Abstract
Several studies recognized an overlap between CFS (chronic fatigue syndrome) and POTS (postural tachycardia syndrome). We compared the autonomic and neurohormonal phenotype of POTS patients with CFS (CFS–POTS) to those without CFS (non-CFS–POTS), to determine whether CFS–POTS represents a unique clinical entity with a distinct pathophysiology. We recruited 58 patients with POTS, of which 47 were eligible to participate. A total of 93% of them reported severe fatigue [CIS (Checklist of Individual Strength), fatigue subscale >36], and 64% (n=30) fulfilled criteria for CFS (CFS–POTS). The prevalence of CFS symptoms (Centers for Disease Control and Prevention criteria) was greater in the CFS–POTS group, but the pattern of symptoms was similar in both groups. Physical functioning was low in both groups (RAND-36 Health Survey, 40±4 compared with 33±3; P=0.153), despite more severe fatigue in CFS–POTS patients (CIS fatigue subscale 51±1 compared with 43±3; P=0.016). CFS–POTS patients had greater orthostatic tachycardia than the non-CFS–POTS group (51±3 compared with 40±4 beats/min; P=0.030), greater low-frequency variability of BP (blood pressure; 6.3±0.7 compared with 4.8±1.0 mmHg2; P=0.019), greater BP recovery from early to late phase II of the Valsalva manoeuvre (18±3 compared with 11±2 mmHg; P=0.041) and a higher supine (1.5±0.2 compared with 1.0±0.3 ng/ml per·h; P=0.033) and upright (5.4±0.6 compared with 3.5±0.8 ng/ml per h; P=0.032) PRA (plasma renin activity). In conclusion, fatigue and CFS-defining symptoms are common in POTS patients. The majority of them met criteria for CFS. CFS–POTS patients have higher markers of sympathetic activation, but are part of the spectrum of POTS. Targeting this sympathetic activation should be considered in the treatment of these patients.
Keywords: autonomic nervous system, blood volume, chronic fatigue syndrome, orthostatic intolerance, postural tachycardia syndrome (POTS), renin
Abbreviations: AFT, autonomic function test; AngI, angiotensin I; BMI, body mass index; BP, blood pressure; BV, blood volume; CDC, Centers for Disease Control and Prevention; CFS, chronic fatigue syndrome; CIS, Checklist of Individual Strength; HR, heart rate; POTS, postural tachycardia syndrome; PRA, plasma renin activity; PV, plasma volume; QSART, quantitative sudomotor axon reflex testing; RAAS, renin–angiotensin–aldosterone system
INTRODUCTION
CFS (chronic fatigue syndrome) is a disabling disorder characterized by persistent or relapsing unexplained fatigue, accompanied by characteristic physical, constitutional and neuropsychological symptoms lasting at least 6 months [1]. The prevalence of CFS varies between 0.007 and 2.5% in the general population, and it is about twice as common in women [2]. The aetiology and pathophysiology of this syndrome remain unknown, but the autonomic nervous system has been proposed to play a role [3,4]. Clinical features of autonomic dysfunction such as orthostatic intolerance, increased sweating, pallor, sluggish pupillary responses, gastrointestinal symptoms and frequency of micturition are often observed in patients with CFS [5,6]. Particularly, neurally mediated hypotension and POTS (postural tachycardia syndrome), two forms of orthostatic intolerance, have been shown to be prevalent in adults and children with CFS [7–11], and it has been proposed that the haemodynamic mechanisms underlying these autonomic conditions may play a role in the pathophysiology of CFS [4,6] and contribute to its symptoms [9,11].
POTS is one of the most frequent forms of chronic orthostatic intolerance in the general population [12], with a 5:1 female/male ratio [13]. POTS is a disabling condition characterized by excessive tachycardia and symptoms upon standing that significantly improve by recumbency [14]. Nevertheless, non-orthostatic symptoms, such as fatigue or CFS-related symptoms, are often major complaints, and in some patients can be chronic and overwhelming [15]. A substantial overlap between POTS and CFS has been consistently reported in the literature [10,11,15,16]. The prevalence of POTS in CFS patients has ranged from 19% [7] to 70% [10], whereas studies in cohorts of patients selected for POTS have shown a prevalence of chronic fatigue between 48 and 77% [15,17], and CFS between 17 and 23% [17,18]. Furthermore, increased sympathetic activation and low BVs (blood volumes) have been proposed as pathophysiological mechanisms in both conditions [4,11,19–21]. The aim of the present study was to compare the clinical, autonomic and neurohumoral features of POTS patients with and without CFS, to determine whether POTS patients with CFS represent a unique clinical entity with a distinct pathophysiology, or are a subset of patients within the spectrum of POTS.
