a 7-point scale (strongly disagree to strongly agree), collapsed into two factors: “intention to smoke” (Factor 1) and “anticipation of relief from withdrawal” (Factor 2).
Table 1.
Statistical Analysis Results for All Outcome Measures
| Conditiona |
Timeb |
Condition × Time |
||||
| F | P | F | p | F | p | |
| Physiological measures | ||||||
| Plasma nicotinec | 19.3 | <.001 | 4.4 | <.01 | 4.4 | <.01 |
| Heart rated | 14.2 | <.01 | 16.3 | <.001 | 10.0 | <.001 |
| Carbon monoxidee | 31.8 | <.001 | 41.4 | <.001 | 53.4 | <.001 |
| Subjective measuresc | ||||||
| QSU Brief | ||||||
| Factor 1 | 5.0 | <.05 | 1.6 | ns | 4.5 | <.01 |
| Factor 2 | 5.3 | <.05 | 0.9 | ns | 2.8 | ns |
| Hughes–Hatsukami withdrawal scale | ||||||
| Urges to smoke a B&M | 5.1 | <.05 | 1.1 | ns | 6.2 | <.001 |
| Irritability/frustration/anger | 2.1 | ns | 2.0 | ns | 3.4 | <.05 |
| Anxious | 2.3 | ns | 3.8 | <0.5 | 1.8 | ns |
| Difficulty concentrating | 0.3 | ns | 0.8 | ns | 0.5 | ns |
| Restlessness | 2.7 | ns | 1.1 | ns | 1.0 | ns |
| Hunger | 6.9 | <.05 | 4.0 | <.05 | 2.1 | ns |
| Impatient | 3.1 | ns | 1.6 | ns | 2.9 | <.05 |
| Craving a B&M | 3.7 | ns | 1.3 | ns | 5.3 | <.01 |
| Drowsy | 5.2 | <.05 | 1.3 | ns | 5.2 | <.01 |
| Depression/feeling blue | 1.7 | ns | 1.9 | ns | 1.3 | ns |
| Desire for sweets | 2.3 | ns | 2.0 | ns | 1.4 | ns |
| Direct Effects of Nicotine | ||||||
| Nauseous | 1.7 | ns | 1.7 | ns | 1.5 | ns |
| Dizzy | 5.6 | <.05 | 1.0 | ns | 0.9 | ns |
| Lightheaded | 5.5 | <.05 | 1.7 | ns | 1.4 | ns |
| Nervous | 1.0 | ns | 1.0 | ns | 0.8 | ns |
| Sweaty | 0.0 | ns | 0.7 | ns | 0.5 | ns |
| Headache | 1.0 | ns | 0.3 | ns | 1.3 | ns |
| Salivation | 0.4 | ns | 0.8 | ns | 0.6 | ns |
| Heart pounding | 1.8 | ns | 0.5 | ns | 0.2 | ns |
| Confused | 0.0 | ns | 1.5 | ns | 1.2 | ns |
| Weak | 0.4 | ns | 0.5 | ns | 0.6 | ns |
| Direct Effects of Tobacco | ||||||
| Was the B&M satisfying? | 9.2 | <.01 | 14.6 | <.001 | 4.9 | <.001 |
| Was the B&M pleasant? | 8.0 | <.05 | 16.6 | <.001 | 4.3 | <.01 |
| Did the B&M taste good? | 7.5 | <.05 | 16.2 | <.001 | 3.5 | <.01 |
| Did the B&M make you dizzy? | 6.9 | <.05 | 2.1 | ns | 1.8 | ns |
| Did the B&M calm you down? | 8.5 | <.05 | 10.1 | <.001 | 3.5 | <.01 |
| Did the B&M help you concentrate? | 5.7 | <.05 | 6.3 | <.001 | 2.5 | <.05 |
| Did the B&M make you feel more awake? | 4.3 | ns | 10.9 | <.001 | 2.9 | <.05 |
| Did the B&M reduce your hunger for food? | 4.6 | <.05 | 4.1 | <.01 | 3.4 | <.05 |
| Did the B&M make you sick? | 1.2 | ns | 0.8 | ns | 1.0 | ns |
| Did the product taste like your own brand of B&M? | 7.1 | <.05 | 19.6 | <.001 | 2.8 | ns |
| Did the product feel like your own brand of B&M? | 8.6 | <.05 | 17.5 | <.001 | 3.8 | <.05 |
| Did the product feel as harsh as your own brand of B&M? | 2.7 | ns | 9.1 | <.001 | 1.3 | ns |
| Did the product feel as mild as your own brand of B&M? | 3.5 | ns | 12.5 | <.001 | 1.3 | ns |
| Would you like to smoke another B&M right now? | 1.4 | ns | 24.9 | <.001 | 3.7 | <.01 |
| Smoking topographyf | ||||||
| Total volume | 0.5 | ns | 4.5 | ns | 1.9 | ns |
| Volume | 0.2 | ns | 5.2 | <.05 | 1.9 | ns |
| Duration | 0.0 | ns | 8.7 | <.05 | 0.7 | ns |
| Interpuff interval | 0.2 | ns | 0.2 | ns | 0.1 | ns |
Note. B&M = Black & Mild; QSU = Questionnaire of Smoking Urges.
