Abstract
Introduction:
Many studies have investigated the association of the dopamine type-2 receptor (DRD2) Taq1A polymorphism with tobacco use and cigarette smoking behaviors, but findings remain equivocal. There is a biological basis for considering that this association differs by sex, and differences in subpopulations might explain some of the contradictory evidence.
Methods:
Our a priori hypothesis was that the association of the DRD2 Taq1A polymorphism with smoking behavior would be more prominent in females than males. We therefore investigated the strength of evidence for an association between the DRD2 Taq1A polymorphism and smoking behavior in a large sample of females and used meta-analytic techniques to synthesize existing published data and explore the role of sex in explaining any heterogeneity between studies.
Results:
We did not observe any strong evidence of association between the DRD2 Taq1A polymorphism and smoking behavior, including smoking initiation, smoking persistence, and smoking rate, either in our female sample or in our meta-analysis of 29 studies, comprising 28 published studies and the data from the present study. Metaregression suggested an association between the proportion of male participants in a study and the individual study effect size, indicating a larger effect size with a greater proportion of male participants for smoking initiation and smoking persistence. This effect did not appear to be due to the inclusion of the data from the present study.
Discussion:
Available evidence does not support an association between the DRD2 Taq1A polymorphism and smoking behavior. Contrary to our a priori hypothesis, we found evidence of a stronger association in males than in females.
Introduction
The human central dopamingeric system is widely considered to play an important role in substance use and the development of subsequent dependence. Evidence of a role for this system extends to a range of psychoactive substances, including opiates, cocaine, nicotine, and alcohol (Koob & Le Moal, 2001; Lingford-Hughes & Nutt, 2003; M. R. Munafò, Johnstone, Murphy, & Walton, 2001). As a result, a great deal of attention has been devoted to determining whether variation in genes with a dopaminergic function could account for the heritable variation in susceptibility to substance abuse. In particular, the dopamine type-2 receptor (DRD2) gene on chromosome 11 (q22–q23) has been studied widely (Blum et al., 1995).
Following a report (Blum et al., 1990) that the A1 allele of the Taq1A polymorphism (rs1800497) of the DRD2 gene, a C-T substitution located in a noncoding region of the DRD2 locus, was associated with alcoholism, several studies have investigated the association of this polymorphism with substance use behavior, including tobacco (Noble et al., 1994), opioid (Lawford et al., 2000), and cocaine (Noble et al., 1993) use. Despite the large number of individual studies investigating smoking behavior, however, results have been equivocal, and a recent meta-analysis suggested no evidence of association with cigarette smoking behavior (M. Munafò, Clark, Johnstone, Murphy, & Walton, 2004), although another meta-analysis did suggest evidence of association with risk of alcohol dependence (M. R. Munafò, Matheson, & Flint, 2007).
A number of pharmacogenetic studies have suggested an association between the DRD2 gene and response to smoking cessation pharmacotherapy. Reduced-function alleles (i.e., those associated with, e.g., reduced mRNA expression) of polymorphisms in the DRD2 gene (e.g., Taq1A1) generally have been shown to predict better response to nicotine replacement therapy (NRT) (Johnstone, Yudkin, Hey, et al., 2004; Lerman et al., 2006; Yudkin et al., 2004), whereas increased-function alleles (e.g., Taq1A2) have been reported to predict better response to bupropion (David et al., 2007; Lerman et al., 2006; Swan et al., 2005). Nevertheless, some studies have failed to demonstrate an effect of DRD2 genotype on smoking cessation (Berlin, Covey, Jiang, & Hamer, 2005). To be able to interpret these findings, we need to know whether the DRD2 gene is associated with smoking behavior variables. If it is, the gene may simply be acting as a proxy for tobacco dependence or some other characteristic in pharmacogenetic studies (as opposed to a more direct moderator of treatment response).
One reason for the lack of association observed in studies of smoking behaviors may be that other factors moderate this association, so that an association is present in some subpopulations but not others. A possible moderating factor is sex, since growing evidence indicates differences between males and females in response to nicotine and in the factors that motivate smoking behaviors. For example, nicotine reinforcement has been observed to control smoking to a greater degree in men than in women (Perkins, 1999; Perkins et al., 1996; Perkins, Donny, & Caggiula, 1999), and sex differences exist in nicotine metabolism (Zeman, Hiraki, & Sellers, 2002) and the development of psychomotor reactivity to environmental smoking cues (Niaura et al., 1998). Although research has suggested that NRT is less effective in women than in men (Perkins, 2001), evidence remains equivocal (M. Munafò, Bradburn, Bowes, & David, 2004). We have reported evidence that the association of the DRD2 Taq1 polymorphism with response to NRT differs between males and females (Yudkin et al., 2004), with the association present only in females. On this basis, it might be expected that any association of the DRD2 Taq1A polymorphism with smoking behavior may be present only in females.
We therefore investigated the strength of evidence for an association between the DRD2 Taq1A polymorphism and smoking behavior in a large sample of females and used meta-analytic techniques to synthesize existing published data and explore the hypothesis that between-study heterogeneity was related to differences in the sex distribution within studies. The large number studies of DRD2 Taq1A polymorphism allowed us to apply a formal test of publication bias, as well as investigate (albeit indirectly) the impact of potential moderating factors such as sex through the use of metaregression. We included studies that reported data on categorical smoking status by genotype and those that reported data on continuously distributed smoking rate by genotype. Although it has been shown that the Taq1A variant alters an amino acid in a protein kinase gene (ANKK1) near the DRD2 locus (Neville, Johnstone, & Walton, 2004), we refer to the variant throughout as the DRD2 Taq1A polymorphism, as this is the nomenclature used in the majority of published studies to date.
Methods
Participants
Participants were originally recruited into the British Women's Heart and Health Study (BWHHS). Between 1999 and 2001, a total of 4,286 women aged 60–79 years were randomly selected from 23 British towns, interviewed and examined, and completed a series of medical questionnaires. The original study design and materials have been reported elsewhere (Lawlor, Bedford, Taylor, & Ebrahim, 2003; Lawlor, Ebrahim, & Davey Smith, 2002). Individuals for whom the ethnicity variable was recorded as “non-White” (n = 9) were removed from all analyses to avoid potential population stratification effects. The resulting sample for the purposes of the present study were analysis dependent and consisted of individuals for whom complete data were available for the analysis of genotypic data on the Taq1A variant (n = 3,648), and covariate data on alcohol consumption (n = 2,940), body mass index (n = 3,957), socioeconomic status (SES) score (n = 2,638), and age (n = 4,285). This provided a working dataset of 2,437 individuals with complete data, from which specific data were available on smoking initiation (n = 2,409), smoking persistence (n = 1,376), and current smoking rate (n = 256).
Measures
Smoking status variables used in the present study included self-report of whether or not the participant had ever smoked cigarettes (“Have you ever smoked cigarettes?”), current smoking status (“Do you smoke at present?”), and among current smokers, the number of cigarettes smoked per day. Other variables used in the present study included age in years, body mass index (BMI) in kg/m2, SES using a composite measure (described in detail below), and alcohol consumption using frequency of consumption. BMI and alcohol consumption have been reported to be associated with both DRD2 Taq1A genotype and smoking status and were included to adjust for potential confounding. Age may be related to genotype and was included to control for possible survivor bias. SES is unlikely to be related to genotype but is a strong predictor of smoking behavior; therefore, adjustment would provide greater precision to any estimate of association.
