Abstract
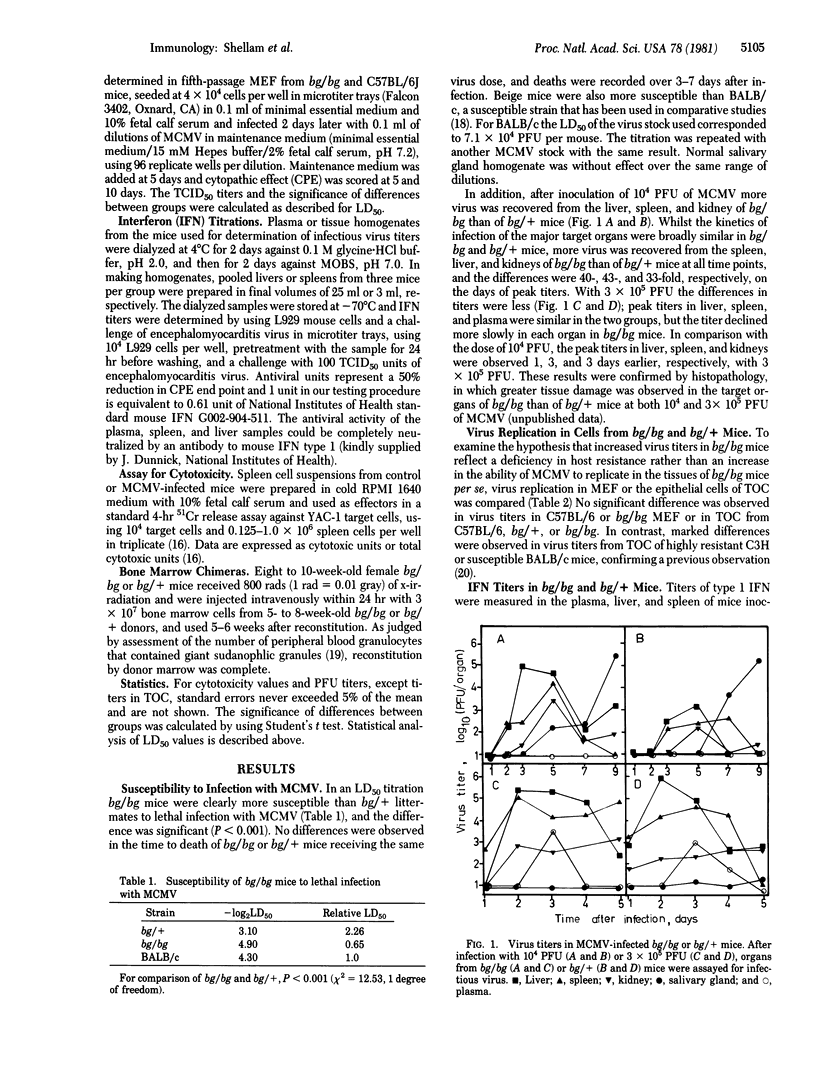
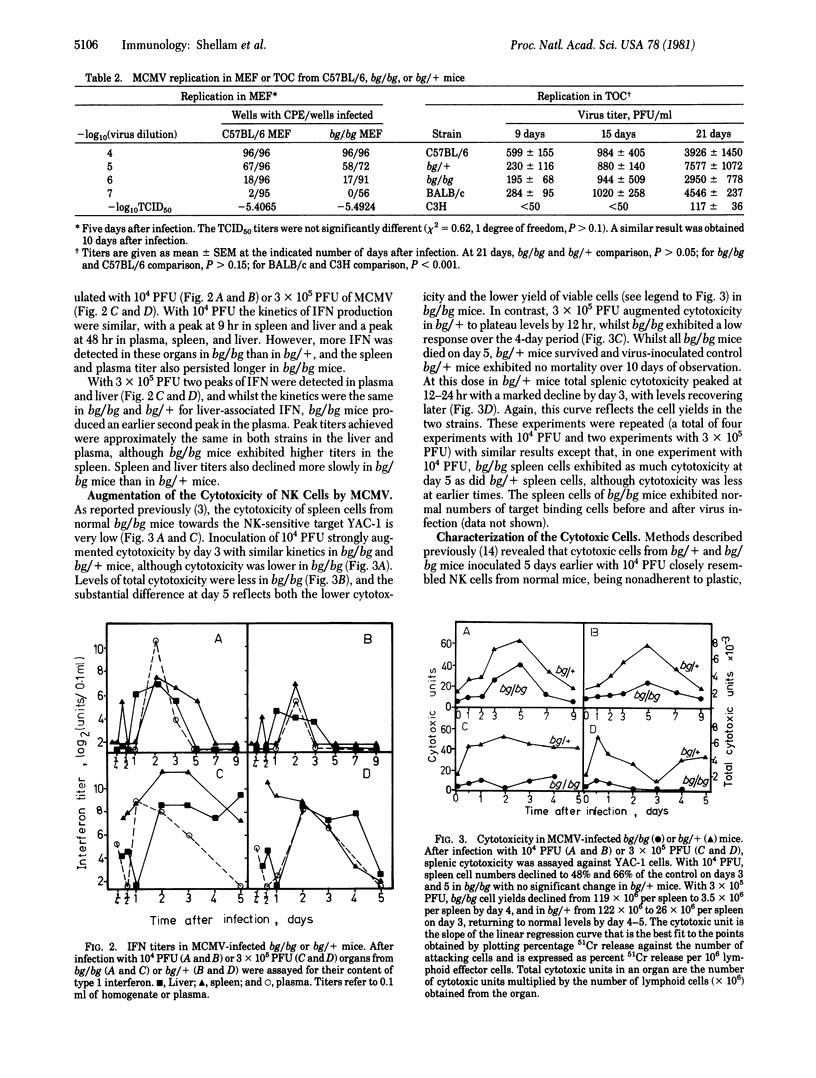
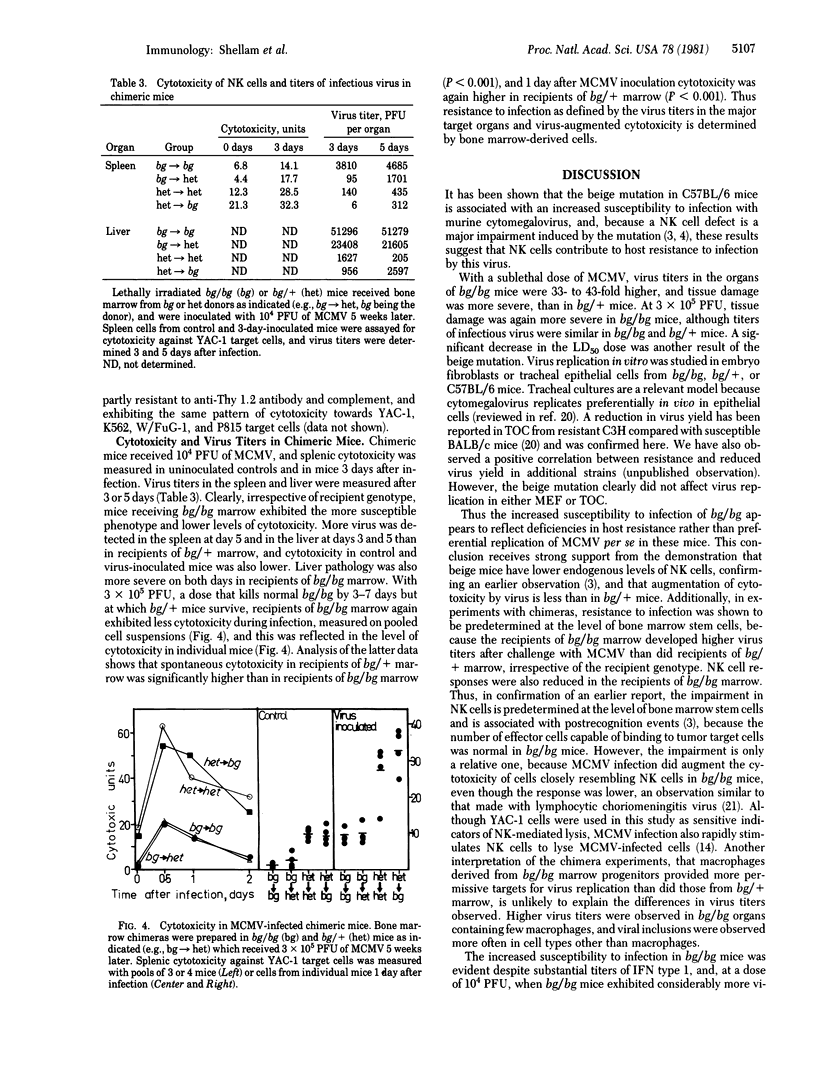
Mice homozygous for the beige gene (bg/bg) are a homologue of the Chédiak-Higashi syndrome of man and are known to be selectively defective in natural killer (NK) cells. We have compared the susceptibility of bg/bg and bg/+ C57BL/6J mice to infection with murine cytomegalovirus (MCMV). Beige mice are more susceptible to lethal infection and develop 33- to 43-fold higher virus titers in the liver, spleen, and kidney than do bg/+ mice after a sublethal infection, although virus replication is the same in vitro in cultured fibroblasts or epithelial cells from these mice. Inoculation with a sublethal dose of virus stimulates a NK cell response, although this is lower in bg/bg mice despite higher titers of interferon type 1 than in bg/+. A dose of MCMV that is lethal only to bg/bg augments cytotoxicity within 12 hr in bg/+ mice, whereas cytotoxicity in bg/bg remains very low. In bone marrow chimeras, recipients of bg/bg marrow were more susceptible to MCMV and had lower NK cell responses after virus inoculation than did recipients of marrow from bg/+ donors. The greater susceptibility of beige mice to the virus suggests that NK cells may contribute to resistance early in McMV infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Shellam G. R., Chalmer J. E. Genetic influences on the augmentation of natural killer (NK) cells during murine cytomegalovirus infection: correlation with patterns of resistance. J Immunol. 1981 Mar;126(3):988–994. [PubMed] [Google Scholar]