MATERIALS AND METHODS
Subjects
We studied 58 consecutive female patients with POTS referred to Vanderbilt University Autonomic Dysfunction Center for disabling orthostatic intolerance between August 2006 and March 2010. A diagnosis of POTS was based on the following criteria: (i) a history of daily orthostatic symptoms for at least 6 months; (ii) HR (heart rate) increase of ≥30 beats/min within the first 10 min of standing; (iii) the absence of orthostatic hypotension [defined as a fall in BP (blood pressure) >20/10 mmHg]; and (iv) the absence of conditions that can explain postural tachycardia, such as acute dehydration, prolonged bed rest or medication [13]. Patients were at least 18 years old and all were screened with a comprehensive medical history, physical examination, 12-lead ECG and routine laboratory studies. Subjects with abnormal renal function, liver function, haematological disease or systemic illnesses that might affect autonomic function, e.g. diabetes, cardiac arrhythmias, adrenocortical disease or other known autonomic disorders, were excluded. The Vanderbilt University Investigational Review Board approved the present study, and written informed consent was obtained from each subject before initiating the study (http://www.ClinicalTrials.gov identifier NCT00580619).
CFS: definition and classification
To fulfil the case definition, fatigue was defined as unexplained, persistent fatigue present for ≥6 months that was not mainly a result of exertion; was not substantially relieved by rest, was of new onset (not lifelong) and resulted in a significant reduction in previous levels of activity [1]. CFS was defined according to CDC (Centers for Disease Control and Prevention) criteria [1], and included a case-defining fatigue associated with at least four of the following ancillary symptoms: impaired short-term memory or concentration, sore throat, tender lymphadenopathy, muscle pain, joint pain, headaches of a new type, sleep disturbance and post-exercise malaise [1]. Patients with exclusionary medical or psychiatric conditions for CFS were not included in the present study [1]. As a result, five patients (two with eating disorders, two with significant fatigue associated with β-blockers and one patient with juvenile rheumatoid arthritis) were excluded. Six other patients with case-defining fatigue but insufficient symptom criteria for CFS (i.e. less than four ancillary symptoms) were not included in the analysis because these patients cannot be clearly differentiated from the CFS group [22]. Among the remaining 47 patients who were enrolled in the study, 30 met CFS criteria (termed CFS–POTS) and the other 17 were classified as non-CFS–POTS.
General protocol
All subjects were admitted to the Vanderbilt General Clinical Research Center and were fed a low-monoamine, caffeine-free diet containing 150 mM/l Na+ and 70 mM/l K+ per day. Medication affecting BP, BV and the autonomic nervous system was withheld for ≥5 half-lives before admission. Fludrocortisone was discontinued for ≥5 days. All participants completed the following evaluations: AFTs (autonomic function tests), posture study with supine and upright catecholamines, and a battery of questionnaires. In addition, a subset of patients underwent BV assessment and QSART (quantitative sudomotor axon reflex testing).
Evaluation of fatigue and functional impairment
Fatigue severity was assessed by a subscale of the CIS (Checklist of Individual Strength) [23] that measures both general and physical fatigue; a score >36 represents severe fatigue [24]. Functional impairment and general health status were assessed by the use of the RAND-36 Health Survey that includes eight domains: limitations in physical activities (physical functioning), role limitation due to physical problems, bodily pain, emotional well-being, role limitation due to emotional problems, energy/fatigue, social functioning and general health [25]. Scores for each domain range from 0 to 100, with higher scores reflecting better health status.
Posture study and AFTs
An orthostatic test was performed with patients fasted, and while they remained supine after an overnight rest, to evaluate haemodynamic and hormonal changes on standing. An indwelling catheter was placed in an antecubital vein at least 30 min before testing. BP and HR were obtained using an automated sphygmomanometer (Dinamap; GE Medical Systems Information Technologies). Subjects were encouraged to stand as long as possible to a maximum of 30 min. During this period, they were allowed to sit at intervals if presyncopal symptoms developed. Blood samples were obtained for plasma noradrenaline (norepinephrine), PRA (plasma renin activity) and aldosterone.
AFTs included the Valsalva manoeuvre and the cold pressor test as described previously [26]. All tests were performed in the morning, 2 h or more after a light breakfast. BP and HR were obtained using an automated oscillometric sphygmomanometer (Dinamap), finger photoplethysmography (Finometer, FMS; or Nexfin, BMEYE), and continuous ECG. Supine baseline data collected during the Valsalva manoeuvre were digitized and recorded using a WINDAQ data acquisition system (14 Bit, 500 Hz; DI220; DATAQ;) and processed off-line using custom-written software in PV-Wave language (PV-Wave; Visual Numerics). Beat-to-beat values of BP and R–R intervals were digitized and analysed to determine the power spectra in the low-frequency (0.04–0.15 Hz) and high-frequency (0.15–0.40 Hz) range, as described previously [27].
