Abstract
Background. Low-level lead exposure is widespread and has been implicated as a chronic kidney disease (CKD) risk factor. However, studies evaluating associations of lead dose with newer, potentially more accurate, estimates of kidney function, in participants with a wide range of glomerular filtration rates (GFRs), are scarce.
Methods. We compared associations of blood lead and estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease (MDRD), Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) and cystatin C single variable, multivariable and combined creatinine/cystatin C equations in 3941 adults who participated in the 1999–2002 National Health and Nutrition Examination Survey cystatin C subsample.
Results. Geometric mean blood lead was 1.7 μg/dL. After multivariable adjustment, differences [95% confidence interval (CI)] in mean eGFR for a doubling of blood lead were −1.9 (−3.2, −0.7), −1.7 (−3.0, −0.5) and −1.4 (−2.3, −0.5) mL/min/1.73 m2, using the cystatin C single variable, multivariable and combined creatinine/cystatin C equations, respectively, reflecting lower eGFR with increased blood lead. The corresponding differences (95% CI) were −0.9 (−1.9, 0.02) and −0.9 (−1.8, 0.01) using the creatinine-based MDRD and CKD-EPI equations, respectively. In participants aged ≥60 years, differences in mean eGFR ranged from −3.0 to −4.5 mL/min/1.73 m2, and odds of reduced eGFR (<60 mL/min/1.73 m2) were increased for all estimates of GFR.
Conclusions. These results support the inclusion of cystatin C-based eGFR in future lead research and provide additional evidence for environmental lead exposure as a CKD risk factor.
Keywords: blood lead, kidney function, lead exposure, NHANES
Introduction
Recent research suggests that environmental lead exposure increases risk for chronic kidney disease (CKD), even at the lower levels currently observed in the USA and other developed countries [1–8]. The association between lead exposure and CKD has been observed in prospective studies, in a variety of populations, and is consistent with experimental and mechanistic evidence [2, 3, 5, 9–14]. Environmental lead exposure remains widespread globally [6, 7, 15]. Moreover, lead accumulated in bone from past exposure remains a source of current endogenous exposure [16]. The increasing prevalence of CKD [17] and the fact that lead exposure is preventable and treatable with chelation in selected settings [18] highlight the need to fully characterize kidney risk from lead exposure. In such research, accurate assessment of kidney function is essential to avoid kidney disease misclassification resulting in underestimation of risk. Equations to estimate glomerular filtration rate (GFR) are the most common method for assessing kidney function clinically and in large epidemiologic studies, where GFR assessment with an exogenous filtration marker is not possible. Ongoing efforts to improve the accuracy of these approaches have resulted in new serum creatinine-based equations and equations incorporating serum cystatin C. However, publications utilizing these newer techniques in research on the impact of lead on the kidney are scarce.
The objective of this study was to evaluate these recently developed GFR-estimating approaches in lead research. Therefore, we compared associations of blood lead level with estimated glomerular filtration rate (eGFR) calculated with recently developed equations to associations using the Modification of Diet in Renal Disease (MDRD) equation [20, 21], a serum creatinine-based equation routinely used in clinical practice and research. We used four new approaches: the creatinine-based Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [22], developed to be more accurate than the MDRD equation at higher GFRs, and three serum cystatin C-based equations: (i) cystatin C only; (ii) cystatin C, age, sex and race and (iii) cystatin C, age, sex, race and serum creatinine [23]. We used data from US adults who participated in the cystatin C subsample of the 1999–2002 National Health and Nutrition Examination Survey (NHANES). To our knowledge, this is the first study to evaluate associations of lead dose with these potentially more accurate eGFR approaches.
Materials and methods
Study population
NHANES 1999–2002 was conducted using a complex multistage sampling design to obtain a representative sample of the noninstitutionalized, civilian US population [24]. The study protocols were approved by the National Center for Health Statistics Institutional Review Board. All participants provided oral and written consent.
In 2006, cystatin C was assayed on stored serum samples from all NHANES 1999–2002 participants aged ≥60 years as well as on a 25% random sample of those aged 12–59 years [25, 26]. The younger group was supplemented with all individuals with a serum creatinine >1.2 mg/dL (SI conversion: multiply by 88.4 for micromoles per liter) in males and >1.0 mg/dL in females [25, 26]. Of 4563 adults aged ≥20 years with cystatin C measures available, we excluded pregnant women and those missing blood lead levels and other variables of interest, leaving 3941 participants with complete data.
Blood lead measurement
Blood lead was measured at the Centers for Disease Control and Prevention's National Center for Environmental Health [24]. Lead was measured in whole blood together with cadmium using a Perkin-Elmer Model SIMAA 6000 simultaneous multielement atomic absorption spectrometer with Zeeman background correction. Strict quality control procedures were followed including confirmation that collection and storage materials were not contaminated. The limit of detection was 0.3 μg/dL [27]; results were below the limit of detection in 0.5% of participants in our study population. For these values, a level equal to the limit of detection divided by the squared root of two was imputed [28, 29]. National Institute of Standards and Technology whole-blood standard reference materials were used for external calibration. The interassay coefficients of variation ranged from 3.1 to 7.0% for concentrations ranging from 2.1 to 29.3 μg/dL [30, 31].