In the BWHHS, the assessment of SES was a composite procedure that involved an extensive series of questionnaire-based, self-reported observations. To incorporate the SES components into working analysis, a single variable, a SES score, was derived from 10 of the component information points. This approach has been described in detail elsewhere (Lawlor, Davey Smith, Rumley, Lowe, & Ebrahim, 2005; Timpson et al., 2005). In general, dichotomized measures of SES in the BWHHS included (a) father had manual social class, (b) no bathroom as a child, (c) no hot water as a child, (d) shared bedrooms as a child, (e) no family car as a child, (f) left school before leaving age, (g) adult manual social class, (h) currently in local housing authority housing, (i) provision for state pension only on retirement, and (j) currently no car access. These variables were assessed as representative of cumulative measures of SES over the life course for the women of the BWHHS. A simple score of the number of life course indicators that each woman was exposed to was created as an ordered categorical variable from 0/1 (most advantaged) to 9/10 (least advantaged). Participants in the two lowest and the two highest scoring categories were combined due to small numbers.
Genotyping
Single nicleotide polymorphisms (SNPs) were genotyped using the KASPar chemistry, which is a competitive allele-specific polymerase chain reaction SNP genotyping system using FRET quencher cassette oligos. All genotyping was performed by KBioscience (www.kbioscience.co.uk). Three stages of internal quality control were used during genotyping. Known locations of non-DNA test controls were used to assure unique plate identity, a small sample of duplicate DNAs were genotyped for all SNPs, and initial assay validations were performed on a subsample of 96 chromosomes before genotyping the whole sample set.
Selection of studies for inclusion
Studies in our meta-analysis included studies of the DRD2 Taq1A polymorphism that reported data on categorical smoking status by genotype and those which reported data on continuously distributed smoking rate by genotype. Studies reporting data on either single-sex or both male and female participants of any ethnic origin were included. Studies using within-subjects repeated measures designs that employed an experimental manipulation (e.g., pharmacogenetic studies) were excluded, as were family-based studies that only reported transmission disequilibrium to affected offspring.
The principal outcome measures were the genotypic odds ratio (OR) for the Taq1A polymorphism and smoking status, coded as ever-smoker versus never-smoker and current smoker versus nonsmoker, and the standardized mean difference (Cohen's d) for the Taq1A polymorphism and cigarettes per day in current smokers, to allow the inclusion of data from the BWHHS, assuming a dominant model of genetic action for the A1 allele.
Search strategy
The search was performed on two databases: PubMed and PsycINFO. These databases were searched from the first date available in each database up to July 31, 2007, using the search terms smok$, nicotine, tobacco, DRD2, dopamine$, D2, Taq1A, and ANKK1. Once articles had been collected, bibliographies were hand searched for additional references.
The abstracts of studies identified by these search strategies were examined with reference to the inclusion and exclusion criteria. Duplications were deleted, and the whole text of each reference was checked to further establish whether the study met the inclusion criteria. Where studies reported previously published data (i.e., duplicate publications), we included only one of the publications, most commonly the original one or the one reporting the largest sample.
Data extraction
For each study, the following data were extracted independently by two authors (MM and SD) using standard forms: (a) authors and year of publication, (b) methods (country of origin, dominant ancestry of sample, case and control sample size, diagnostic criteria or classification of smoking status, statement of Hardy–Weinberg equilibrium (HWE), and method of genotyping), and (c) data (number of participants in control and case groups, M and SD of cigarettes per day in genotype groups, mean age, and sex ratio). Genotype frequencies were used to calculate whether or not these variables deviated significantly from HWE among controls. Ancestry was coded as European, East Asian, or Other (which included cases in which ancestry was stated as mixed, or when it was not stated). Additional information, including presentation of results in a consistent format, was obtained through contact with study authors (see Acknowledgments). Discrepancies between the two data abstractors were resolved by mutual consent.
Data analyses
Primary data from the BWHHS were analyzed using logistic and linear regression models, for categorical and continuous smoking behavior variables, respectively. For categorical variables (i.e., smoking status), ORs were calculated per DRD2 Taq1A allele assuming linearity and also were grouped as presence (A1A1 and A1A2) or absence (A2A2) or the A1 (T) allele. For continuous variables (i.e., smoking rate), the effect of number of DRD2 Taq1A alleles was assessed using linear regression and genotype also grouped as presence (A1A1 and A1A2) or absence (A2A2) or the A1 (T) allele. This grouping assumes a dominant model of genetic action of the A1 allele, consistent with existing functional data and the majority of studies to date. We repeated all analyses adjusting for the effects of age, BMI, SES, and alcohol consumption. Unadjusted and adjusted results are reported in the text.
Secondary data (studies identified from our systematic search), together with data from our primary study, were analyzed within both a fixed-effects and a random-effects framework. For the fixed-effects analyses, individual study effect sizes were pooled using inverse variance methods to generate a summary effect size and 95% CI. A fixed-effects framework assumes that the effect of genotype is constant across studies, and between-study variation is considered to be due to chance or random variation. For the random-effects analyses, effect sizes were pooled using DerSimonian and Laird methods. A random-effects framework assumes that between-study variation is due to both chance or random variation and an individual study effect. Random-effects models are more conservative than fixed-effects models and generate a wider CI. The significance of the pooled effect sizes was determined using a Z test. We used a chi-square and I2 to test between-study heterogeneity, with the latter providing a measure of the proportion of variation that is explained by between-study variation (Higgins, Thompson, Deeks, & Altman, 2003).
Results from both models are presented because, although random-effects models are used when between-study heterogeneity is apparent (and this was likely among the included studies given our expectation of sex differences), they do not “fix” the problem. A random-effects framework, compared with a fixed-effects framework, reduces the weight for each individual study proportional to the difference in effect size of an individual study from the pooled effect size estimate for all other studies. Because heterogeneity may be the result of ascertainment bias, a random-effects model combining several small positive studies (and an absence of small negative studies, e.g., due to publication bias) with a large null study will tend to underweight the latter, resulting in overestimation of the true effect.
The effect size of the first published study was compared with the pooled effect size of the remaining studies using a Z test, because evidence indicated a substantially greater estimate of effect size in the first published study (Trikalinos, Ntzani, Contopoulos-Ioannidis, & Ioannidis, 2004). Metaregression of individual study effect size against year of publication also was conducted.
Stratified analyses by sample ancestry were conducted to assess the potential moderating effect of this variable. Studies with samples of predominantly European or East Asian ancestry were combined separately, and the difference in pooled effect size was determined using a Z test.
A metaregression of individual study effect size against the proportion of male participants in individual study samples was conducted to assess the potential moderating effect of sex. The significance of the effect size of proportion of male participants was determined using a Z test.
Funnel plots were created to assess potential ascertainment bias by plotting the natural logarithm of individual study effect size against the SE of the natural logarithm of individual study effect size. Ascertainment bias also was assessed using the Egger test (Egger, Davey Smith, Schneider, & Minder, 1997).
Data were analyzed with Stata Statistical Software version 9.2 and Comprehensive Meta-Analysis version 2 statistical software. Exact p values are given throughout.
Results
Characteristics of participants in new sample
Participants were, on average, aged 68 years (SD = 5, range = 59–80), had a BMI of 27.6 kg/m2 (SD = 5.0, range = 15.2–58.8), had a median SES score of 4 (interquartile range = 3–6, range = 0/1–9/10), and consumed alcohol once or twice a month. The distribution of DRD2 Taq1 genotypes (A1A1: n = 1,608, 66%; A1A2: n = 735, 30%; A2A2: n = 103, 4%) did not deviate significantly from HWE (p = .10). Participant characteristics are presented in Table 1.