BV assessment
We determined PV (plasma volume) in a subset of 28 patients (20 CFS–POTS and eight non-CFS–POTS patients) by the indicator dye-dilution technique using 131I-labelled human serum albumin (Volumex; Daxor), as described previously [21]. Total BV was calculated from measured PV and haematocrit. Erythocyte volume was calculated as the difference between total BV and PV. Ideal PV and total BV were determined for each individual based on normative data considering height, weight and gender [21].
QSART
A subset of 31 patients (21 CFS–POTS and ten non-CFS–POTS patients) underwent QSART testing to compare the prevalence of neural sudomotor abnormalities between these two groups. QSART testing was performed using the QSweat device (WR Electronics), as described previously [28]. Capsules were placed at four standard sites: distal forearm, proximal leg, distal leg and foot. A 10% acetylcholine solution was iontophoresed using a current of 2.0 mA. Results were recorded in microlitres of sweat volume. Results were deemed to be abnormal if one or more sites were below the fifth percentile for age and gender using published normative data or if there was a proximal distal gradient with a distal site <one-third of the volume of the proximal site. Results were also analysed by raw sweat volume.
Hormone measurements
Plasma noradrenaline were collected in plastic syringes, immediately transferred to chilled vacuum tubes with sodium heparin (BD, Franklin Lakes, NJ, U.S.A.), and placed on ice. Plasma was separated by centrifugation at −4 °C and stored at −70 °C in collection tubes with 6% GSH (Sigma) until the assay was performed. Concentrations of noradrenaline were measured by batch alumina extraction followed by HPLC for separation with electrochemical detection and quantification [29]. PRA was assessed by the conversion of angiotensinogen into AngI (angiotensin I) and expressed as ng of AngI produced/ml of plasma per h. Plasma aldosterone was measured by RIA [21].
Statistical methods
Frequency tables were generated for categorical variables. Continuous variables are expressed as means±S.E.M. Normal distribution of data was assessed using the Kolmogorov–Smirnov test. Comparisons between patients with CFS–POTS and non-CFS–POTS were analysed by Student's t tests if they had normal distribution. Otherwise, a Mann–Whitney U test was used. The χ2 test and Fisher's exact test were used for categorical comparisons of data.
All the tests were two-tailed, and a P<0.05 was considered significant. Analyses were performed with SPSS for Windows, version 17.0.
RESULTS
Patient characteristics and CFS symptoms
We recruited 58 patients with POTS, of which 47 were eligible to participate. The majority of them (64%, n=30) fulfilled criteria for CFS (CFS–POTS), whereas the remainder (36%, n=17) were classified as non-CFS–POTS. Clinical characteristics of both groups are shown in Table 1. There was no significant difference in age, BMI (body mass index), serum electrolytes, calculated osmolality or plasma creatinine between groups. CFS–POTS patients tended to have a longer duration of disease (estimated from the onset of orthostatic symptoms) as compared with those with non-CFS–POTS, but this trend did not reach statistical significance (P=0.231). The proportion of patients taking β-blockers or fludrocortisone acetate before admission was similar between groups.
Table 1. Clinical characteristics of patients with CFS–POTS and non-CFS–POTS.
Values are means±S.E.M., unless otherwise stated.
| Clinical characteristics | Non-CFS–POTS (n=17) | CFS–POTS (n=30) | P value |
|---|---|---|---|
| Age (years) | 33±2 | 30±2 | 0.121 |
| BMI (kg/m2) | 23±1 | 24±1 | 0.877 |
| Duration of disease (months)* | 55±14 | 72±11 | 0.231 |
| Prior medication use† | |||
| β-Blockers (n) | 11 (65%) | 19 (63%) | 0.925 |
| Fludrocortisone acetate (n) | 4 (24%) | 11 (37%) | 0.353 |
| Haematocrit (%) | 40±1 | 40±1 | 0.321 |
| Na+ (mM/l) | 139±0.6 | 140±0.4 | 0.296 |
| K+ (mM/l) | 4.0±0.1 | 3.9±0.1 | 0.480 |
| Calculated plasma osmolality (mM/l) | 286±1 | 288±1 | 0.301 |
| Plasma creatinine (mg/dl) | 0.8±0.03 | 0.8±0.02 | 0.420 |
*Duration of disease calculated from the onset of orthostatic symptoms.
†Medications reported within 6 months before admission.
Fatigue and CFS-related symptoms
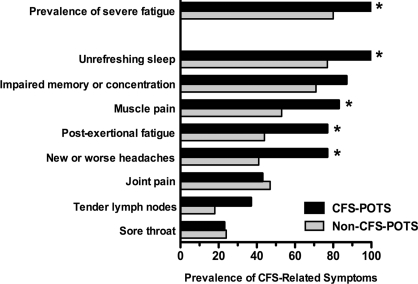
Severe fatigue (CIS, fatigue subscale >36) was observed in 93% of POTS patients. By definition, all subjects with CFS–POTS reported severe fatigue (Figure 1), which was greater in severity than in those without CFS (51±1 compared with 43±3 respectively; P=0.016). The majority of non-CFS–POTS patients (80%), however, also experienced severe fatigue, but none of them met the case definition of fatigue, either because it was reported as ‘lifelong’ (82%, n=14), mainly related to exercise (53%, n=9), non-disabling (29%, n=5) and lasting <6 months (29%, n=5).