- Chalmer J. E., Mackenzie J. S., Stanley N. F. Resistance to murine cytomegalovirus linked to the major histocompatibility complex of the mouse. J Gen Virol. 1977 Oct;37(1):107–114. doi: 10.1099/0022-1317-37-1-107. [DOI] [PubMed] [Google Scholar]
- Dawkins H. J., Shellam G. R. Augmentation of cell-mediated cytotoxicity to a rat lymphoma. I. Stimulation of non-T-cell cytotoxicity in vivo by tumour cells. Int J Cancer. 1979 Aug;24(2):235–243. doi: 10.1002/ijc.2910240216. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Bujak J. S., Patten E., Wolff S. M. Granulocyte function in the Chediak-Higashi syndrome of mice. Blood. 1974 Feb;43(2):201–206. [PubMed] [Google Scholar]
- Grundy J. E., Mackenzie J. S., Stanley N. F. Influence of H-2 and non-H-2 genes on resistance to murine cytomegalovirus infection. Infect Immun. 1981 Apr;32(1):277–286. doi: 10.1128/iai.32.1.277-286.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haliotis T., Roder J., Klein M., Ortaldo J., Fauci A. S., Herberman R. B. Chédiak-Higashi gene in humans I. Impairment of natural-killer function. J Exp Med. 1980 May 1;151(5):1039–1048. doi: 10.1084/jem.151.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Ho M. Role of specific cytotoxic lymphocytes in cellular immunity against murine cytomegalovirus. Infect Immun. 1980 Mar;27(3):767–776. doi: 10.1128/iai.27.3.767-776.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kees U., Blanden R. V. A single genetic element in H-2K affects mouse T-cell antiviral function in poxvirus infection. J Exp Med. 1976 Feb 1;143(2):450–455. doi: 10.1084/jem.143.