Table 1.
Characteristics of participants
| Never-smokers (n = 1,380) |
Ever-smokers (n = 1,029) |
Nonsmokers (n = 1,124) |
Current smoker (n = 252) |
|||||
| Characteristic | M | SD | M | SD | M | SD | M | SD |
| Age, years | 69 | 6 | 69 | 6 | 69 | 6 | 67 | 5 |
| Body mass index, kg/m2 | 27.52 | 4.82 | 27.61 | 5.05 | 27.99 | 5.06 | 26.26 | 4.67 |
| socioeconomic status score | 4 | 2 | 5 | 2 | 5 | 2 | 5 | 2 |
| No. of cigarettes per day | – | – | 12 | 7 | – | – | 12 | 7 |
| Alcohol | n | % | n | % | n | % | n | % |
| Daily | 259 | 25 | 205 | 14 | 242 | 21 | 54 | 21 |
| Weekends | 207 | 20 | 262 | 19 | 212 | 19 | 51 | 21 |
| Monthly | 89 | 9 | 165 | 12 | 110 | 10 | 12 | 5 |
| Occasionally | 335 | 33 | 505 | 37 | 395 | 35 | 89 | 35 |
| Never | 139 | 13 | 243 | 18 | 165 | 15 | 46 | 18 |
Note. SES, socioeconomic status.
Association of DRD2 genotype with smoking behavior in new sample
Data on smoking status and smoking rate grouped by DRD2 Taq1A genotype are presented in Table 2.
Table 2.
Smoking status and smoking rate by DRD2 Taq1A genotype
|
DRD2 Taq1A genotype |
|||||||
| A1A1 | A1A2 | A2A2 | OR/β | 95% CI | p value | ||
| Ever smoked (n = 2,409) | 43/101 | 307/723 | 679/1,585 | Unadjusted | 1.01 | 0.88–1.17 | 0.88 |
| 43% | 43% | 43% | Adjusted | 1.01 | 0.88–1.17 | 0.85 | |
| Smoke at present (n = 1,376) | 11/52 | 69/404 | 172/920 | Unadjusted | 0.97 | 0.76–1.24 | 0.80 |
| 21% | 17% | 19% | Adjusted | 0.97 | 0.75–1.25 | 0.83 | |
| Cigarettes per day, n = 256 | M = 8 (SD = 7), n = 12 | M = 12 (SD = 7), n = 69 | M = 12 (SD = 7), n = 175 | Unadjusted | −1.19 | −2.71–0.34 | 0.13 |
| Adjusted | −0.81 | −0.79–0.22 | 0.28 | ||||
Smoking initiation.
Logistic regression indicated no association between A1 allele frequency and the likelihood of being an ever-smoker (unadjusted OR = 1.01, 95% CI = 0.88–1.22, p = .88; adjusted OR = 1.01, 95% CI = 0.88–1.17, p = .85). Grouping the genotypes containing the minor A1 (T) allele (A2A2 vs. A2A1 + A1A1) did not alter these results substantially (unadjusted: p = .86; adjusted: p = .89).
Smoking persistence.
Logistic regression indicated no association between A1 allele frequency and the likelihood of being a current smoker (unadjusted OR = 0.97, 95% CI = 0.76–1.24, p = .80; adjusted OR = 0.97, 95% CI = 0.75–1.25, p = .83). Grouping the genotypes containing the minor A1 (T) allele (A2A2 vs. A2A1 + A1A1) did not alter these results substantially (unadjusted: p = .60; adjusted: p = .61).
Smoking rate.
Linear regression among current smokers indicated no association between A1 allele frequency and mean number of cigarettes per day (unadjusted β = −1.19, 95% CI = −2.71 to 0.34, p = .12; adjusted β = −0.81, 95% CI = −2.29 to 0.66, p = .28). Grouping the genotypes containing the minor A1 (T) allele (A2A2 vs. A2A1 + A1A1) did not alter these results substantially (unadjusted: p = .31; adjusted: p = .64).
Description of studies in meta-analysis
A total of 28 studies published between 1994 and 2007, comprising k = 34 independent samples, were identified by the search strategy, met the inclusion criteria, and contributed to the meta-analysis (Bierut et al., 2000; Comings et al., 1996, 1997; Connor et al., 2007; Costa-Mallen et al., 2000; David et al., 2007; Erblich, Lerman, Self, Diaz, & Bovbjerg, 2004, 2005; Freire, Marques, Hutz, & Bau, 2006; Hamajima et al., 2002; Johnstone, Yudkin, Griffiths, et al., 2004; Johnstone, Yudkin, Hey, et al., 2004; Lee, 2003; Lerman et al., 1999, 2003; Morton et al., 2006; Noble et al., 1994; Preuss, Zill, Koller, Bondy, & Sokya, 2007; Qi, Tan, Xing, Miao, & Lin, 2002; Robinson et al., 2006; Sabol et al., 1999; Singleton et al., 1998; Spitz et al., 1998; Swan et al., 2005; Timberlake et al., 2007; Ton et al., 2007; Wu, Hudmon, Detry, Chamberlain, & Spitz, 2000; Yoshida et al., 2001). Data from the present study also were included in the meta-analysis. Study characteristics are described in Table 3.
Table 3.
Characteristics of included studies
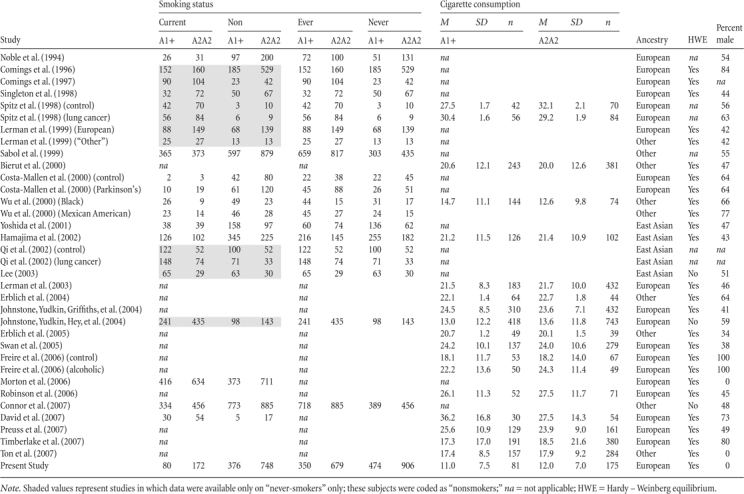 |
A total of 20 samples reported data on participants of predominantly European ancestry, 5 on participants of predominantly East Asian ancestry, and 9 on participants of “Other” ancestry. Two samples reported DRD2 genotype frequencies for control subjects that deviated significantly from HWE (Lee, 2003; Timberlake et al., 2006).
Meta-analysis
Separate analyses were performed for smoking initiation (ever vs. never), smoking persistence (current vs. nonsmoker), and smoking rate (cigarettes per day).
Smoking initiation.
When all samples with relevant data (k = 21) were pooled using a fixed-effects model, we found evidence for an association between the DRD2 Taq1A genotype and likelihood of being an ever-smoker (OR = 1.09, 95% CI = 1.01–1.17, Z = 2.18, p = .030). We found strong evidence of between-study heterogeneity (I2 = 76.68, χ2[20] = 85.74, p < .001), however. When these data were analyzed within a random-effects framework, the point estimate (indicating a 9% greater odds of smoking per A1 allele) remained identical to that in the fixed-effects analyses but the p value was consistent with the null hypothesis (Figure 1).