Estimates of GFR
Serum creatinine was measured using a kinetic rate Jaffé method with a Hitachi Model 704 multichannel analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN) [24]. Serum creatinine concentrations were calibrated to standard creatinine [32]. The interassay coefficients of variation were 2.7 and 2.2% at mean creatinine concentrations of 1.67 and 6.51 mg/dL, respectively, for 1999–2000 [33] and 4.4 and 1.5% at mean creatinine concentrations of 0.68 and 7.0 mg/dL, respectively, for 2001–2002 [34]. Serum cystatin C was measured using an automated particle-enhanced nephelometric assay (Dade Behring N Latex Cystatin C run on a Dade Behring Nephelometer II; Siemens Healthcare Diagnostics, Deerfield, IL) [24]. The assay range was 0.23–7.25 mg/L. The interassay coefficients of variation were 5.05 and 4.87% at mean cystatin C concentrations of 0.97 and 1.90 mg/L, respectively [35]. The following equations were used to estimate GFR:
MDRD eGFR = 175 × (serum creatinine)−1.154 × age−0.203 × 1.212 (if black) × 0.742 (if female) [20]
CKD-EPI eGFR = 141 × min(Scr/κ, 1)α × max(Scr/κ, 1)−1.209 × 0.993Age × 1.159 (if black) × 1.018 (if female), where Scr is serum creatinine, κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1 and max indicates the maximum of Scr/κ or 1 [22]
Cystatin C single variable eGFR = 76.7 × serum cystatin C−1.19 [23]
Cystatin C multivariable eGFR = 127.7 × (serum cystatin C)−1.17 × age−0.13 × 1.06 (if black) × 0.91 (if female) [23]
Combined cystatin C/creatinine eGFR = 177.6 × serum creatinine−0.65 × serum cystatin C−0.57 × age−0.20 × (0.82 if female) × (1.11 if black) [23]
Other variables
Information on age, sex, race/ethnicity, education, smoking, income and alcohol consumption was based on self-report [24]. Body mass index was calculated by dividing measured weight in kilograms by measured height in meters squared. Serum cotinine was measured by an isotope-dilution high-performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometric method. Hypertension was defined as a mean systolic blood pressure ≥140 mmHg or mean diastolic blood pressure ≥90 mmHg, based on three blood pressure measurements obtained during the medical examination, or a self-reported physician diagnosis. Diabetes mellitus was defined as a fasting glucose ≥126 mg/dL, a non-fasting glucose ≥200 mg/dL or a self-reported physician diagnosis.
Statistical analysis
Data were obtained from the NHANES Web site [24] and merged and analyzed using STATA 10 (StataCorp, College Station, TX). Statistical analyses were performed using the survey commands in STATA 10 with specific weights for the cystatin C subsample to account for the complex sampling design.
Distribution of blood lead was right skewed and log-transformed for the analyses. Tertile cut-points were based on weighted distributions in the whole study population. In separate linear regression models for each equation, differences in mean eGFR were estimated comparing each of the two higher tertiles of blood lead to the lowest tertile and for doubling of lead levels. These linear regression models were conducted in all participants and in those <60 and ≥60 years of age. In separate logistic regression models for each equation, odds ratios for reduced eGFR (<60 mL/min/1.73 m2) were estimated only in participants ≥60 years because few participants <60 years of age had reduced eGFRs (103 participants with the MDRD equation and 53–60 participants with the other equations). Model adjustment was based on biological plausibility and known lead and kidney confounders. Adjustment for cadmium was performed since this is another proximal tubule nephrotoxicant for which exposure is common environmentally. P-values for linear trend in logistic and linear regression models were obtained by including blood lead tertiles coded as ordinal variables; results obtained by entering blood lead as tertile medians were similar. To assess the dose–response relationship in a flexible manner, we also estimated odds ratios for reduced eGFR by modeling blood lead levels with restricted quadratic splines with knots at the 5th, 50th and 95th percentiles. Spline models were conducted only in participants ≥60 years due to the small number of participants <60 years of age with eGFR <60 mL/min/1.73 m2.
Results
The geometric mean blood lead was 1.7 μg/dL for all participants, 2.2 μg/dL for participants aged ≥60 years and 1.6 μg/dL for participants aged <60 years. Corresponding values for blood cadmium were 0.45, 0.50 and 0.44 μg/L, respectively. Median eGFR levels were lowest using the MDRD equation (Table 1). The weighted prevalence of reduced eGFR (<60 mL/min/1.73 m2) was 8.4% for the MDRD equation, 6.5% for the CKD-EPI equation and 6.6%, 8.6% and 6.4% for the three cystatin C equations, respectively, consistent with previous prevalence estimates [17]. Geometric mean [95% confidence interval (CI)] blood lead levels for participants with cystatin C multivariable eGFR <60 and ≥60 mL/min/1.73 m2 were 2.3 (2.1, 2.5) and 1.7 (1.6, 1.7) μg/dL, respectively. Mean blood lead levels were also significantly higher in participants with reduced kidney function using the other equations (data not shown). All eGFR measures were highly correlated, particularly for eGFR levels calculated with the same serum measure: correlations between eGFR calculated using the MDRD equation and eGFR using CKD-EPI, cystatin C single variable, cystatin C multivariable and cystatin C/creatinine equations were 0.95, 0.71, 0.72 and 0.93, respectively (Appendices 1–3).
Table 1.