Figure 1. Prevalence of severe fatigue and CFS-related symptoms.
Severe fatigue was defined as a CIS fatigue severity subscale score >36. *P<0.05.
CFS-related symptoms were common in both groups. The mean number of ancillary symptoms was 5.3±0.2 [median (interquartile range), 5 (4–7)] in patients with CFS–POTS, and 3.8±0.5 [median (interquartile range), 4 (3–5)] in the non-CFS group. Only one non-CFS–POTS patient had no CFS-related symptoms. The pattern of case-defining symptoms was similar in the two groups. Unrefreshing sleep, impaired memory or concentration and muscle pain were the most common symptoms in both groups; whereas joint pain, tender lymph nodes and sore throat were the least prevalent (Figure 1). CFS–POTS patients, however, had a higher prevalence of sleep disturbances, muscle pain, post-exertional fatigue and headaches as compared with those without CFS.
Consistent with the CFS definition, the CFS–POTS group had significantly lower scores on the fatigue/energy domain, indicating more severe fatigue (Table 2). Most notably, both groups reported extremely low scores in the role limitation due to physical health domain, underscoring their severe disability. There was also a trend towards increased perception of bodily pain (lower scores) in POTS patients with CFS, but did not reach significance (P=0.057).
Table 2. General health status and functional impairment (RAND-36 health survey).
Scores are means±S.E.M.; range 0–100. Low scores indicate more severe conditions.
| Scale | Non-CFS–POTS (n=17) | CFS–POTS (n=30) | P value |
|---|---|---|---|
| Physical health | |||
| Physical functioning | 40.1±4.1 | 32.8±3.1 | 0.153 |
| Role limitation due to physical health | 2.9±2.9 | 0.9±0.9 | 0.676 |
| Bodily pain | 65.8±6.1 | 54.1±3.7 | 0.057 |
| Mental health | |||
| Emotional well-being | 66.1±4.9 | 68.0±3.4 | 0.796 |
| Role limitation due to emotional problems | 39.6±11.5 | 37.9±8.6 | 0.844 |
| Other | |||
| General health | 31.8±3.7 | 27.8±2.4 | 0.351 |
| Energy/fatigue | 31.5±6.1 | 16.8±3.0 | 0.037 |
| Social functioning | 36.8±5.9 | 32.6±5.1 | 0.499 |
Autonomic testing, power spectral densities and neurohormonal profile
Supine BP and HR were similar in the two groups (Table 3). All patients had postural tachycardia within the first 10 min of upright posture, but ten (59%) non-CFS and 16 (53%) CFS–POTS patients could not complete the 30-min test due to orthostatic symptoms. Of them, one non-CFS and six CFS–POTS patients developed neurally mediated hypotension after prolonged standing. The mean standing time (i.e. orthostatic tolerance) was similar in both groups. After 10 min standing, both groups had similar increases in HR (49±3 compared with 40±5 beats/min in the CFS–POTS and non-CFS–POTS group respectively; P=0.114), in systolic BP (5±2 compared with 4±3 mmHg in the CFS–POTS and non-CFS–POTS group respectively; P=0.868) and in diastolic BP (6±2 and 4±2 mmHg in the CFS–POTS and non-CFS–POTS group respectively; P=0.256). At the time of maximal orthostatic tolerance, HR had a greater increase in CFS–POTS patients as compared with the non-CFS–POTS group (P=0.030; Table 3), whereas BP remained similar in the two groups (113/76±4/2 compared with 106/72±4/3 mmHg in the CFS–POTS and non-CFS–POTS group respectively; P>0.05).
Table 3. Autonomic and neurohormonal profile in CFS–POTS and non-CFS–POTS.