Figure 1.
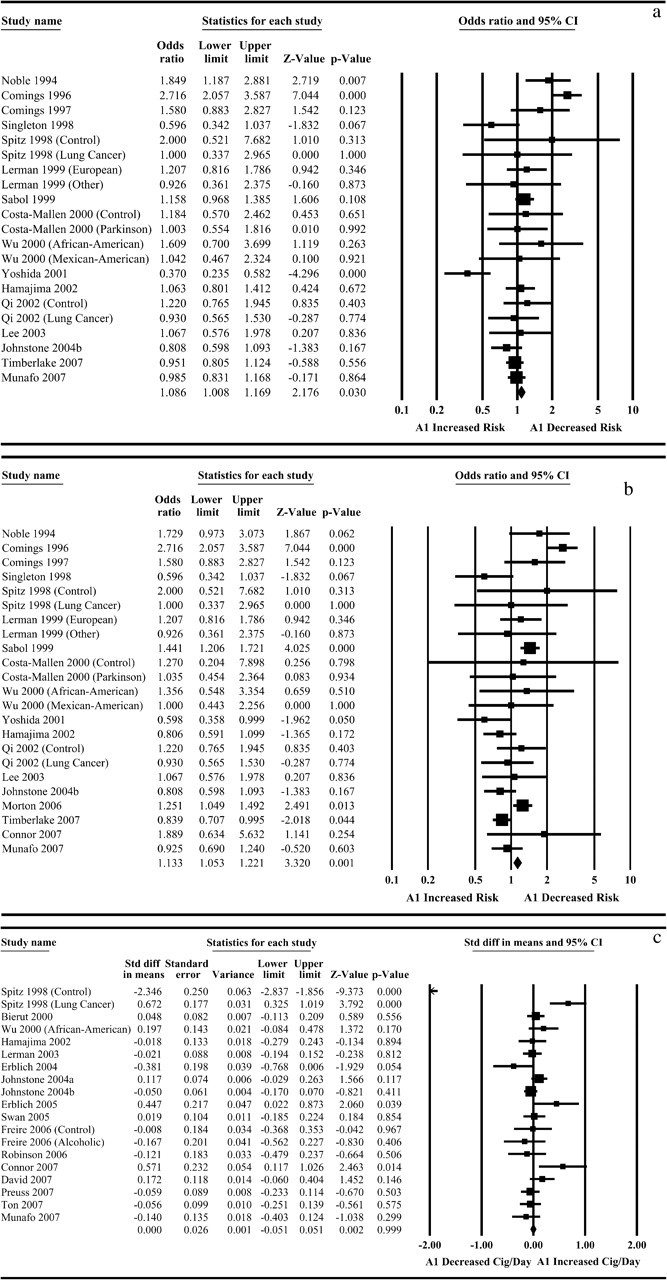
Meta-analysis of association of DRD2 Taq1A1 allele with smoking initiation, smoking persistence, and smoking rate. Meta-analysis indicates marginal evidence for association of the DRD2 Taq1A1 allele with smoking initiation (a) and smoking persistence (b), but not smoking rate (c). In all cases there was evidence of between-study heterogeneity, and analyses within a random-effects framework were consistent with the null hypothesis.
When the first published study (Noble et al., 1994) was removed from the analysis (k = 20), these results were not altered substantially, although the statistical evidence for association within a fixed-effects framework was marginal (p = .081). Metaregression indicated a significant association between effect size estimate and year of publication (Z = −5.09, p < .001), suggesting decreasing effect size with increasing year of publication (Figure 2).
Figure 2.
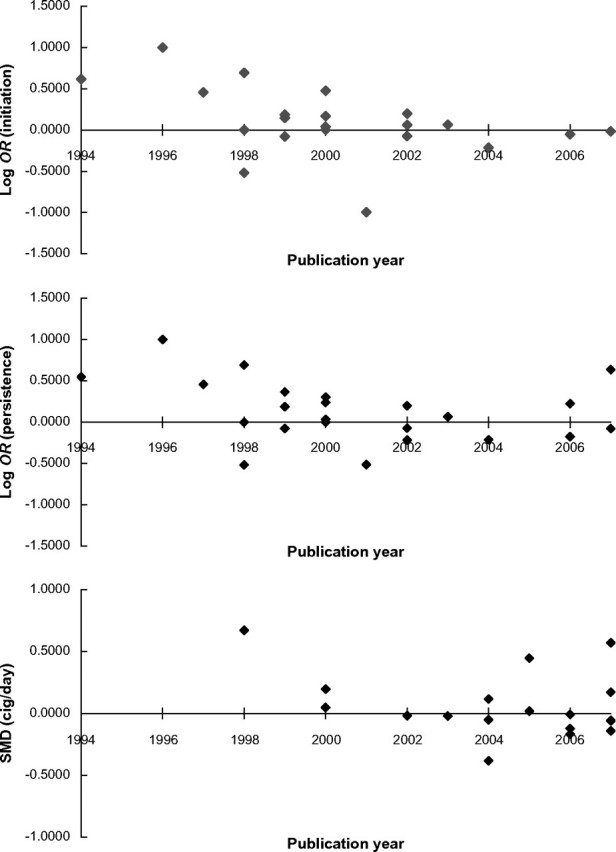
Metaregression of publication year against effect size for smoking initiation, smoking persistence, and smoking rate. Metaregression indicates a significant association between individual study publication year and effect size estimate for smoking initiation (top) and smoking persistence (middle), but not smoking rate (bottom). Where this was observed, the effect reflected a reduction in effect size estimate over time.
When studies that recruited samples of predominantly European ancestry were analyzed separately (k = 11), we found evidence of association, with a 20% greater odds of ever smoking per A1 allele (OR = 1.20, p = .001), although there was strong statistical evidence of between-study heterogeneity (I2 = 82.33, p < .001). When these data were analyzed within a random-effects framework, the odds of ever smoking per A1 allele remained increased (23%), but the p value was consistent with the null hypothesis (OR = 1.23, p = .19). Consistent with the main analysis, metaregression indicated a significant association between effect size estimate and year of publication (Z = −5.29, p < .001), suggesting decreasing effect size with increasing year of publication. When studies that recruited samples of predominantly East Asian ancestry were analyzed separately (k = 5), we found no strong evidence of association in either the fixed-effects (OR = 0.89, p = .23) or the random-effects (OR = 0.86, p = .49) model.
Metaregression, excluding two studies (Comings et al., 1997; Wu et al., 2000) comprising three samples in which data were not available, indicated a positive association between effect size and proportion of male participants (Z = 3.71, p < .001), suggesting increasing effect size with increasing proportion of male participants (<50% male: k = 7, OR = 0.93, 95% CI = 0.84–1.03, Z = 1.38, p = .17; >50% male: k = 11, OR = 1.32, 95% CI = 1.17–1.48, Z = 4.60, p < .001; Figure 3). Excluding the data from the present study did not alter these results substantially.
Figure 3.