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Roder J., Haliotis T., Korec S., Jett J. R., Herberman R. B., Katz P., Fauci A. S. Chédiak-Higashi gene in humans. II. The selectivity of the defect in natural-killer and antibody-dependent cell-mediated cytotoxicity function. J Exp Med. 1980 May 1;151(5):1049–1058. doi: 10.1084/jem.151.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärre K., Klein G. O., Kiessling R., Klein G., Roder J. C. Low natural in vivo resistance to syngeneic leukaemias in natural killer-deficient mice. Nature. 1980 Apr 17;284(5757):624–626. doi: 10.1038/284624a0. [DOI] [PubMed] [Google Scholar]
- Murphy E. D., Harrison D. E., Roths J. B. Giant granules of beige mice. A quantitative marker for granulocytes in bone marrow transplantation. Transplantation. 1973 May;15(5):526–530. [PubMed] [Google Scholar]
- Mäntyjärvi R. A., Selgrade M. J., Collier A. M., Hu S., Pagano J. S. Murine cytomegalovirus infection of epithelial cells in mouse tracheal ring organ culture. J Infect Dis. 1977 Sep;136(3):444–448. doi: 10.1093/infdis/136.3.444. [DOI] [PubMed] [Google Scholar]
- Nedrud J. G., Collier A. M., Pagano J. S. Cellular basis for susceptibility to mouse cytomegalovirus: evidence from tracheal organ culture. J Gen Virol. 1979 Dec;45(3):737–744. doi: 10.1099/0022-1317-45-3-737. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Manischewitz J. E., Ennis P. A. Role of cytotoxic T lymphocytes in murine cytomegalovirus infection. J Gen Virol. 1980 Apr;47(2):503–508. doi: 10.1099/0022-1317-47-2-503. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Manischewitz J. E. The role of natural killer cells and antibody-dependent cell-mediated cytotoxicity during murine cytomegalovirus infection. J Exp Med. 1979 Dec 1;150(6):1549–1554. doi: 10.1084/jem.150.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder J. C., Lohmann-Matthes M. L., Domzig W., Wigzell H. The beige mutation in the mouse. II. Selectivity of the natural killer (NK) cell defect. J Immunol. 1979 Nov;123(5):2174–2181. [PubMed] [Google Scholar]