Median (interquartile range) levels of blood lead and eGFR by participant characteristics using different estimating equations (BMI, body mass index)a
| Characteristic | n (weighted %) | Blood lead level (μg/dL)a | MDRD | CKD-EPI | Cystatin C single variable | Cystatin C multivariable | Cystatin C/creatinine |
| Overall | 3941 (100.0) | 1.7 (1.1, 2.5) | 85.0 (73.1, 99.6) | 94.5 (80.0, 108.6) | 95.7 (80.5, 109.8) | 93.4 (77.1, 108.4) | 93.8 (80.2, 108.3) |
| Age <60 years | 1332 (77.3) | 1.6 (1.0, 2.3) | 89.3 (77.4, 103.4) | 100.8 (87.1, 112.6) | 100.0 (86.9, 113.4) | 98.7 (85.8, 113.5) | 99.5 (87.4, 112.6) |
| Age ≥60 years | 2609 (22.7) | 2.2 (1.6, 3.1) | 70.5 (59.0, 82.5) | 73.4 (60.3, 85.0) | 76.7 (63.0, 89.3) | 70.7 (57.7, 82.0) | 73.2 (60.9, 84.2) |
| Male | 2010 (49.2) | 2.1 (1.4, 3.0) | 86.2 (74.1, 99.8) | 94.4 (81.0, 107.5) | 93.1 (79.5, 104.7) | 96.2 (79.7, 110.9) | 95.0 (82.0, 108.0) |
| Female | 1931 (50.8) | 1.4 (0.9, 2.1) | 83.5 (71.3, 99.6) | 94.5 (78.6, 109.5) | 97.1 (81.5, 113.4) | 91.5 (74.2, 106.1) | 92.7 (78.3, 108.5) |
| White | 2113 (72.8) | 1.7 (1.1, 2.4) | 82.2 (70.9, 93.5) | 91.4 (77.6, 104.2) | 93.1 (78.6, 106.3) | 90.8 (75.0, 103.3) | 90.7 (78.0, 102.8) |
| Black | 705 (10.2) | 1.7 (1.2, 2.8) | 97.7 (79.7, 111.1) | 104.7 (84.5, 121.1) | 103.1 (84.7, 117.3) | 106.9 (86.1, 123.2) | 104.4 (87.5, 120.2) |
| Mexican American | 861 (6.9) | 1.7 (1.1, 2.8) | 102.0 (86.8, 117.9) | 111.8 (97.1, 121.8) | 108.0 (93.1, 121.4) | 109.4 (93.0, 122.9) | 112.4 (96.3, 125.9) |
| Other race/ethnicity | 262 (10.0) | 1.9 (1.0, 2.7) | 92.9 (77.6, 109.9) | 102.7 (86.2, 115.2) | 100.0 (84.7, 115.3) | 98.0 (83.1, 116.1) | 103.5 (85.5, 117.9) |
| <High school | 1482 (21.5) | 1.9 (1.3, 3.1) | 91.3 (74.6, 105.8) | 99.0 (80.2, 115.0) | 93.1 (75.8, 108.0) | 90.3 (71.3, 109.6) | 94.9 (78.1, 113.2) |
| High school graduation | 934 (27.5) | 1.9 (1.2, 2.8) | 85.4 (72.4, 100.3) | 95.7 (79.2, 109.4) | 93.1 (79.5, 106.3) | 91.4 (75.6, 104.5) | 92.2 (78.8, 108.6) |
| >High school | 1525 (51.0) | 1.5 (1.0, 2.2) | 83.0 (72.9, 95.3) | 93.2 (80.1, 105.2) | 98.6 (82.6, 111.5) | 96.2 (80.6, 110.7) | 93.8 (81.1, 106.7) |
| BMI <25 kg/m2 | 1209 (36.2) | 1.7 (1.1, 2.7) | 86.1 (73.8, 100.4) | 96.1 (81.8, 110.5) | 100.0 (85.8, 115.3) | 98.1 (82.9, 114.5) | 98.1 (84.2, 110.8) |
| BMI 25–29 kg/m2 | 1504 (34.1) | 1.8 (1.1, 2.6) | 83.2 (72.0, 97.9) | 92.7 (77.9, 106.7) | 94.4 (79.5, 108.0) | 93.3 (77.5, 107.0) | 92.0 (78.6, 107.3) |
| BMI ≥30 kg/m2 | 1228 (29.7) | 1.6 (1.0, 2.3) | 85.5 (73.1, 101.2) | 94.0 (80.0, 108.1) | 89.3 (74.9, 103.1) | 86.8 (71.0, 101.8) | 90.2 (77.8, 105.6) |
| Never smoker | 1921 (49.1) | 1.4 (0.9, 2.1) | 83.9 (72.1, 98.9) | 94.4 (78.7, 108.8) | 98.6 (81.5, 113.4) | 96.1 (78.2, 111.8) | 94.6 (80.2, 110.4) |
| Former smoker | 1330 (26.4) | 1.8 (1.3, 2.7) | 80.7 (70.5, 91.4) | 89.1 (76.5, 101.4) | 94.4 (79.5, 108.0) | 91.2 (75.2, 103.7) | 90.7 (77.1, 102.2) |
| Current smoker | 690 (24.5) | 2.1 (1.5, 3.2) | 91.1 (77.9, 104.5) | 102.1 (88.0, 115.8) | 91.8 (79.5, 103.1) | 91.7 (77.5, 105.7) | 97.6 (83.7, 110.7) |
| Cotinine <0.3 ng/mLa | 2717 (60.6) | 1.5 (1.0, 2.3) | 82.6 (70.9, 95.9) | 91.4 (77.3, 104.9) | 97.1 (81.5, 111.5) | 94.5 (76.7, 108.8) | 92.4 (78.6, 106.8) |
| Cotinine 0.3–2.9 ng/mLa | 333 (9.3) | 1.4 (1.0, 2.3) | 88.3 (72.6, 99.2) | 100.0 (81.7, 111.8) | 95.7 (80.5, 109.8) | 96.2 (80.1, 113.5) | 98.6 (81.0, 110.3) |
| Cotinine 3.0–99.0 ng/mLa | 275 (8.4) | 2.0 (1.2, 2.7) | 90.5 (76.4, 105.0) | 102.1 (84.9, 116.7) | 100.0 (79.5, 113.4) | 98.6 (79.9, 116.9) | 98.2 (83.5, 117.0) |
| Cotinine ≥100 ng/mLa | 616 (21.6) | 2.2 (1.6, 3.4) | 90.4 (76.7, 103.7) | 100.8 (85.0, 112.9) | 89.3 (79.5, 100.0) | 90.7 (77.4, 103.2) | 94.7 (82.5, 108.0) |
| Never alcohol drinker | 1383 (28.5) | 1.4 (1.0, 2.1) | 83.7 (70.0, 98.9) | 93.2 (76.0, 106.5) | 89.3 (73.2, 106.3) | 85.7 (68.5, 105.2) | 89.5 (73.9, 107.5) |
| Former alcohol drinker | 501 (7.9) | 1.9 (1.3, 2.7) | 84.1 (71.1, 99.5) | 88.6 (75.0, 102.2) | 89.3 (74.0, 104.7) | 86.3 (67.9, 103.7) | 88.7 (72.9, 104.9) |
| Current alcohol drinker | 2057 (63.5) | 1.8 (1.2, 2.6) | 85.6 (73.8, 100.0) | 95.8 (82.0, 110.3) | 97.1 (84.7, 111.5) | 96.9 (82.0, 110.7) | 95.6 (83.3, 109.2) |
| Diabetes | 547 (6.8) | 2.0 (1.2, 2.8) | 81.2 (63.8, 104.4) | 86.2 (67.0, 104.9) | 82.6 (65.6, 104.7) | 75.9 (62.7, 103.0) | 83.5 (65.2, 103.0) |
| No diabetes | 3394 (93.2) | 1.7 (1.1, 2.5) | 85.1 (73.4, 99.5) | 94.8 (80.4, 108.7) | 95.7 (81.5, 109.7) | 94.5 (78.4, 109.1) | 94.1 (81.0, 108.4) |
| Hypertension | 2210 (35.3) | 1.9 (1.3, 2.7) | 78.3 (65.5, 91.1) | 84.5 (69.0, 98.8) | 85.8 (71.6, 100.0) | 81.6 (65.7, 97.4) | 84.4 (69.8, 98.0) |
| No hypertension | 1731 (64.7) | 1.6 (1.0, 2.4) | 88.6 (76.4, 102.0) | 99.8 (85.6, 111.8) | 100.0 (85.8, 113.4) | 98.5 (84.5, 113.5) | 99.3 (86.1, 112.1) |
Conversion factors for units: to convert lead to micromoles per liter, multiply by 0.0483; to convert cotinine to nanomoles per liter, multiply by 5.68. Kidney outcomes in mL/min/1.73 m2.