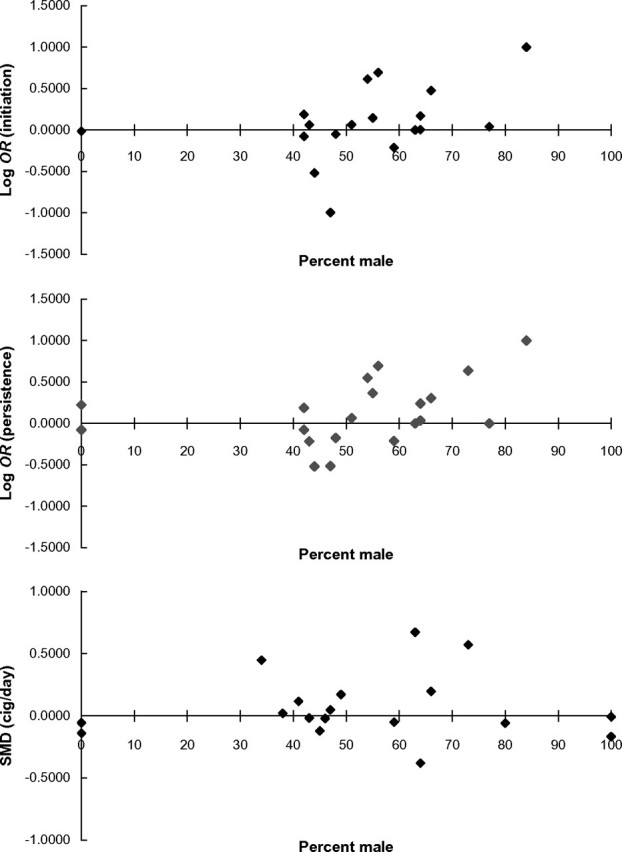
Metaregression of proportion of male participants against effect size for smoking initiation, smoking persistence, and smoking rate. Metaregression indicates a significant association between individual study proportion of male participants and effect size estimate for smoking initiation (top), smoking persistence (middle), but not smoking rate (bottom). Removal of the data from the primary study we report did not alter these results substantially.
A visual inspection of a funnel plot of 1/SE against effect size estimate did not indicate any evidence of ascertainment bias among the entire sample of studies that contributed to the analysis of smoking initiation. Egger's test also did not indicate any evidence of ascertainment bias, t(19) = 0.16, p = .87.
Smoking persistence.
When all samples (k = 23) with relevant data were included, we found evidence for an association between the DRD2 Taq1A genotype and the odds of being a current smoker (OR = 1.13, 95% CI = 1.05–1.22, Z = 3.32, p = .001) in the fixed-effects model. We found strong statistical evidence of between-study heterogeneity (I2 = 74.64, χ2[22] = 87.76, p < .001), however. When these data were analyzed within a random-effects framework the point estimate (indicating an 11% greater odds of current smoking per A1 allele) remained similar to that in the fixed-effects model but the p value was consistent with the null hypothesis (OR = 1.11, 95% CI = 0.94–1.33, Z = 1.21, p = .23). These results are presented graphically in Figure 1.
When the first published study (Noble et al., 1994) was removed from the analysis (k = 22), these results were not altered substantially. Metaregression indicated a negative association between effect size estimate and year of publication (Z = −5.50, p < .001), suggesting decreasing effect size with increasing year of publication. These data are presented graphically in Figure 2.
When studies that recruited samples of predominantly European ancestry were analyzed separately (k = 13), we found evidence for an association (OR = 1.26, p < .001), although there was evidence of significant between-study heterogeneity (I2 = 76.97, p < .001). When these data were analyzed within a random-effects framework, the p value was consistent with the null hypothesis (OR = 1.25, p = .11). Consistent with the main analysis, metaregression indicated a significant association between effect size estimate and year of publication (Z = −4.12, p < .001), suggesting decreasing effect size with increasing year of publication. When studies that recruited samples of predominantly East Asian ancestry were analyzed separately (k = 5), we found no evidence of association in either the fixed-effects (OR = 0.88, p = .19) or the random-effects (OR = 0.88, p = .26) model.
Metaregression, excluding two studies (Comings et al., 1997; Wu et al., 2000) comprising three samples in which data were not available, indicated a positive association between effect size and proportion of male participants (Z = 2.42, p = .015), suggesting increasing effect size with increasing proportion of male participants (<50% male: k = 8, OR = 0.96, 95% CI = 0.87–1.06, Z = 0.86, p = .39; >50% male: k = 12, OR = 1.45, 95% CI = 1.29–1.64, Z = 6.03, p < .001). Excluding the data from the present study did not alter these results substantially. These data are presented graphically in Figure 3.
A visual inspection of a funnel plot of 1/SE against effect size estimate did not indicate any evidence of possible ascertainment bias among the entire sample of studies that contributed to the analysis of smoking persistence. Egger's test also did not indicate any evidence of ascertainment bias, t(21) = 0.13, p = .90.
Smoking rate.
When all studies (k = 19) with relevant data were included, we found no evidence of an association between DRD2 Taq1A genotype and smoking rate (d = .00, 95% CI = −0.05 to 0.05, Z = 0.00, p = 1.00). There was strong statistical evidence of between-study heterogeneity (I2 = 85.80, χ2[18] = 126.80, p < .001), and the evidence for association remained nonsignificant (d = –.03, 95% CI = −0.18 to 0.11, Z = 0.45, p = .65). These results are presented graphically in Figure 1.
When the first published study (Spitz et al., 1998), comprising two samples, was removed from the analysis (k = 17), these results were not altered substantially. Metaregression indicated no evidence of an association between effect size estimate and year of publication (p = .71). These data are presented graphically in Figure 2.
When samples that recruited participants of predominantly European ancestry were analyzed separately (k = 13), we found no evidence of an association (d = –.01, p = .11). Only one sample recruited participants of predominantly East Asian ancestry, precluding a separate meta-analysis for this subgrouping.
Metaregression indicated no evidence of an association between effect size and proportion of male participants (Z = 20.07, p = .95). These data are presented graphically in Figure 3.
A visual inspection of a funnel plot of 1/SE against effect size estimate did not indicate any evidence of possible ascertainment bias among the entire sample of studies that contributed to the analysis of smoking rate. Egger's test also did not indicate any evidence of ascertainment bias, t(17) = 0.62, p = .55.
Discussion
We did not observe any evidence of an association between the DRD2 Taq1A polymorphism and smoking behavior, including smoking initiation, smoking persistence, and smoking rate, in a large sample of older females. In a meta-analysis of 29 studies, comprising 28 published studies and the data from the present study, we observed some evidence of a positive association with smoking initiation and persistence, though not smoking rate. However, considerable between-study heterogeneity was present, and p values in the random-effect models were consistent with the null hypothesis. Heterogeneity was explained in part by year of publication, with studies published more recently having weaker effects than earlier publications. A possible interpretation of these null results might be that no association exists. Intriguingly, however, metaregression suggested an association between the proportion of male participants in a study and the individual study effect size, indicating a larger effect size with a greater proportion of male participants. This effect did not appear to be due to the inclusion of the data from the present study, except in the analysis of smoking rate data, where removal of these data rendered the results of the metaregression nonsignificant.
The finding of greater effects in males was contrary to our a priori hypothesis. We felt that, based on previous evidence of sex differences in the effects of NRT and in nicotine metabolism, any effect modification by sex would relate to stronger effects in females. As a result, our findings should be interpreted as not supporting an association between the DRD2 Taq1A polymorphism and smoking behavior in either sex until further large studies with sufficient statistical power to determine a sex × genotype interaction within the same study population have been completed.