Condition factors: active, sham.
Time factors: levels vary according to measure.
dfcondition = (1, 15); dftime = (9, 135); dfcond×time = (9, 135).
dfcondition = (1, 13); dftime = (9, 117); dfcond×time = (9, 117).
dfcondition = (1, 15); dftime = (6, 90); dfcond×time = (6, 90).
dfcondition = (1, 14); dftime = (1, 14); dfcond×time = (1, 14).
Smoking Topography Measures
An adapted mouthpiece was used to fit the wider circumference of a cigarillo, as well as the plastic or wood tip. Specifically, participants’ usual tip type was placed on the proximal end of the mouthpiece (for direct contact with smokers’ lips) and the cigarillo was placed on the distal end. The mouthpiece was also was connected to a pressure transducer via tubing; inhalation-induced pressure changes were amplified, digitized, and sampled at 1,000 Hz. Software (Borgwaldt, Richmond, VA) converts signals to air flow (ml/s) and integrates these data, producing measures of puff number, volume, duration, and IPIs.
Data Analysis
Due to human error or device malfunctioning, HR and topography data are based on n = 14 participants. Plasma values below the limit of quantification were replaced with 2.0 ng/ml. For each B&M bout, HR data were averaged into bins: 5 min prior to product administration (baseline), the first (+2.5) and last (+5) 2.5 min during smoking, and the first (+10) and second (+15) 5-min periods post-smoking. For subjective, CO, and blood measures, the timepoints were baseline and 5 (except CO), 15, 30, and 45 min post-administration for each smoking bout. These data were analyzed using a condition (2; ACTIVE and SHAM) by time (levels varied depending on measure) repeated measures analysis of variance. Topography data were averaged within each session to obtain a single value for each variable and analyzed using condition and bout (1, 2) as the within-subject factors. Huynh–Feldt corrections were used to adjust for violations of the sphericity assumption (Huynh & Feldt, 1976). Differences between means were examined using Tukey’s Honestly Significant Difference (HSD; Keppel, 1991) test. Comparisons for which p < .05 are reported as significant.
Results
Statistical analysis results for all measures are displayed in Table 1.
Physiological Measures
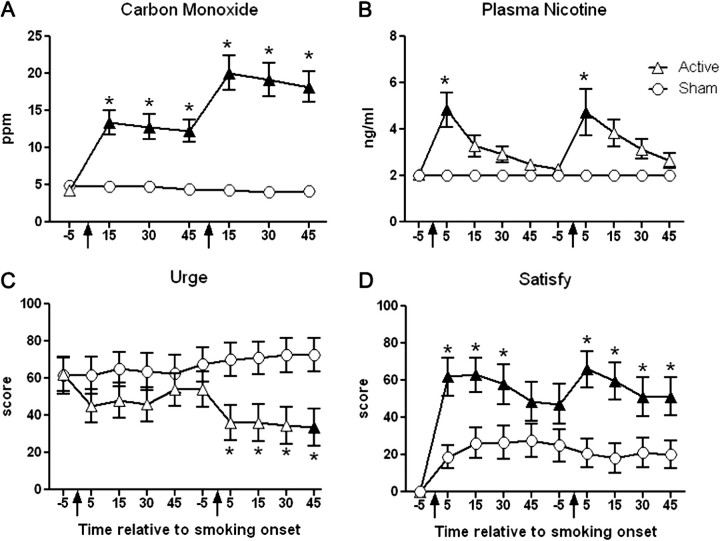
Carbon monoxide (Figure 1A) did not differ across timepoints for SHAM, but increased significantly at every timepoint for ACTIVE (p < .05, Tukey’s HSD), relative to baseline. Specifically, for SHAM, average (±SEM) CO levels were 4.9 ± 0.5 ppm at baseline and 4.2 ± 0.4 ppm by the end of session. For ACTIVE, CO levels were 4.3 ± 0.6 ppm at baseline but increased at 15 min following the first (13.4 ± 1.7 ppm) and second (20.1 ± 2.3 ppm) smoking bouts (all ps < .05, Tukey’s HSD). Average CO for ACTIVE was significantly higher than for SHAM at all timepoints (p < .05, Tukey’s HSD).