Values are means±S.E.M. DBP, diastolic BP; SBP, systolic BP; LF, low frequency; HF, high frequency; RRI, R–R heart rate interval. Control values from the Autonomic Dysfunction Center Database at Vanderbilt University are presented as a reference.
| Non-CFS POTS | CFS-POTS | Controls | |||||
|---|---|---|---|---|---|---|---|
| Test | Value | n | Value | n | P value | Value | n |
| Orthostatic stress test | |||||||
| Supine | |||||||
| SBP (mmHg) | 104±3 | 17 | 108±2 | 30 | 0.385 | 100±2 | 22 |
| DBP (mmHg) | 67±2 | 17 | 67±2 | 30 | 0.963 | 65±2 | 22 |
| HR (beats/min) | 75±3 | 17 | 73±1 | 30 | 0.495 | 62±2 | 22 |
| 10 min standing | |||||||
| SBP (mmHg) | 109±4 | 15 | 113±4 | 28 | 0.509 | 100±3 | 22 |
| DBP (mmHg) | 72±2 | 15 | 73±2 | 28 | 0.755 | 69±2 | 22 |
| HR (beats/min) | 115±6 | 15 | 122±3 | 28 | 0.244 | 85±2 | 22 |
| Total standing time (min) | 21±2 | 17 | 20±2 | 30 | 0.902 | 27±2 | 22 |
| ΔHR at maximal tolerance (beats/min)* | 40±4 | 17 | 51±3 | 30 | 0.030 | 26±3 | 22 |
| Valsalva manoeuvre† | |||||||
| Early phase II ΔSBP (mmHg) | −24±4 | 17 | −24±3 | 27 | 0.838 | −14±3 | 21 |
| Late phase II ΔSBP (mmHg)‡ | 11±2 | 17 | 18±3 | 27 | 0.041 | 10±2 | 21 |
| Phase II ΔHR (beats/min) | 37±4 | 17 | 42±3 | 27 | 0.642 | 25±3 | 21 |
| Phase IV overshoot ΔSBP (mmHg) | 31±5 | 17 | 36±3 | 30 | 0.298 | 12±3 | 21 |
| Valsalva manoeuvre ratio | 1.95±0.41 | 17 | 2.01±0.06 | 30 | 0.580 | 1.62±0.07 | 21 |
| Cold pressor ΔSBP (mmHg)† | 20±2 | 17 | 20±2 | 29 | 1.000 | 20±3 | 20 |
| LFRRI (ms2) | 371±92 | 17 | 564±97 | 30 | 0.293 | 921±161 | 22 |
| HFRRI (ms2) | 322±109 | 17 | 338±58 | 30 | 0.598 | 1372±321 | 22 |
| LFRRI/HFRRI | 2.0±0.3 | 17 | 2.3±0.2 | 30 | 0.406 | 1.1±0.2 | 22 |
| LFSBP (mmHg2) | 4.8±1.0 | 17 | 6.3±0.7 | 30 | 0.019 | 3.5±0.6 | 22 |
| Plasma noradrenaline (pg/ml) | |||||||
| Supine | 173±27 | 17 | 203±27 | 30 | 0.232 | 189±15 | 22 |
| Upright | 779±131 | 17 | 635±51 | 30 | 0.587 | 388±18 | 22 |
*HR changes (ΔHR) from supine to standing at the time of maximal orthostatic tolerance.
†BP and HR responses are given as the change (Δ) compared with baseline.
‡BP recovery from early to late phase II of the Valsalva manoeuvre.
Both groups had an exaggerated decrease in systolic BP during early phase II of the Valsalva manoeuvre (Table 3). BP recovery from early to late phase II, however, was greater in the CFS–POTS group (P=0.041; Table 3). The pressor response to pain stimulus (cold pressor test) did not differ between the two groups. Both the high- and low-frequency components of HRV (heart rate variability) at supine rest were greatly decreased in both groups compared with normal controls (Table 3). CFS–POTS patients had greater supine resting BP variability in the low-frequency (LFSBP) component compared with those with non-CFS–POTS (P=0.019; Table 3).
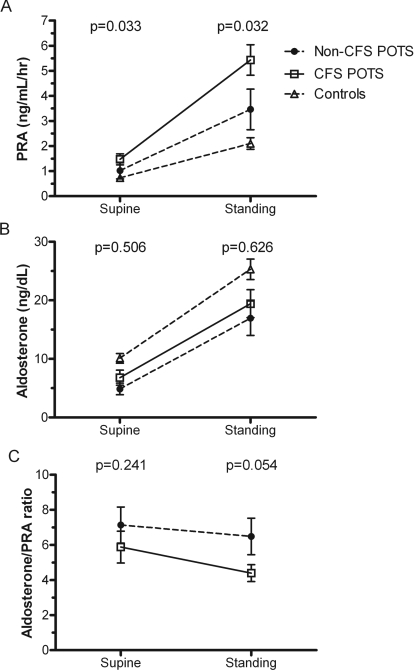
Patients with CFS–POTS and non-CFS–POTS had a significant increase in plasma noradrenaline with standing (P<0.01; Table 3), but no differences were found between groups in supine or upright plasma noradrenaline. Supine and upright PRA were significantly higher in the CFS–POTS group compared with those with non-CFS–POTS [supine, 1.5±0.2 compared with 1.0±0.3 ng/ml per h respectively (P=0.033); upright, 5.4±0.6 compared with 3.5±0.8 ng/ml per h respectively (P=0.032)] (Figure 2A). Plasma aldosterone tended to be higher in CFS–POTS compared with non-CFS–POTS patients (Figure 2B), but the differences did not reach significance for either supine (6.8±1.3 compared with 4.9±1.0 ng/dl respectively; P=0.506) or standing (19.4±2.4 compared with 17.0±3.0 ng/dl respectively; P=0.626). There was a trend towards lower supine aldosterone/PRA ratios in CFS–POTS patients compared with the non-CFS–POTS group (5.9±0.9 compared with 7.1±1.0 respectively; P=0.245) (Figure 2C), particularly upon standing (4.4±0.5 compared with 6.5±1.0 respectively; P=0.054).