In the overall sample, multivariable adjusted differences (95% CI) in mean eGFR for a doubling of blood lead were −1.9 (−3.2, −0.7), −1.7 (−3.0, −0.5) and −1.4 (−2.3, −0.5) mL/min/1.73 m2 for the cystatin C single variable, cystatin C multivariable and cystatin C/creatinine equations, respectively (Table 2). The corresponding differences (95% CI) were −0.9 (−1.9, 0.02) and −0.9 (−1.8, 0.01) using the creatinine-based MDRD and CKD-EPI equations, respectively. For comparison with studies that used serum creatinine and cystatin C as kidney outcomes without incorporating them into estimating equations, fully adjusted mean differences (95% CI) for a doubling of blood lead for serum creatinine and cystatin C were 0.05 (0.02, 0.07) mg/dL and 0.04 (0.02, 0.07) mg/L, respectively. The difference in mean level (95% CI) for a doubling of blood lead using the traditional Cockcroft–Gault equation for creatinine clearance [36] was −2.2 (−3.5, −0.8); however, median creatinine clearance was 107.7 mL/min indicating a substantial overestimation of GFR. After correction for body surface area, an approach reported to be more accurate [37] and providing a more comparable result, the difference in mean level (95% CI) was −1.4 (−2.4, −0.3) although the median (98.3 mL/min/1.73 m2) remained higher than those using the eGFR equations.
Table 2.
Differences (95% confidence interval) in mean eGFR (mL/min/1.73 m2) by blood lead levelsa
| Blood lead, μg/dLb | Mean eGFR in all participants | All participantsa | Age <60a | Age ≥60a |
| MDRD | ||||
| Tertile 1 (≤1.3)c | 91.4 | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
| Tertile 2 (>1.3–2.2)c | 84.5 | −1.7 (−3.7, 0.2) | −1.1 (−3.5, 1.3) | −3.8 (−5.8, −1.9) |
| Tertile 3 (>2.2)c | 83.2 | −2.4 (−4.5, −0.3) | −0.8 (−3.4, 1.7) | −7.1 (−9.5, −4.8) |
| P trend | 0.03 | 0.5 | <0.001 | |
| Doubling of lead level | −0.9 (−1.9, 0.02) | −0.2 (−1.2, 0.9) | −3.3 (−4.8, −1.9) | |
| CKD-EPI | ||||
| Tertile 1 (≤1.3)c | 99.8 | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
| Tertile 2 (>1.3–2.2)c | 91.2 | −1.3 (−2.9, 0.3) | −0.9 (−2.8, 1.0) | −3.1 (−5.0, −1.2) |
| Tertile 3 (>2.2)c | 88.4 | −1.8 (−3.7, 0.1) | −0.3 (−2.7, 2.0) | −6.1 (−8.3, −3.9) |
| P trend | 0.07 | 0.7 | <0.001 | |
| Doubling of lead level | −0.9 (−1.8, 0.01) | −0.2 (−1.2, 0.8) | −3.0 (−4.2, −1.8) | |
| Cystatin C single variable | ||||
| Tertile 1 (≤1.3)c | 100.6 | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
| Tertile 2 (>1.3–2.2)c | 93.7 | −1.6 (−4.2, 1.0) | −1.2 (−4.3, 2.0) | −4.5 (−6.7, −2.3) |
| Tertile 3 (>2.2)c | 88.2 | −3.3 (−5.3, −1.4) | −2.2 (−4.9, 0.4) | −7.8 (−10.3, −5.2) |
| P trend | 0.001 | 0.09 | <0.001 | |
| Doubling of lead level | −1.9 (−3.2, −0.7) | −1.3 (−2.8, 0.3) | −4.5 (−5.6, −3.3) | |
| Cystatin C multivariable | ||||
| Tertile 1 (≤1.3)c | 98.8 | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
| Tertile 2 (>1.3–2.2)c | 91.4 | −1.2 (−3.6, 1.2) | −1.0 (−3.9, 2.0) | −3.7 (−5.7, −1.8) |
| Tertile 3 (>2.2)c | 86.9 | −2.9 (−4.7, −1.1) | −1.9 (−4.5, 0.6) | −6.8 (−9.0, −4.6) |
| P trend | 0.003 | 0.1 | <0.001 | |
| Doubling of lead level | −1.7 (−3.0, −0.5) | −1.1 (−2.7, 0.4) | −4.0 (−5.0, −2.9) | |
| Cystatin C/creatinine | ||||
| Tertile 1 (≤1.3)c | 100.6 | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
| Tertile 2 (>1.3–2.2)c | 91.8 | −1.7 (−3.6, 0.3) | −1.2 (−3.6, 1.2) | −4.2 (−6.0, −2.4) |
| Tertile 3 (>2.2)c | 88.7 | −2.8 (−4.3, −1.2) | −1.3 (−3.3, 0.7) | −7.6 (−9.8, −5.4) |
| P trend | 0.001 | 0.2 | <0.001 | |
| Doubling of lead level | −1.4 (−2.3, −0.5) | −0.7 (−1.7, 0.4) | −3.9 (−5.2, −2.7) | |
Models adjusted for survey year, age (years modeled as restricted cubic spline with five knots), sex, race/ethnicity, body mass index (kg/m2), education (<high school, high school, >high school), smoking status (never, former, current), cotinine category, alcohol intake (never, former, current), hypertension (yes, no), diabetes mellitus (yes, no) and blood cadmium (ln μg/L).