Figure 1.
Mean data (± 1 SEM) for expired-air carbon monoxide (A), plasma nicotine (B), Hughes–Hatsukami item “Urges to smoke a B&M,” and Direct Effects of Tobacco Scale item “Was the B&M satisfying?” Arrows indicate product administration; filled symbols, a significant difference from baseline; and asterisks, a significant difference between ACTIVE and SHAM conditions at that timepoint (p < .05, Tukey’s Honestly Significant Difference). B&M = Black &Mild.
A significant interaction was observed for plasma nicotine (Figure 1B). Nicotine concentration for SHAM was 2.0 ± 0.0 ng/ml at all timepoints (relative to baseline, all ns, Tukey’s HSD). Plasma nicotine for ACTIVE increased at every timepoint, relative to baseline, with the greatest increases observed 5 min after Bouts 1 (5.3 ± 0.8 ng/ml) and 2 (4.9 ± 0.9 ng/ml; p < .05, Tukey’s HSD). Additionally, nicotine levels at both 5-min post-smoking timepoints were significantly different between ACTIVE and SHAM (p < .05, Tukey’s HSD).
A significant interaction was also observed for HR. For SHAM, HR remained stable relative to baseline (71.1 ± 2.2 bpm) at every timepoint (ns, Tukey’s HSD). For ACTIVE, however, HR increased significantly from baseline (70.3 ± 2.1 bpm) at 5 (80.7 ± 2.3 bpm) and 10 (79.2 ± 2.1 bpm) min following Bout 1 administration and at 5 (77.6 ± 2.6 bpm) min following Bout 2 administration (p < .05, Tukey’s HSD). Relative to SHAM, HR for ACTIVE was significantly faster at the 5- and 10-min timepoints of both bouts (p < .05, Tukey’s HSD).
Subjective Measures
Several Hughes–Hatsukami items revealed a significant condition by time interaction (see Table 1). Figure 1C displays data for “urges to smoke a B&M,” the item with the largest F value. Average ratings did not differ across timepoints for SHAM (ns, Tukey’s HSD), but for ACTIVE, decreased by 45 min post-smoking Bout 2 (33.5 ± 10.2), relative to baseline (61.9 ± 9.1; p < .05, Tukey’s HSD). Average ratings at 5, 15, 30, and 45 min following Bout 2 were significantly different across conditions (p < .05, Tukey’s HSD). For “craving a B&M,” ratings did not differ across timepoints within ACTIVE or SHAM but were significantly different between conditions at 5 (37.8 ± 9.7 for ACTIVE vs. 69.0 ± 9.5 for SHAM) and 45 (34.6 ± 9.7 for ACTIVE vs. 67.1 ± 9.7 for SHAM) minutes following Bout 2. The other three items (“irritability/frustration/anger,” “impatient,” and “drowsy”) presented a similar pattern of results: no differences across timepoints for SHAM, decreased ratings relative to baseline for ACTIVE, and lower scores for ACTIVE relative to SHAM. Nonetheless, post-hoc tests did not reveal any significant differences across timpoints or between conditions (ns, Tukey’s HSD).
A significant main effect of time was revealed for “anxious” and “hunger.” For “anxious,” average ratings (collapsed across condition) decreased from 24.8 ± 5.7 at baseline to 17.0 ± 4.9 by 45 min post-smoking (ns, Tukey’s HSD). Average “hunger” ratings (collapsed across condition) increased from 41.8 ± 5.5 at baseline to 56.0 ± 7.6 at 45 min post-smoking (ns, Tukey’s HSD). The item “hunger” also revealed a main effect of condition: 36.6 ± 3.0 for ACTIVE and 57.6 ± 2.8 for SHAM.