Figure 2. Orthostatic changes in (A) PRA, (B) plasma aldosterone and (C) the aldosterone/PRA ratio in patients with CFS and non-CFS–POTS.
Control values from the Vanderbilt Autonomic Dysfunction Center database are included for reference. Values are expressed as means±S.E.M. The reported P values are for Mann–Whitney U tests comparing CFS–POTS with non-CFS–POTS patients.
BV
CFS–POTS and non-CFS–POTS patients had similar PVs (2659±134 and 2787±130 ml respectively; P=0.580). The calculated deficit in PV compared with normative data was similar in both groups [−132±66 ml (5.3±2.5%) and −36±119 ml (1.3±4.2%) in the CFS–POTS and non-CFS–POTS group respectively; P=0.458]. The proportion of subjects with PV deficits >8% was non-significantly higher in CFS–POTS than in the non-CFS–POTS group (35 compared with 25% respectively).
There was no significant difference in the calculated erythocyte volume between CFS and non-CFS–POTS patients (1334±74 compared with 1331±72 ml respectively; P=0.980). Both groups experienced a significant deficit in erythocyte volume compared with normative data [−330±30 ml (20.2±1.9%) compared with −302±65 ml (18.4±3.9%) in the CFS–POTS and non-CFS–POTS group respectively; P=0.657], but were not different from each other.
The calculated total BV was similar between CFS–POTS and non-CFS–POTS patients (3878±137 compared with 4118±184 ml respectively; P=0.341). There was no significant difference in the calculated deficit from predicted values in total BV between the CFS and non-CFS–POTS groups (−441±82 compared with −338±154 ml respectively; P=0.526). The percentage deficit in total BV for the non-CFS–POTS group was borderline (−7.7±3.5%; normal range, 0–8%), whereas patients with CFS–POTS had a mild deficit (−10.4±2%; mild deficit range, 8–16%). This difference in percentage deficit, however, was not statistically significant (P=0.468). The proportion of subjects with deficits in total BV was non-significantly higher in the CFS–POTS group compared with the non-CFS–POTS group (55 compared with 37%).
QSART
Abnormal QSART results were observed in 52% of CFS–POTS patients tested (11 of 21) and 50% of patients with non-CFS–POTS (5 of 10). In both groups, the most affected sites were the foot [81% of CFS–POTS (n=9) and 80% of non-CFS–POTS (n=4)] and the distal leg [64% of CFS–POTS (n=7) and 100% of non-CFS–POTS (n=5)]. The forearm was the least affected site in both CFS and non-CFS–POTS patients [27% (n=3) and 20% (n=1) respectively]. The most common pattern seen in both groups was a distal pattern affecting two to three sites (four CFS–POTS and two non-CFS–POTS patients). However, there were not significant differences in mean sweat volumes between patients with CFS–POTS and non-CFS–POTS at the four standard sites: forearm (0.73±0.13 compared with 0.64±0.19 L; P=0.760), proximal leg (0.61±0.09 compared with 0.47±0.08 litre; P=0.347), distal leg (0.63±0.11 compared with 0.36±0.09 litre; P=0.476) and foot (0.40±0.07 compared with 0.33±0.08 litre; P=0.416).
DISCUSSION
The main findings from this study were that: (i) the majority of patients with POTS fulfilled the criteria for CFS. Furthermore, severe fatigue and CFS-defining symptoms were also common features in POTS patients not meeting criteria for CFS; (ii) the pattern of CFS symptoms was similar in both groups, although as expected some CFS-defining symptoms were more prevalent in patients with CFS–POTS; (iii) physical functioning was similarly low in both groups, despite more severe fatigue in CFS–POTS patients; (iv) CFS–POTS patients had higher markers of sympathetic overactivity, including increased orthostatic tachycardia, resting low-frequencySBP, and greater BP recovery during late phase II of the Valsalva manoeuvre and higher supine and upright PRA; (v) POTS patients with and without CFS showed a similar mild decrease in erythocyte volume and borderline deficit in total BV; and (vi) Sudomotor abnormalities were frequent in both groups, with no differences in the pattern of sudomotor denervation or sweat volumes. These findings suggest that CFS–POTS is not a separate clinical entity distinct from non-CFS–POTS. We propose that CFS–POTS is part of the spectrum of this syndrome, associated with greater sympathetic activation and/or a more severe form of this condition.