Blood lead levels (ug/dL). Conversion factors for units: to convert lead to micromoles per liter, multiply by 0.0483.
Blood lead levels (μg/dL).
In participants aged ≥60 years, differences in mean eGFR for a doubling of blood lead ranged from −3.0 to −4.5 mL/min/1.73 m2 across equations (Table 2). In younger participants, the differences were smaller (range −0.2 to −2.2 mL/min/1.73 m2) and, although not statistically significant, were larger for the cystatin C single and multivariable estimates.
The adjusted odds ratios (95% CI) for reduced eGFR (<60 mL/min/1.73 m2) for increasing blood lead levels in participants ≥60 years of age were similar for all equations (Table 3 and Figure 1). In models adjusted for all covariates except cadmium, differences in mean kidney outcome and odds ratios for reduced kidney function were consistent with fully adjusted models but were generally stronger.
Table 3.
Odds ratios (95% confidence interval) for reduced eGFR (<60 mL/min/1.73 m2) by blood lead levels for participants ≥60 years of agea
| Cases/noncases (weighted %) | Odds ratios | |
| MDRD | ||
| ≤1.3b | 78/358 (20.6) | 1.00 (reference) |
| >1.3–2.2b | 179/655 (23.8) | 1.29 (0.87, 1.93) |
| >2.2b | 391/948 (31.2) | 1.90 (1.26, 2.87) |
| P trend | 0.002 | |
| Doubling of lead level | 1.38 (1.17, 1.63) | |
| CKD-EPI | ||
| ≤1.3b | 76/360 (19.6) | 1.00 (reference) |
| >1.3–2.2b | 164/670 (21.1) | 1.14 (0.76, 1.71) |
| >2.2b | 382/957 (29.2) | 1.78 (1.18, 2.69) |
| P trend | 0.003 | |
| Doubling of lead level | 1.37 (1.15, 1.62) | |
| Cystatin C single variable | ||
| ≤1.3b | 68/368 (15.8) | 1.00 (reference) |
| >1.3–2.2b | 146/688 (19.2) | 1.25 (0.86, 1.82) |
| >2.2b | 332/1007 (24.4) | 1.57 (1.01, 2.46) |
| P trend | 0.040 | |
| Doubling of lead level | 1.41 (1.17, 1.70) | |
| Cystatin C multivariable | ||
| ≤1.3b | 97/339 (22.4) | 1.00 (reference) |
| >1.3–2.2b | 214/620 (27.3) | 1.48 (1.04, 2.12) |
| >2.2b | 423/916 (33.0) | 2.02 (1.28, 3.17) |
| P trend | 0.004 | |
| Doubling of lead level | 1.53 (1.31, 1.80) | |
| Cystatin C/creatinine | ||
| ≤1.3b | 70/366 (17.4) | 1.00 (reference) |
| >1.3–2.2b | 163/671 (20.6) | 1.33 (0.95, 1.86) |
| >2.2b | 354/985 (27.2) | 2.00 (1.29, 3.08) |
| P trend | 0.003 | |
| Doubling of lead level | 1.46 (1.21, 1.75) | |
Models adjusted for survey year, age (years modeled as restricted cubic spline with five knots), sex, race/ethnicity, body mass index (kg/m2), education (<high school, high school, >high school), smoking status (never, former, current), cotinine category, alcohol intake (never, former, current), hypertension (yes, no), diabetes mellitus (yes, no) and blood cadmium (ln μg/L).
Blood lead levels (μg/dL). Conversion factors for units: to convert lead to micromoles per liter, multiply by 0.0483.
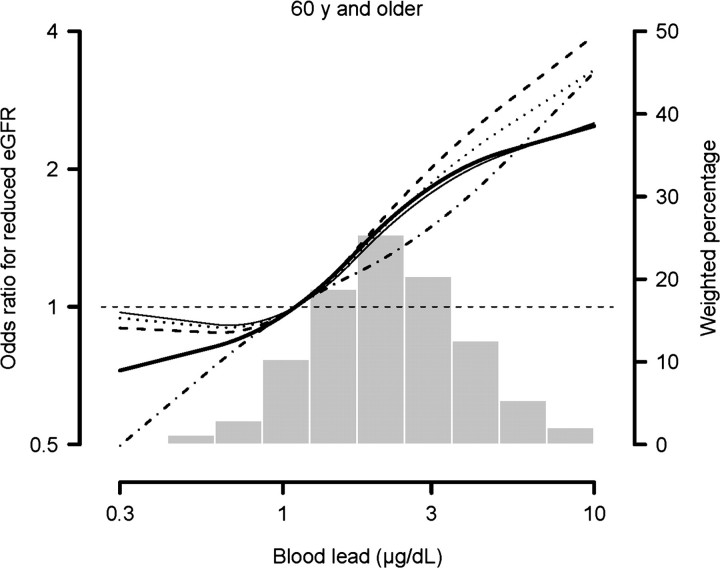
Fig. 1.
Odds ratios for reduced eGFR with blood lead levels modeled with restricted quadratic splines with knots at the 5th, 50th and 95th percentiles. MDRD: thick solid line; CKD-EPI: thin solid line; cystatin C single variable: dashed and dotted line; cystatin C multivariable: dashed line; cystatin C/creatinine combined: dotted line.
Discussion
In this large representative sample of US adults, higher blood lead levels were associated with lower eGFR levels and reduced eGFR (<60 mL/min/1.73 m2) with all equations examined. Mean differences in eGFR by blood lead levels were larger with cystatin C compared to creatinine equations in analyses in all participants reflecting results in those <60 years of age. For all equations, differences in eGFR levels with increasing blood lead levels were larger for participants ≥60 years of age. In this age group, mean eGFR differences for a doubling in blood lead levels ranged from −3.0 mL/min/1.73 m2 with the CKD-EPI equation to −4.5 mL/min/1.73 m2 with the cystatin C single variable equation. Odds of reduced eGFR for a doubling of blood lead level, examined in participants ≥60 years, were consistently increased with all equations.