Factor 1 was significant for a condition by time interaction. In the ACTIVE condition, average scores at baseline were 21.1 ± 2.4, and decreased to 15.8 ± 2.7 at 5 min, 16.9 ± 2.7 at 15 min, 17.1 ± 2.4 at 30 min, and 18.6 ± 2.7 at 45 min after the first smoking bout (ns, Tukey’s HSD). Also relative to baseline, scores decreased to 13.7 ± 2.9 at 5 min, 13.5 ± 3.0 at 15 min, 12.9 ± 3.0 at 30 min, and 12.9 ± 2.9 at 45 min after the second smoking bout (ns, Tukey’s HSD). In the SHAM condition, however, scores were increased relative to baseline (18.8 ± 3.0) at every timepoint (ns, Tukey’s HSD) following the first smoking bout (22.2 ± 2.3 at 5 min, 22.4 ± 2.3 at 15 min, 22.1 ± 2.3 at 30 min, and 22.1 ± 2.4 at 45 min), as well as following the second smoking bout (22.6 ± 2.3 at 5 min, 22.6 ± 2.3 at 15 min, 22.4 ± 2.4 at 30 min, and 22.1 ± 2.4 at 45 min). Scores at all timepoints following Bout 2 were significantly different between conditions (p < .05, Tukey’s HSD). For Factor 2, a main effect of condition was observed such that scores were significantly lower for ACTIVE (6.8 ± 0.6) than for SHAM (10.3 ± 0.6).
Two DENS items were significant for a main effect of condition; average ACTIVE scores were significantly higher than SHAM scores for “dizzy” (4.8 ± 1.0 vs. 1.1 ± 0.2) and “lightheaded” (6.5 ± 1.3 vs. 1.2 ± 0.3).
Significant dose by time interactions were revealed for most DETS items. Some of these items assessed sensory characteristics of B&M smoking (e.g., taste, satisfy). Figure 1D presents the responses to one such item, “Was the B&M satisfying?” (item with the largest F value). Generally speaking, scores on this item were increased at every timepoint relative to baseline for both conditions, although to a greater degree in the ACTIVE condition. For instance, ratings for SHAM were 0.1 ± 0.1 at baseline, 18.8 ± 6.3 at 5 min, 26.2 ± 8.3 at 15 min, 26.4 ± 9.0 at 30 min, and 27.6 ± 9.1 at 45 min following Bout 1, and 20.7 ± 7.8 at 5 min, 18.2 ± 8.0 at 15 min, 21.0 ± 8.2 at 30 min, and 20.3 ± 7.5 at 45 min following Bout 2 (ns, Tukey’s HSD). Ratings for ACTIVE were 0.3 ± 0.1 at baseline, 62.0 ± 10.3 at 5 min, 62.9 ± 9.2 at 15 min, 57.9 ± 10.8 at 30 min, and 48.6 ± 10.5 at 45 min following bout 1, and 66.1 ± 9.8 at 5 min, 59.4 ± 10.1 at 15 min, 51.3 ± 10.4 at 30 min, and 51.2 ± 10.3 at 45 min following Bout 2 (all ps < .05, Tukey’s HSD). Consequently, ratings for ACTIVE were significantly higher than those for SHAM at every post-smoking timepoint except 45 min following Bout 1. This same pattern of results (nonsignificant increases from baseline for SHAM, significant increases from baseline for ACTIVE, and significant differences between ACTIVE and SHAM at most post-smoking timepoints) was observed for the items assessing “pleasant,” “taste good,” “calm,” and “awake.” A similar pattern was also observed for the items assessing “concentrate” and “reduce hunger,” though few significant differences between conditions were observed.
For the items “Did the product feel like your own brand of B&M?” and “Would you like to smoke another B&M right now?” ratings increased significantly from baseline for both conditions, although no significant differences between conditions were observed (ns, Tukey’s HSD). For the item assessing “feel like own brand,” ratings for ACTIVE were 81.5 ± 8.2, 77.6 ± 9.0, 69.2 ± 10.6, and 62.8 ± 11.3 from 5 to 45 min following Bout 1, relative to 0.1 ± 0.1 at baseline (all ps < .05, Tukey’s HSD). At these same timepoints following Bout 2, ratings for ACTIVE were 80.4 ± 7.6, 75.6 ± 9.4, 69.2 ± 10.2, and 67.1 ± 10.4, relative to baseline (all ps < .05, Tukey’s HSD). Average ratings, relative to baseline (0.0 ± 0.0), for SHAM were 51.4 ± 10.9 at 5 min, 42.9 ± 10.7 at 15 min, 39.7 ± 9.8 at 30 min, and 39.4 ± 9.9 at 45 min following Bout 1, and 45.7 ± 10.7 at 5 min, 40.8 ± 10.4 at 15 min, 34.3 ± 10.3 at 30 min, and 35.0 ± 10.2 at 45 min following Bout 2 (all ps < .05, Tukey’s HSD).