Fatigue is often a major complaint of patients with POTS and, in some patients, can be chronic and overwhelming [15]. Thus, a substantial overlap between POTS and CFS has been consistently reported in the literature [10,11,15,16,18]. Most of these studies, however, have focused on the clinical features of orthostatic intolerance in patients with CFS. It is, as yet, unclear if and to what extent the hallmark symptoms of CFS are also main features of POTS. To address this question, we evaluated CFS symptoms in patients with POTS, and compared the CFS phenotype of subjects with POTS who met CFS criteria with those who did not. We found that severe fatigue was highly prevalent in patients with POTS, even among subjects who did not meet the criteria for CFS. Only three out of 44 patients reported levels of fatigue comparable with healthy subjects (<36 in the CIS, fatigue subscale) [30]. Accordingly, the majority of our POTS patients (64%) met the CDC criteria for CFS. This is in contrast with previous studies in which the prevalence of fatigue (48–77%), and subjects fulfilling CFS criteria (17–23%) were lower [15,17,18]. Possible explanations for these discrepancies may include differences in methods of symptom assessment (chart review compared with validated questionnaires), clinical heterogeneity of POTS patients or patient selection bias. In this regard, because we are a tertiary referral centre for the management of these disorders, it is possible that we preferentially enrol patients severely affected with POTS, which may explain the high prevalence of fatigue.
CFS-related symptoms followed the same pattern in both POTS groups. Although there were some differences in the prevalence of symptoms such as unrefreshing sleep, muscle pain, post-exertional fatigue and headaches; we could not find a distinctive clinical presentation of CFS in POTS. Moreover, the pattern of CFS symptoms in our cohort was similar to that observed in large international epidemiological and clinical datasets [22,31], raising the possibility that common pathophysiological mechanisms may underlie CFS-related symptoms in POTS and other CFS populations. We also found greatly impaired physical functioning in both groups, despite greater fatigue in POTS patients with CFS. Costigan et al. [32] observed that a higher burden of orthostatic symptoms was the only factor independently associated with functional impairment in CFS patients. Hence, we speculate that orthostatic intolerance in POTS may be a more important determinant of functional impairment than fatigue. Taken together, these findings suggest that POTS is a symptoms complex that is not only associated with orthostatic intolerance, but is also associated with CFS symptoms that may arise from a common pathophysiology.
Several studies have shown enhanced sympathetic activity in CFS patients as compared with healthy subjects [4,33,34], and the same was true in POTS patients; those who met CFS criteria had higher markers of sympathetic activity. The orthostatic tachycardia in POTS is easily explained by this increase in sympathetic activity and the corresponding decrease in vagal tone evidenced by the decrease in high frequency power spectra of HR. It is less clear how, or if, sympathetic activation relates to the symptoms of fatigue, and this may be an area of future research.
Supine and upright PRA were higher in CFS–POTS than in non-CFS–POTS patients. Low BV is normally a potent stimulus for renin release, and in our patients, BVs were similarly low in both groups, but tended to be lower in the CFS–POTS group. Aldosterone, on the other hand, was similar in both groups, so that there was a trend towards lower aldosterone/PRA ratios in the CFS–POTS group, suggesting decreased aldosterone responsiveness to PRA stimulation [21]. These findings raise the possibility that impairment of the RAAS (renin–angiotensin–aldosterone system) may contribute to the lower BVs in CFS–POTS [21], and the higher PRA may be a compensatory response to more severe hypovolaemia [35]. Given the results of the autonomic testing in CFS–POTS, which pointed to enhanced sympathetic activation, it is tempting to speculate that the increased PRA in this group may be, at least in part, the result of enhanced renal sympathetic activation. Similar results were reported by Wyller et al. [36], who found higher PRA in adolescents with CFS as compared with healthy subjects. Garland et al. [13] showed that POTS patients with higher supine PRA also had higher orthostatic tachycardia, and lower aldosterone/PRA ratio as compared with POTS with lower supine PRA. Nevertheless, we cannot exclude the possibility that the differences in PRA between groups may be due to differences in other components of RAAS such as AngII (angiotensin II), which has been shown to be increased in some patients with POTS [37].
A partial autonomic neuropathy has been proposed as playing a role in the pathophysiology of POTS [18]. We used QSART to assess the integrity of postganglionic cholinergic sudomotor fibres [15,28]. Patients with CFS–POTS and non-CFS–POTS had a similar high prevalence of abnormal sudomotor responses (52 and 50% respectively), which is in agreement with the prevalence reported in other POTS cohorts [15,28].