Lead is a widespread environmental toxicant [15, 38]. In the human body, lead accumulates in bone and the biological half-life is on the order of decades [16]. Thus, although exposure to lead has decreased in developed countries after the institution of public health measures banning lead in gasoline, paint and solder, the body burden of lead resulting from past exposures remains an important source of endogenous exposure [16, 18]. Moreover, exogenous exposure continues to occur through folk remedies, glazed pottery, industrial sources, lead paint, active smoking and exposure to secondhand smoke [6, 39, 40]. Certain populations are disproportionately exposed to lead, especially workers in occupations such as construction and residents in low socioeconomic status communities [6]. Globally, exposure remains higher in developing countries [41–43]. Given the magnitude of exposure, the impact of lead dose on kidney function is a substantial public health concern.
Our results are consistent with publications in other NHANES analyses using the MDRD equation to estimate GFR [7, 8]. The CKD-EPI equation was recently published [22], and to our knowledge, there are no publications examining associations between blood lead and GFR estimated with this equation. A few studies have examined associations between blood lead and kidney function using serum cystatin C or single variable cystatin C-based eGFR equations [1, 4, 44, 45]. In a cross-sectional study of Swedish women, higher blood lead levels were associated with lower serum cystatin C-based eGFR [4, 46]. Associations were comparable to estimates using creatinine clearance as the kidney outcome [4]. An association between blood lead level and serum cystatin C was observed in Belgian adolescents [1]. In US adolescents, blood lead levels were associated with decreased cystatin C-based eGFR [47] levels; the association with creatinine-based eGFR was not statistically significant [44]. In a cross-sectional study of European children, on the other hand, higher blood lead levels were associated with lower serum cystatin C and creatinine levels and these paradoxical associations were attributed to hyperfiltration [45].
Strengths of our study include those related to NHANES data: a relatively large sample size; representation of the US noninstitutionalized civilian population; high-quality, standardized laboratory procedures and extensive quality control. This is also one of the few data sets to date that includes serum creatinine and cystatin C and blood lead. Limitations include lack of GFR measurement using an exogenous filtration marker. The GFR-estimating equations used in this study have important limitations and differences. The MDRD equation systematically underestimates GFR at higher levels; the CKD-EPI equation was developed to be more accurate in this range [22]. Both equations use serum creatinine, which is generated from muscle metabolism and overestimates GFR in individuals with low muscle mass such as the elderly [19]. Cystatin C is a 120-amino acid cysteine protease inhibitor that is freely filtered at the glomerulus and reabsorbed and catabolized in the proximal tubules [19]. It is produced and secreted by all nucleated cells [19]; the resulting lack of muscle mass confounding may increase its accuracy as a kidney filtration marker [48, 49]. Research to assess accuracy of cystatin C-based eGFR is ongoing [50, 51]. Associations between cystatin C and age, sex, race, nutritional factors, body composition and inflammatory markers, that persisted after adjustment for GFR, have recently been reported [35, 52]. Thus, the use of multivariable equations that incorporate age, sex and race as well as equations that use both creatinine and cystatin C may provide more accurate estimations of GFR [23, 51].
Second, reverse causation, specifically increased blood lead levels as a result of reduced kidney excretion, cannot be excluded due to the cross-sectional study design. However, the temporal relation between lead exposure and CKD onset and/or progression is a critical factor in determining causality. Longitudinal data in both CKD patient and general populations have reported lead dose to be a predictor of kidney function decline for follow-up periods as long as 4 to >6 years, respectively [2, 3, 5, 9–11]. Further, reverse causality should be most prominent in populations with CKD. However, analyses to address this in the Normative Aging Study population found that blood lead was positively associated with serum creatinine even over the normal range where a substantial decrease in lead excretion is unlikely [3, 5]. In addition, the impact of lead chelation on kidney function in CKD patients provides evidence against reverse causality [10]. Third, cumulative lead dose could not be analyzed. Blood lead reflects current exogenous exposure as well as endogenous exposure from accumulated body burden. Bone lead is a better marker of cumulative lead exposure [16] but has never been measured in NHANES. Fourth, our study may be subject to survival bias, due to increased mortality of CKD patients, and to other selection biases which could underestimate the effect of lead on kidney function, such as exclusion of institutionalized participants and need for mobility to attend the examination portion of the NHANES evaluation. The specific criteria used to derive the cystatin C subsample could also result in selection bias. Finally, residual confounding by recently reported factors, including nutritional factors and inflammatory markers, whose associations with cystatin C persist after adjustment for GFR, may also affect our study [35, 52].
In conclusion, in this large representative sample of US adults, higher blood lead levels were associated with lower eGFR and increased odds of reduced eGFR, irrespective of the endogenous marker of GFR and estimating equation used. In all participants, larger differences in mean eGFR for a doubling of blood lead were observed with the cystatin C equations. In participants aged ≥60 years, the association between lead and reduced eGFR was observed throughout the range of blood lead levels with no apparent threshold. Given the global burden of CKD, it is essential to conduct research on potential risk factors that are common and preventable, including lead exposure. These results support the inclusion of cystatin C-based eGFR in future lead research and provide additional evidence for environmental lead exposure as a CKD risk factor.
Acknowledgments
This work was supported by the National Institutes of Health National Institute of Environmental Health Sciences (grants 2 and 3 ES007198) and the National Institute for Occupational Safety and Health (grant T42 OH008428 from the Education and Research Center for Occupational Safety and Health at the Johns Hopkins Bloomberg School of Public Health). Support for J.T.S. was provided by the Occupational Physicians Scholarship Fund and is currently provided by the National Institute of Environmental Health Sciences (grant 5T32ES015459-02). The results presented in this paper have not been published previously in whole or part, except in abstract form. Aspects of this work were accepted as an abstract at the American Society of Nephrology Annual Conference, in San Diego, CA, in October/November 2009. The abstract can be accessed online at http://www.asn-online.org/education_and_meetings/renal_week/archives/RW09Abstracts.pdf. SA-PO2758, 742A.
Conflict of interest statement. None declared.
Appendix
Appendix 1.