Smoking Topography Measures
A significant effect of time was observed for duration and volume. Collapsed across conditions, the average volume for Bout 1 was 92.6 ± 7.4 ml and for Bout 2 was 123.0 ± 16.6 ml. Collapsed across condition, average puff durations for Bouts 1 and 2 were 2.0 ± 0.1 and 2.4 ± 0.2 s, respectively.
Discussion
This study is among the first to evaluate of the effects of cigarillos. Under the controlled conditions reported here, cigarillo smoking delivers nicotine that is measurable via blood plasma and in doses that are physiologically active as indicated by increased heart rate. Mean peak nicotine concentration for ACTIVE was 6.0 ± 0.9 ng/ml after the first bout and 5.3 ± 0.9 ng/ml after the second bout. HR increased significantly for ACTIVE, with an average maximum change of 10.7 ± 1.2 bpm for Bout 1 and 11.0 ± 1.7 for Bout 2. By way of comparison, 12 puffs from a cigarette results in mean peak plasma concentrations of 14.3 ng/ml and a mean peak HR change of 26.0 ± 9.0 bpm (Benowitz, Porchet, Sheiner, & Jacob, 1988).
In this study, users were exposed to considerable amounts of CO; average boosts for ACTIVE were 9.1 ± 1.3 ppm for the first bout and 7.8 ± 1.1 ppm for the second bout. In contrast, eight puffs from a cigarette increase average CO levels by 5.6 ± 0.6 ppm and 5.8 ± 1.1 after two consecutive smoking bouts (Breland, Buchhalter, Evans, & Eissenberg, 2002). The physiological effects demonstrated here are similar to other work, where ad lib cigarillo smoking increased plasma nicotine concentration, heart rate, and expired air CO (Pickworth et al., 2010).
For subjective effects, neither condition reliably reduced withdrawal symptoms typically observed following a period of nicotine/tobacco abstinence (few significant differences pre- to post-smoking within condition). In contrast, a variety of positive effects related to product sensory characteristics (e.g., “taste good,” “pleasant”) were produced by ACTIVE but not SHAM. Overall, the pattern of results observed here (i.e., delivery of physiologically active nicotine concentrations, increases in direct product effects without withdrawal symptom alleviation) may reflect the relatively low frequency of tobacco use in our sample (50% smoke <2 cigarillos/day and ≤6 cigarettes/day). Nonetheless, the nicotine delivery profile suggests that cigarillo smoking may support tobacco/nicotine dependence, especially in individuals who also smoke cigarettes (e.g., concurrent use may lead to increased plasma nicotine exposure as compared with cigarillo use alone). Unfortunately, very little is known about the dependence level and smoking behavior of cigarillo users. This lack of information makes determining the generalizability of our results difficult and also highlights a growing need for national tobacco use surveillance programs.
Funding
Support provided to author AN by the National Center on Minority Health and Health Disparities and Virginia Commonwealth University's Department of Psychology
Declaration of Interests
None declared.
Acknowledgments
We thank Barbara Kilgalen, R.N., and Janet Austin, M.S., for their diligent efforts on data collection and management. All work performed at Virginia Commonwealth University.