Researchers are interested in the Taq1A variant in part because it may alter the function of the nearby DRD2 gene. The SNP has been reported to affect DRD2 availability in postmortem striatal samples (Noble, Blum, Ritchie, Montgomery, & Sheridan, 1991; Thompson et al., 1997), and evidence from in vivo studies indicates an association between the A1 allele and lower mean relative glucose metabolic rate in dopaminergic regions in the human brain (Noble, Gottschalk, Fallon, Ritchie, & Wu, 1997). Positron emission tomography (PET) scan studies have indicated that this allele also is associated with low receptor density (Jonsson et al., 1999). Evidence that the Taq1A variant alters an amino acid in the ANKK1 protein kinase gene, near the DRD2 locus (Neville et al., 2004), does not rule out an effect on the DRD2 gene. Data from the HapMap project reveal that the variant is in linkage disequilibrium with other variants in the DRD2 gene, but not with variants in the ANKK1 gene, although other data appear to contradict this finding (Gelernter et al., 2006). Thus, it may be that additional functional variants in DRD2 are contributing to any association with smoking behavior.
Another, more speculative, possibility is that ANKK1 may exert an effect on dopaminergic neurotransmission itself. The function of many proteins can be influenced or regulated by a process of phosphorylation of key amino acid residues within the protein. This process can influence factors such as the affinity of the protein for ligands that bind to it, such as dopamine to its transporter in this instance. Phosphorylation also can influence other aspects of activity, and kinases catalyze these phosphorylation processes. ANKK1 might therefore be a kinase that acts on the transporter to influence its activity. If this were to be so, it might explain how a polymorphism in a gene that was not the transporter itself might relate to dopaminergic activity, so that the polymorphism in ANKK1 may influence the activity or regulation of the kinase, thereby influencing the activity of the transporter (D. J. K. Balfour, personal communication, 23 June 2006). Data do not currently exist to test this possibility directly.
An important question that our data are not able to answer is why any effects of the DRD2 Taq1A polymorphism may operate only in males or be stronger in males. Recent imaging data suggest one possibility, however, which is that dopamine release following stimulant challenge, as measured by PET with high–specific activity [11C] raclopride, appears to be greater in males than in females, with corresponding differences between males and females in the subjective ratings of the positive effects of the challenge (Munro et al., 2006). Therefore, increased dopaminergic neurotransmission in response to drug challenge among males, in particular in the striatum, may result in greater scope for individual differences due to genetic factors to exert an influence on drug-seeking and drug-taking behavior. Imaging genetic study designs offer one means by which this possibility could be tested. Another potential mechanism that may explain our findings relates to hormonal differences between males and females. Estrogen interacts with activity at dopamine D2 receptor sites in the striatum (Lammers et al., 1999), a D2 receptor–rich area of the brain, whereas females also show greater estrogen-induced dopamine activation (Carpenter, Upadhyaya, LaRowe, Saladin, & Brady, 2006; Dluzen & Anderson, 1997; Lammers et al., 1999). The generally higher estrogen levels among females may therefore be a protective factor against suboptimal dopamine functioning. That is, a genetic variant associated with reduced dopaminergic activity might result in a relatively greater deficit among males than among females, thereby leading to a greater likelihood of persistent smoking, given the agonist effects of nicotine on dopamine release.
The present study has several limitations, in particular the new primary data that we report, that should be considered when interpreting these results. We originally hypothesized that any effect of DRD2 Taq1A genotype would be stronger in females, hence our choice of a large representative sample of women. However, the absence of men did not permit us to test explicitly for any putative sex × genotype interaction. Nevertheless, our new data were consistent with the findings from other studies included in the meta-analysis. Second, data on smoking behaviors were obtained by self-report and lack biochemical validation. Although the survey procedures were intended to maximize accurate responding and most other studies reporting this association also lack biochemical validation of smoking behavior, nondifferential misreporting may have weakened any true association. Third, the smoking behavior phenotypes available to us were relatively crude and do not capture important aspects of smoking behavior such as degree of dependence (except perhaps through smoking rate). However, those phenotypes that were available were broadly comparable with those available in other published studies, which allowed us to conduct a meta-analysis of these studies. Indeed, the principal limitation of our meta-analysis (as opposed to our analysis of the primary data that we report) is that the procedures used allow only comparable data to be combined. Fourth, our new data were drawn from a relatively old sample, and genetic effects may become more prominent as cigarette smoking becomes more socially unacceptable, so that these effects may have been masked in our data. However, the observed negative correlation between effect size estimate and year of publication would argue against this possibility.
Despite these limitations, our results provide some support for the suggestion that the mechanisms of smoking behavior and dependence differ between males and females and that the genetic influences on these mechanisms also may differ, although the apparent direction of effect we observed in our meta-analysis is contrary to our original hypothesis, so that these findings must be regarded as tentative and preliminary. If an association exists between the DRD2 Taq1A polymorphism and smoking behavior, it may operate only in males or be stronger in males. In general, it should not be assumed that genetic associations will be comparable in males and females. This possibility requires further study, in particular in a large, adequately powered primary sample with sufficient power to test explicitly for differences between males and females. Future studies also should investigate sex differences in genetic associations with more complex phenotypes, such as measures of dependence and smoking trajectories, and potential mediating mechanisms, for example, through the use of imaging genetic study designs.
Funding
The British Women's Heart and Health Study was funded by the U.K. Department of Health and the British Heart Foundation. DAL is funded by a U.K. Department of Health career scientist award. NJT is funded by the U.K. Medical Research Council. The views expressed in this publication are those of the authors and not necessarily of any funding body.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
The authors thank Ivan Berlin, Laura Bierut, Jason Connor, Paola Costa-Mallen, Maria Teresa Freire, Nobuyuki Hamajima, Elaine Johnstone, Ulrich Preuss, Jason Robinson, David Timberlake, and Thanh Ton for releasing data in a format that enabled their inclusion in the meta-analysis and to Garrett Sullivan for his assistance in collating articles for inclusion in the meta-analysis. They also thank the general practitioners and participants in the British Women's Heart and Health Study and staff who have collected and maintained data on the study.