Correlation coefficients for eGFR by equation in all participants (n = 3941)a
| MDRD | CKD-EPI | Cystatin C single variable | Cystatin C multivariable | Cystatin C/creatinine | |
| CKD-EPI | 0.95 | ||||
| Cystatin C single variable | 0.71 | 0.76 | |||
| Cystatin C multivariable | 0.72 | 0.79 | 0.97 | ||
| Cystatin C/creatinine | 0.93 | 0.94 | 0.90 | 0.92 |
P-value <0.001 for all correlations.
Appendix 2.
Correlation coefficients for eGFRs by equation in participants aged ≥60 years (n = 2609)a
| MDRD | CKD-EPI | Cystatin C single variable | Cystatin C multivariable | Cystatin C/creatinine | |
| CKD-EPI | 0.96 | ||||
| Cystatin C single variable | 0.72 | 0.76 | |||
| Cystatin C multivariable | 0.73 | 0.76 | 0.98 | ||
| Cystatin C/creatinine | 0.94 | 0.94 | 0.91 | 0.92 |
P-value <0.001 for all correlations.
Appendix 3.
Correlation coefficients for eGFRs by equation in participants aged <60 years (n = 1332)a
| MDRD | CKD-EPI | Cystatin C single variable | Cystatin C multivariable | Cystatin C/creatinine | |
| CKD-EPI | 0.94 | ||||
| Cystatin C single variable | 0.58 | 0.62 | |||
| Cystatin C multivariable | 0.60 | 0.64 | 0.96 | ||
| Cystatin C/creatinine | 0.92 | 0.91 | 0.84 | 0.86 |
P-value <0.001 for all correlations.
References
- 1.Staessen JA, Nawrot T, Hond ED, et al. Renal function, cytogenetic measurements, and sexual development in adolescents in relation to environmental pollutants: a feasibility study of biomarkers. Lancet. 2001;357:1660–1669. doi: 10.1016/s0140-6736(00)04822-4. [DOI] [PubMed] [Google Scholar]
- 2.Yu CC, Lin JL, Lin-Tan DT. Environmental exposure to lead and progression of chronic renal diseases: a four-year prospective longitudinal study. J Am Soc Nephrol. 2004;15:1016–1022. doi: 10.1097/01.asn.0000118529.01681.4f. [DOI] [PubMed] [Google Scholar]
- 3.Kim R, Rotnitsky A, Sparrow D, et al. A longitudinal study of low-level lead exposure and impairment of renal function. The Normative Aging Study. JAMA. 1996;275:1177–1181. [PubMed] [Google Scholar]
- 4.Akesson A, Lundh T, Vahter M, et al. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ Health Perspect. 2005;113:1627–1631. doi: 10.1289/ehp.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsaih SW, Korrick S, Schwartz J, et al. Lead, diabetes, hypertension, and renal function: The Normative Aging Study. Environ Health Perspect. 2004;112:1178–1182. doi: 10.1289/ehp.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekong EB, Jaar BG, Weaver VM. Lead-related nephrotoxicity: a review of the epidemiologic evidence. Kidney Int. 2006;70:2074–2084. doi: 10.1038/sj.ki.5001809. [DOI] [PubMed] [Google Scholar]
- 7.Muntner P, Menke A, DeSalvo KB, et al. Continued decline in blood lead levels among adults in the United States: The National Health and Nutrition Examination Surveys. Arch Intern Med. 2005;165:2155–2161. doi: 10.1001/archinte.165.18.2155. [DOI] [PubMed] [Google Scholar]
- 8.Navas-Acien A, Tellez-Plaza M, Guallar E, et al. Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol. 2009;170:1156–1164. doi: 10.1093/aje/kwp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JL, Lin-Tan DT, Yu CC, et al. Environmental exposure to lead and progressive diabetic nephropathy in patients with type II diabetes. Kidney Int. 2006;69:2049–2056. doi: 10.1038/sj.ki.5001505. [DOI] [PubMed] [Google Scholar]
- 10.Lin JL, Lin-Tan DT, Hsu KH, Yu CC. Environmental lead exposure and progression of chronic renal diseases in patients without diabetes. N Engl J Med. 2003;348:277–286. doi: 10.1056/NEJMoa021672. [DOI] [PubMed] [Google Scholar]
- 11.Lin JL, Lin-Tan DT, Li YJ, et al. Low-level environmental exposure to lead and progressive chronic kidney diseases. Am J Med. 2006;119:707.e1–707.e9. doi: 10.1016/j.amjmed.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Khalil-Manesh F, Gonick HC, Cohen AH. Experimental model of lead nephropathy I. Continuous high-dose lead administration. Kidney Int. 1992;41:1192–1203. doi: 10.1038/ki.1992.181. [DOI] [PubMed] [Google Scholar]
- 13.Khalil-Manesh F, Gonick HC, Cohen AH. Experimental model of lead nephropathy III. Continuous low-level lead administration. Arch Environ Health. 1993;48:271–278. doi: 10.1080/00039896.1993.9940372. [DOI] [PubMed] [Google Scholar]
- 14.Vaziri ND. Mechanisms of lead-induced hypertension and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2008;295:H454–H465. doi: 10.1152/ajpheart.00158.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Department of Health and Human Services. Fourth National Report on Human Exposure to Environmental Chemicals. Centers for Disease Control and Prevention; http://www.cdc.gov/exposurereport/pdf/FourthReport.pdf (13 June 2010, date last accessed) [Google Scholar]
- 16.Hu H. Bone lead as a new biologic marker of lead dose: recent findings and implications for public health. Environ Health Perspect. 1998;106(Suppl 4):961–967. doi: 10.1289/ehp.98106s4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the united states. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 18.Weaver VM, Jaar BG. In: Lead Nephropathy and Lead-Related Nephrotoxicity. Basow DS, editor. UpToDate. http://www.utdol.com/online/content/topic.do?topicKey=renldis/14437&selectedTitle=1∼7&source=search_result (5 May 2010, date last accessed) [Google Scholar]
- 19.Fried LF. Creatinine and cystatin C: what are the values? Kidney Int. 2009;75:578–580. doi: 10.1038/ki.2008.688. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Greene T, et al. Chronic Kidney Disease Epidemiology Collaboration: expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Center for Health Statistics. National Health and Nutrition Examination Survey. Questionnaires, Datasets, and Related Documentation. Centers for Disease Control and Prevention.; http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm (13 June 2010, date last accessed) [Google Scholar]
- 25.National Center for Health Statistics. National Health and Nutrition Examination Survey. Documentation, Codebook, and Frequencies; Surplus Specimen Laboratory Component: Cystatin C (Surplus Sera) Centers for Disease Control and Prevention.; http://www.cdc.gov/nchs/data/nhanes/nhanes_99_00/sscyst_a.pdf (13 June 2010, date last accessed) [Google Scholar]