- Roder J. C. The beige mutation in the mouse. I. A stem cell predetermined impairment in natural killer cell function. J Immunol. 1979 Nov;123(5):2168–2173. [PubMed] [Google Scholar]
- Santoli D., Koprowski H. Mechanisms of activation of human natural killer cells against tumor and virus-infected cells. Immunol Rev. 1979;44:125–163. doi: 10.1111/j.1600-065x.1979.tb00269.x. [DOI] [PubMed] [Google Scholar]
- Selgrade M. K., Ahmed A., Sell K. W., Gershwin M. E., Steinberg A. D. Effect of murine cytomegalovirus on the in vitro responses of T and B cells to mitogens. J Immunol. 1976 May;116(5):1459–1465. [PubMed] [Google Scholar]
- Starr S. E., Allison A. C. Role of T lymphocytes in recovery from murine cytomegalovirus infection. Infect Immun. 1977 Aug;17(2):458–462. doi: 10.1128/iai.17.2.458-462.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge J. E., Meyers K. M., Prieur D. J., Starkey J. R. Role of NK cells in tumour growth and metastasis in beige mice. Nature. 1980 Apr 17;284(5757):622–624. doi: 10.1038/284622a0. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Kiessling R. W. Natural killer cell response to lymphocytic choriomeningitis virus in beige mice. Scand J Immunol. 1980;11(4):363–367. doi: 10.1111/j.1365-3083.1980.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Yap K. L., Ada G. L., McKenzie I. F. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978 May 18;273(5659):238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]