References
- Baker F, Dye JT, Denniston MM, Ainsworth SR. Risk perception and cigar smoking behavior. American Journal of Health Behavior. 2001;25:106–114. doi: 10.5993/ajhb.25.2.3. Retrieved from http://www.cinahl.com/cgi-bin/refsvc?jid=182&accno=2001033782. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Sheiner L, Jacob P. Nicotine absorption and cardiovascular effects with smokeless tobacco use: Comparison with cigarettes and nicotine gum. Clinical Pharmacology and Therapeutics. 1988;44:23–28. doi: 10.1038/clpt.1988.107. doi:10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- Breland AB, Buchhalter AR, Evans SE, Eissenberg T. Evaluating acute effects of potential reduced-exposure products for smokers: Clinical laboratory methodology. Nicotine & Tobacco Research. 2002;4:S131–S140. doi: 10.1080/1462220021000032780. doi:10.1080/1462220021000032780. [DOI] [PubMed] [Google Scholar]
- Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine & Tobacco Research. 2006;8:727–738. doi: 10.1080/14622200600789585. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigars: Fact sheet. 2007. Retrieved from http://www.cdc.gov/tobacco/factsheets/cigars_factsheet.htm. [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. doi:10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Evans SE, Blank M, Sams C, Weaver MF, Eissenberg T. Transdermal nicotine-induced tobacco abstinence symptoms suppression: Nicotine dose and smokers’ gender. Experimental and Clinical Psychopharmacology. 2006;14:121–135. doi: 10.1037/1064-1297.14.2.121. doi:10.1037/1064-1297.14.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Feyerabend C, Vesey C, Jarvis M, Russell MAH. Effect of transdermal nicotine patches on cigarette smoking: A double-blind crossover study. Psychopharmacology. 1992;106:421–427. doi: 10.1007/BF02245429. Retrieved from http://www.springer.com/biomed/neuroscience/journal/213. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Hoffmann D. The relevance of tobacco-specific nitrosamines to human cancer. Cancer Surveys. 1989;8:273–294. Retrieved from http://www.researchgate.net/journal/0261-2429_Cancer_surveys. [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. Retrieved from http://archpsyc.ama-assn.org.proxy.library.vcu.edu/ [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Estimation of the box correction for degrees of freedom from sample data in randomized block and split-plot designs. Journal of Educational Statistics. 1976;1:69–82. doi:10.3102/10769986001001069. [Google Scholar]
- Keppel G. Design and analysis: A researcher’s handbook. Englewood Cliffs, NJ: Prentice Hall; 1991. [Google Scholar]
- Kure EH, Ryberg D, Hewer A, Phillips DH, Skaug V, Baera R, et al. p53 Mutations in lung tumors: Relationship to gender and lung DNA adduct levels. Carcinogenesis. 1996;17:2201–2205. doi: 10.1093/carcin/17.10.2201. doi:10.1093/carcin/17.10.2201. [DOI] [PubMed] [Google Scholar]
- Lakier JB. Smoking and cardiovascular disease. American Journal of Medicine. 1992;93:8S–12S. doi: 10.1016/0002-9343(92)90620-q. Retrieved from http://www.elsevier.com/wps/find/journaldescription.cws_home/525049/description. [DOI] [PubMed] [Google Scholar]
- Maxwell JC. The Maxwell report: Cigar industry in 2008. Richmond, VA: 2009. Retrieved from http://tobaccodocuments.org/pm/1000305800–5814.html. [Google Scholar]
- Naidong W, Shou W, Chen Y-L, Jiang X. Novel liquid chromatographic-tandem mass spectrometric methods using silica columns and aqueous-organic mobile phases for quantitative analysis of polar ionic analytes in biological fluids. Journal of Chromatography B. 2001;754:387–399. doi: 10.1016/s0378-4347(01)00021-4. doi:10.1016/S0378-4347(01)00021-4. [DOI] [PubMed] [Google Scholar]
- Pickworth W, Martin R, Canlas L, Smith VM, Malson J, Fabian L. Toxin delivery after Black & Mild little cigar smoking. 2010, June. Poster presented at the 72nd annual meeting for the College on Problems of Drug Dependence, Scottsdale, AZ. [Google Scholar]
- Shanks TG, Burns DM. In: Cigars: Health effects and trends. Burns DM, Cummings KM, Hoffman D, editors. 1998. Monograph 9 (DHHS Publ No. 98-4302. 105–160). Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health. Retrieved from http://cancercontrol.cancer.gov/tcrb/monographs/9/index.html. [Google Scholar]
- U.S. Department of Health and Human Services. The National Survey on Drug Use and Health. 2008. Retrieved from http://www.hhs.gov/ [Google Scholar]
- World Health Organization. The world health report 2002—Reducing risks, promoting healthy life. Quantifying selected major risks to health, chapter 4: Quantifying selected major risks to health. Geneva, Switzerland: Author; 2002. Retrieved from http://www.who.int/whr/2002/en/ [Google Scholar]
- Zang EA, Wynder EL. Differences in lung cancer risk between men and women: Examination of the evidence. Cancer Research. 1996;56:772–778. doi: 10.1093/jnci/88.3-4.183. doi:10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]