- 26.National Center for Health Statistics. National Health and Nutrition Examination Survey. Documentation, Codebook, and Frequencies; Surplus Specimen Laboratory Component: Cystatin C (Surplus Sera) Centers for Disease Control and Prevention.; http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/sscyst_b.pdf (13 June 2010, date last accessed) [Google Scholar]
- 27.Department of Health and Human Services. Fourth National Report on Human Exposure to Environmental Chemicals. Centers for Disease Control and Prevention.; http://www.cdc.gov/exposurereport/pdf/FourthReport.pdf (13 June 2010, date last accessed), p. 514. [Google Scholar]
- 28.Department of Health and Human Services. Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Centers for Disease Control and Prevention.; http://www.cdc.gov/exposurereport/pdf/FourthReport.pdf (13 June 2010, date last accessed), p. 6. [Google Scholar]
- 29.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- 30.National Center for Environmental Health. Laboratory Procedure Manual. Cadmium and Lead. Centers for Disease Control and Prevention.; http://www.cdc.gov/nchs/data/nhanes/nhanes_99_00/lab06_met_lead_and_cadmium.pdf (13 June 2010, date last accessed) [Google Scholar]
- 31.National Center for Environmental Health. Laboratory Procedure Manual. Cadmium and Lead. Centers for Disease Control and Prevention.; http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/l06_b_met_lead_and_cadmium.pdf (13 June 2010, date last accessed) [Google Scholar]
- 32.Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50:918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 33.National Center for Health Statistics. National Health and Nutrition Examination Survey. Laboratory Procedure Manual; Biochemistry Profile in Refrigerated Serum, 1999–2000. Centers for Disease Control and Prevention.; http://www.cdc.gov/nchs/data/nhanes/nhanes_99_00/lab18_met_biochemistry_profile.pdf (13 June 2010, date last accessed) [Google Scholar]
- 34.National Center for Health Statistics. National Health and Nutrition Examination Survey. Laboratory Procedure Manual; Biochemistry Profile in Refrigerated Serum, 2001–2002. Centers for Disease Control and Prevention.; http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/l18_b_met_biochemistry_profile.pdf (13 June 2010, date last accessed) [Google Scholar]
- 35.Kottgen A, Selvin E, Stevens LA, et al. Serum cystatin C in the United States: the third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2008;51:385–394. doi: 10.1053/j.ajkd.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 37.Shoker A, Hossain MA, Koru-Sengul T, et al. Performance of creatinine clearance equations on the original Cockcroft-Gault population. Clin Nephrol. 2006;66:89–97. doi: 10.5414/cnp66089. [DOI] [PubMed] [Google Scholar]
- 38.Skerfving S, Bergdahl IA. Lead. In: Nordberg GF, editor. Handbook on Toxicology of Metals. Amsterdam, Netherlands: Elsevier; 2007. pp. 599–643. [Google Scholar]
- 39.Mannino DM, Albalak R, Grosse S, Repace J. Second-hand smoke exposure and blood lead levels in U.S. children. Epidemiology. 2003;14:719–727. doi: 10.1097/01.EDE.0000081998.02432.53. [DOI] [PubMed] [Google Scholar]
- 40.Mannino DM, Homa DM, Matte T, Hernandez-Avila M. Active and passive smoking and blood lead levels in U.S. adults: data from the Third National Health and Nutrition Examination Survey. Nicotine Tob Res. 2005;7:557–564. doi: 10.1080/14622200500185264. [DOI] [PubMed] [Google Scholar]
- 41.Nichani V, Li WI, Smith MA, et al. Blood lead levels in children after phase-out of leaded gasoline in Bombay, India. Sci Total Environ. 2006;363:95–106. doi: 10.1016/j.scitotenv.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 42.Clark CS, Rampal KG, Thuppil V, et al. The lead content of currently available new residential paint in several Asian countries. Environ Res. 2006;102:9–12. doi: 10.1016/j.envres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Zheng L, Wu K, Li Y, et al. Blood lead and cadmium levels and relevant factors among children from an e-waste recycling town in China. Environ Res. 2008;108:15–20. doi: 10.1016/j.envres.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Fadrowski JJ, Navas-Acien A, Tellez-Plaza M, et al. Blood lead level and kidney function in US adolescents: the third National Health and Nutrition Examination Survey. Arch Intern Med. 2010;170:75–82. doi: 10.1001/archinternmed.2009.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Burbure C, Buchet JP, Leroyer A, et al. Renal and neurologic effects of cadmium, lead, mercury, and arsenic in children: evidence of early effects and multiple interactions at environmental exposure levels. Environ Health Perspect. 2006;114:584–590. doi: 10.1289/ehp.8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64:25–30. doi: 10.1080/00365510410003723. [DOI] [PubMed] [Google Scholar]
- 47.Filler G, Lepage N. Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula? Pediatr Nephrol. 2003;18:981–985. doi: 10.1007/s00467-003-1271-5. [DOI] [PubMed] [Google Scholar]
- 48.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 49.Roos JF, Doust J, Tett SE, Kirkpatrick CM. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children—a meta-analysis. Clin Biochem. 2007;40:383–391. doi: 10.1016/j.clinbiochem.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 50.Madero M, Sarnak MJ, Stevens LA. Serum cystatin C as a marker of glomerular filtration rate. Curr Opin Nephrol Hypertens. 2006;15:610–616. doi: 10.1097/01.mnh.0000247505.71915.05. [DOI] [PubMed] [Google Scholar]
- 51.Tidman M, Sjostrom P, Jones I. A comparison of GFR estimating formulae based upon s-cystatin C and s-creatinine and a combination of the two. Nephrol Dial Transplant. 2008;23:154–160. doi: 10.1093/ndt/gfm661. [DOI] [PubMed] [Google Scholar]
- 52.Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]