References
- Berlin I, Covey LS, Jiang H, Hamer D. Lack of effect of D2 dopamine receptor TaqI A polymorphism on smoking cessation. Nicotine & Tobacco Research. 2005;7:725–728. doi: 10.1080/14622200500259176. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Rice JP, Edenberg HJ, Goate A, Foroud T, Cloninger CR, et al. Family-based study of the association of the dopamine D2 receptor gene (DRD2) with habitual smoking. American Journal of Medical Genetics. 2000;90:299–302. doi: 10.1002/(sici)1096-8628(20000214)90:4<299::aid-ajmg7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Blum K, Noble EP, Sheridan PJ, Montgomery A, Ritchie T, Jagadeeswaran P, et al. Allelic association of human dopamine D2 receptor gene in alcoholism. Journal of the American Medical Association. 1990;263:2055–2060. [PubMed] [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Comings DE. Dopamine D2 receptor gene variants: Association and linkage studies in impulsive-addictive-compulsive behaviour. Pharmacogenetics. 1995;5:121–141. doi: 10.1097/00008571-199506000-00001. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, Brady KT. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: A review. Nicotine & Tobacco Research. 2006;8:627–638. doi: 10.1080/14622200600910793. [DOI] [PubMed] [Google Scholar]
- Comings DE, Ferry L, Bradshaw-Robinson S, Burchette R, Chiu C, Muhleman D. The dopamine D2 receptor (DRD2) gene: A genetic risk factor in smoking. Pharmacogenetics. 1996;6:73–79. doi: 10.1097/00008571-199602000-00006. [DOI] [PubMed] [Google Scholar]
- Comings DE, Gade R, Wu S, Chiu C, Dietz G, Muhleman D, et al. Studies of the potential role of the dopamine D1 receptor gene in addictive behaviors. Molecular Psychiatry. 1997;2:44–56. doi: 10.1038/sj.mp.4000207. [DOI] [PubMed] [Google Scholar]
- Connor JP, Young RM, Lawford BR, Saunders JB, Ritchie TL, Noble EP. Heavy nicotine and alcohol use in alcohol dependence is associated with D2 dopamine receptor (DRD2) polymorphism. Addictive Behaviors. 2007;32:310–319. doi: 10.1016/j.addbeh.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Costa-Mallen P, Costa LG, Smith-Weller T, Franklin GM, Swanson PD, Checkoway H. Genetic polymorphism of dopamine D2 receptors in Parkinson's disease and interactions with cigarette smoking and MAO-B intron 13 polymorphism. Journal of Neurology, Neurosurgery, and Psychiatry. 2000;69:535–537. doi: 10.1136/jnnp.69.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Brown RA, Papandonatos GD, Kahler CW, Lloyd-Richardson EE, Munafò MR, et al. Pharmacogenetic clinical trial of sustained-release bupropion for smoking cessation. Nicotine & Tobacco Research. 2007;9:821–833. doi: 10.1080/14622200701382033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE, Anderson LI. Estrogen differentially modulates nicotine-evoked dopamine release from the striatum of male and female rats. Neuroscience Letters. 1997;230:140–142. doi: 10.1016/s0304-3940(97)00487-4. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich J, Lerman C, Self DW, Diaz GA, Bovbjerg DH. Stress-induced cigarette craving: Effects of the DRD2 TaqI RFLP and SLC6A3 VNTR polymorphisms. Pharmacogenomics Journal. 2004;4:102–109. doi: 10.1038/sj.tpj.6500227. [DOI] [PubMed] [Google Scholar]
- Erblich J, Lerman C, Self DW, Diaz GA, Bovbjerg DH. Effects of dopamine D2 receptor (DRD2) and transporter (SLC6A3) polymorphisms on smoking cue-induced cigarette craving among African-American smokers. Molecular Psychiatry. 2005;10:407–414. doi: 10.1038/sj.mp.4001588. [DOI] [PubMed] [Google Scholar]
- Freire MT, Marques FZ, Hutz MH, Bau CH. Polymorphisms in the DBH and DRD2 gene regions and smoking behavior. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:93–97. doi: 10.1007/s00406-005-0610-x. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Yu Y, Weiss R, Brady K, Panhuysen C, Yang BZ, et al. Haplotype spanning TTC12 and ANKK1, flanked by the DRD2 and NCAM1 loci, is strongly associated to nicotine dependence in two distinct American populations. Human Molecular Genetics. 2006;15:3498–3507. doi: 10.1093/hmg/ddl426. [DOI] [PubMed] [Google Scholar]
- Hamajima N, Ito H, Matsuo K, Saito T, Tajima K, Ando M, et al. Association between smoking habits and dopamine receptor D2 taqI A A2 allele in Japanese males: A confirmatory study. Journal of Epidemiology. 2002;12:297–304. doi: 10.2188/jea.12.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone EC, Yudkin P, Griffiths SE, Fuller A, Murphy M, Walton R. The dopamine D2 receptor C32806T polymorphism (DRD2 Taq1A RFLP) exhibits no association with smoking behaviour in a healthy UK population. Addiction Biology. 2004;9:221–226. doi: 10.1080/13556210412331292226. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Yudkin PL, Hey K, Roberts SJ, Welch SJ, Murphy MF, et al. Genetic variation in dopaminergic pathways and short-term effectiveness of the nicotine patch. Pharmacogenetics. 2004;14:83–90. doi: 10.1097/00008571-200402000-00002. [DOI] [PubMed] [Google Scholar]
- Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, et al. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Molecular Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Lammers CH, D’Souza U, Qin ZH, Lee SH, Yajima S, Mouradian MM. Regulation of striatal dopamine receptors by estrogen. Synapse. 1999;34:222–227. doi: 10.1002/(SICI)1098-2396(19991201)34:3<222::AID-SYN6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Lawford BR, Young RM, Noble EP, Sargent J, Rowell J, Shadforth S, et al. The D(2) dopamine receptor A(1) allele and opioid dependence: Association with heroin use and response to methadone treatment. American Journal of Medical Genetics. 2000;96:592–598. doi: 10.1002/1096-8628(20001009)96:5<592::aid-ajmg3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Bedford C, Taylor M, Ebrahim S. Geographical variation in cardiovascular disease, risk factors, and their control in older women: British Women's Heart and Health Study. Journal of Epidemiology and Community Health. 2003;57:134–140. doi: 10.1136/jech.57.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Davey Smith G, Rumley A, Lowe GD, Ebrahim S. Associations of fibrinogen and C-reactive protein with prevalent and incident coronary heart disease are attenuated by adjustment for confounding factors. British Women's Heart and Health Study. Thrombosis and Haemostasis. 2005;93:955–963. doi: 10.1160/TH04-12-0805. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Ebrahim S, Davey Smith G. Socioeconomic position in childhood and adulthood and insulin resistance: Cross sectional survey using data from British women's heart and health study. British Medical Journal. 2002;325:805. doi: 10.1136/bmj.325.7368.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS. Gender-specific molecular heterosis and association studies: Dopamine D2 receptor gene and smoking. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2003;118:55–59. doi: 10.1002/ajmg.b.10036. [DOI] [PubMed] [Google Scholar]
- Lerman C, Caporaso NE, Audrain J, Main D, Bowman ED, Lockshin B, et al. Evidence suggesting the role of specific genetic factors in cigarette smoking. Health Psychology. 1999;18:14–20. doi: 10.1037//0278-6133.18.1.14. [DOI] [PubMed] [Google Scholar]
- Lerman C, Jepson C, Wileyto EP, Epstein LH, Rukstalis M, Patterson F, et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: Results of two randomized clinical trials. Neuropsychopharmacology. 2006;31:231–242. doi: 10.1038/sj.npp.1300861. [DOI] [PubMed] [Google Scholar]
- Lerman C, Shields PG, Wileyto EP, Audrain J, Hawk LH, Jr., Pinto A, et al. Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health Psychology. 2003;22:541–548. doi: 10.1037/0278-6133.22.5.541. [DOI] [PubMed] [Google Scholar]
- Lingford-Hughes A, Nutt D. Neurobiology of addiction and implications for treatment. British Journal of Psychiatry. 2003;182:97–100. doi: 10.1192/bjp.182.2.97. [DOI] [PubMed] [Google Scholar]
- Morton LM, Wang SS, Bergen AW, Chatterjee N, Kvale P, Welch R, et al. DRD2 genetic variation in relation to smoking and obesity in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Pharmacogenetics and Genomics. 2006;16:901–910. doi: 10.1097/01.fpc.0000230417.20468.d0. [DOI] [PubMed] [Google Scholar]
- Munafò M, Bradburn M, Bowes L, David S. Are there sex differences in transdermal nicotine replacement therapy patch efficacy? A meta-analysis. Nicotine & Tobacco Research. 2004;6:769–776. doi: 10.1080/14622200410001696556. [DOI] [PubMed] [Google Scholar]
- Munafò M, Clark T, Johnstone E, Murphy M, Walton R. The genetic basis for smoking behavior: A systematic review and meta-analysis. Nicotine & Tobacco Research. 2004;6:583–597. doi: 10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Johnstone E, Murphy M, Walton R. New directions in the genetic mechanisms underlying nicotine addiction. Addiction Biology. 2001;6:109–117. doi: 10.1080/13556210020040181. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Matheson IJ, Flint J. Association of the DRD2 gene Taq1A polymorphism and alcoholism: A meta-analysis of case-control studies and evidence of publication bias. Molecular Psychiatry. 2007;12:454–461. doi: 10.1038/sj.mp.4001938. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, et al. Sex differences in striatal dopamine release in healthy adults. Biological Psychiatry. 2006;59:966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: A novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Human Mutation. 2004;23:540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenow DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: Effects of gender and cue type. Addictive Behaviors. 1998;23:209–224. doi: 10.1016/s0306-4603(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Noble EP, Blum K, Khalsa ME, Ritchie T, Montgomery A, Wood RC, et al. Allelic association of the D2 dopamine receptor gene with cocaine dependence. Drug and Alcohol Dependence. 1993;33:271–285. doi: 10.1016/0376-8716(93)90113-5. [DOI] [PubMed] [Google Scholar]
- Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Archives of General Psychiatry. 1991;48:648–654. doi: 10.1001/archpsyc.1991.01810310066012. [DOI] [PubMed] [Google Scholar]
- Noble EP, Gottschalk LA, Fallon JH, Ritchie TL, Wu JC. D2 dopamine receptor polymorphism and brain regional glucose metabolism. American Journal of Medical Genetics. 1997;74:162–166. [PubMed] [Google Scholar]
- Noble EP, St Jeor ST, Ritchie T, Syndulko K, St Jeor SC, Fitch RJ, et al. D2 dopamine receptor gene and cigarette smoking: A reward gene? Medical Hypotheses. 1994;42:257–260. doi: 10.1016/0306-9877(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Nicotine self-administration. Nicotine & Tobacco Research. 1999;1(Suppl. 2):S133–S137. doi: 10.1080/14622299050011951. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Perkins KA, D’Amico D, Sanders M, Grobe JE, Wilson A, Stiller RL. Influence of training dose on nicotine discrimination in humans. Psychopharmacology (Berlin) 1996;126:132–139. doi: 10.1007/BF02246348. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: Review of human and animal evidence. Nicotine & Tobacco Research. 1999;1:301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Preuss UW, Zill P, Koller G, Bondy B, Sokya M. D2 dopamine receptor gene haplotypes and their influence on alcohol and tobacco consumption magnitude in alcohol-dependent individuals. Alcohol and Alcoholism. 2007;42:258–266. doi: 10.1093/alcalc/agm030. [DOI] [PubMed] [Google Scholar]
- Qi J, Tan W, Xing D, Miao X, Lin D. Study on the association between smoking behavior and dopamine receptor D2 gene polymorphisms among lung cancer cases. Zhonghua Liu Xing Bing Xue Za Zhi. 2002;23:370–373. [PubMed] [Google Scholar]
- Robinson JD, Lam CY, Minnix JA, Wetter DW, Tomlinson GE, Minna JD, et al. The DRD2 TaqI-B polymorphism and its relationship to smoking abstinence and withdrawal symptoms. Pharmacogenomics Journal. 2007;7:266–274. doi: 10.1038/sj.tpj.6500427. [DOI] [PubMed] [Google Scholar]
- Sabol SZ, Nelson ML, Fisher C, Gunzerath L, Brody CL, Hu S, et al. A genetic association for cigarette smoking behavior. Health Psychology. 1999;18:7–13. doi: 10.1037//0278-6133.18.1.7. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Thomson JH, Morris CM, Court JA, Lloyd S, Cholerton S. Lack of association between the dopamine D2 receptor gene allele DRD2*A1 and cigarette smoking in a United Kingdom population. Pharmacogenetics. 1998;8:125–128. doi: 10.1097/00008571-199804000-00005. [DOI] [PubMed] [Google Scholar]
- Spitz MR, Shi H, Yang F, Hudmon KS, Jiang H, Chamberlain RM, et al. Case-control study of the D2 dopamine receptor gene and smoking status in lung cancer patients. Journal of the National Cancer Institute. 1998;90:358–363. doi: 10.1093/jnci/90.5.358. [DOI] [PubMed] [Google Scholar]
- Swan GE, Valdes AM, Ring HZ, Khroyan TV, Jack LM, Ton CC, et al. Dopamine receptor DRD2 genotype and smoking cessation outcome following treatment with bupropion SR. Pharmacogenomics Journal. 2005;5:21–29. doi: 10.1038/sj.tpj.6500281. [DOI] [PubMed] [Google Scholar]
- Thompson J, Thomas N, Singleton A, Piggott M, Lloyd S, Perry EK, et al. D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: Reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics. 1997;7:479–484. doi: 10.1097/00008571-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Timberlake DS, Haberstick BC, Lessem JM, Smolen A, Ehringer M, Hewitt JK, et al. An association between the DAT1 polymorphism and smoking behavior in young adults from the National Longitudinal Study of Adolescent Health. Health Psychology. 2006;25:190–197. doi: 10.1037/0278-6133.25.2.190. [DOI] [PubMed] [Google Scholar]
- Timpson NJ, Lawlor DA, Harbord RM, Gaunt TR, Day IN, Palmer LJ, et al. C-reactive protein and its role in metabolic syndrome: Mendelian randomisation study. Lancet. 2005;366:1954–1959. doi: 10.1016/S0140-6736(05)67786-0. [DOI] [PubMed] [Google Scholar]
- Ton TG, Rossing MA, Bowen DJ, Srinouanprachan S, Wicklund K, Farin FM. Genetic polymorphisms in dopamine-related genes and smoking cessation in women: A prospective cohort study. Behavioral and Brain Functions. 2007;3:22. doi: 10.1186/1744-9081-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trikalinos TA, Ntzani EE, Contopoulos-Ioannidis DG, Ioannidis JP. Establishment of genetic associations for complex diseases is independent of early study findings. European Journal of Human Genetics. 2004;12:762–769. doi: 10.1038/sj.ejhg.5201227. [DOI] [PubMed] [Google Scholar]
- Wu X, Hudmon KS, Detry MA, Chamberlain RM, Spitz MR. D2 dopamine receptor gene polymorphisms among African-Americans and Mexican-Americans: A lung cancer case-control study. Cancer Epidemiology Biomarkers and Prevention. 2000;9:1021–1026. [PubMed] [Google Scholar]
- Yoshida K, Hamajima N, Kozaki K, Saito H, Maeno K, Sugiura T, et al. Association between the dopamine D2 receptor A2/A2 genotype and smoking behavior in the Japanese. Cancer Epidemiology, Biomarkers & Prevention. 2001;10:403–405. [PubMed] [Google Scholar]
- Yudkin P, Munafò M, Hey K, Roberts S, Welch S, Johnstone E, et al. Effectiveness of nicotine patches in relation to genotype in women versus men: Randomised controlled trial. British Medical Journal. 2004;328:989–990. doi: 10.1136/bmj.38050.674826.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman MV, Hiraki L, Sellers EM. Gender differences in tobacco smoking: Higher relative exposure to smoke than nicotine in women. Journal of Women's Health and Gender Based Medicine. 2002;11:147–153. doi: 10.1089/152460902753645281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