We excluded patients who were bedridden or severely deconditioned from the present study. Nonetheless, we cannot rule out that milder forms of cardiovascular deconditioning may contribute to the abnormalities observed in POTS [8,35,38]. Deconditioning, however, is unlikely to explain the differences between CFS–POTS and non-CFS–POTS because functional impairment was similar in both groups. Another limitation of this study is the potential referral and selection bias towards the most severe cases of POTS, which may have had an impact on the prevalence of fatigue- and CFS-related symptoms. Also, we did not control for the phase of the menstrual cycle in which patients were evaluated. However, in a previous study, the menstrual cycle did not affect orthostatic HR responses, BV or PRA at 30 min on standing in POTS women [35]. Finally, a relatively small number of non-CFS–POTS patients were included in the BV assessments that may have introduced a type II error (false negative results).
In conclusion, patients with CFS–POTS and non-CFS–POTS had a similar clinical, autonomic and neurohumoral profile. Severe fatigue and CFS-related symptoms were common features in patients with POTS, and the majority of them fulfilled the criteria for CFS. Both groups showed a similar CFS phenotype with some differences in the relative prevalence of case-defining symptoms. CFS in POTS, however, was associated with the evidence of increase sympathetic tone when supine, and greater increases in sympathetic activity and PRA during standing. Because there were no distinguishing features between POTS patients with and without CFS, we propose that CFS–POTS is not a separate clinical entity distinct from POTS without CFS. Rather, CFS seems to be part of the spectrum of POTS, associated with greater sympathetic activation and/or a more severe form of this condition. In future studies, pharmacological approaches aimed at reducing this sympathetic activation could be used to determine if it has a causative role in the fatigue, and may represent a novel approach to the treatment of CFS–POTS patients.
AUTHOR CONTRIBUTION
Luis Okamoto, Satish Raj, Alfredo Gamboa, Cyndya Shibao, David Robertson and Italo Biaggioni contributed to the conception and design of the study. Luis Okamoto, Satish Raj, Amanda Peltier, Alfredo Gamboa, Cyndya Shibao, André Diedrich and Bonnie Black contributed to the study and acquired the data. Luis Okamoto analysed the data and wrote the paper. Italo Biaggioni supervised the conduct of the study and writing of the paper. Satish Raj, Amanda Peltier, Alfredo Gamboa, Cyndya Shibao, André Diedrich, Bonnie Black and Italo Biaggioni contributed to the revision of the paper.
ACKNOWLEDGEMENTS
We thank the patients who volunteered for these studies and the Clinical Research Center staff who made the study possible.
FUNDING
This work was supported by the National Institutes of Health [grant numbers RO1 NS055670, RO1 HL102387, PO1 HL56693, UL1 RR024975], the Paden Dysautonomia Fund, and the National Heart, Lung and Blood Institute, National Institutes of Health [grant numbers K23 HL103976 (to C.S.), K23 HL095905 (to A.G.)].
References
- 1.Fukuda K., Straus S. E., Hickie I., Sharpe M. C., Dobbins J. G., Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Jason L. A., Richman J. A., Rademaker A. W., Jordan K. M., Plioplys A. V., Taylor R. R., McCready W., Huang C. F., Plioplys S. A community-based study of chronic fatigue syndrome. Arch. Intern. Med. 1999;159:2129–2137. doi: 10.1001/archinte.159.18.2129. [DOI] [PubMed] [Google Scholar]
- 3.Freeman R. The chronic fatigue syndrome is a disease of the autonomic nervous system. Sometimes. Clin. Auton. Res. 2002;12:231–233. doi: 10.1007/s10286-002-0058-2. [DOI] [PubMed] [Google Scholar]
- 4.Wyller V. B., Saul J. P., Walløe L., Thaulow E. Sympathetic cardiovascular control during orthostatic stress and isometric exercise in adolescent chronic fatigue syndrome. Eur. J. Appl. Physiol. 2008;102:623–632. doi: 10.1007/s00421-007-0634-1. [DOI] [PubMed] [Google Scholar]
- 5.Parish J. G. Early outbreaks of ‘epidemic neuromyasthenia’. Postgrad. Med. J. 1978;54:711–717. doi: 10.1136/pgmj.54.637.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newton J. L., Okonkwo O., Sutcliffe K., Seth A., Shin J., Jones D. E. J. Symptoms of autonomic dysfunction in chronic fatigue syndrome. Q. J. Med. 2007;100:519–526. doi: 10.1093/qjmed/hcm057. [DOI] [PubMed] [Google Scholar]
- 7.Schondorf R., Benoit J., Wein T., Phaneuf D. Orthostatic intolerance in the chronic fatigue syndrome. J. Auton. Nerv. Syst. 1999;75:192–201. doi: 10.1016/s0165-1838(98)00177-5. [DOI] [PubMed] [Google Scholar]
- 8.Freeman R., Komaroff A. L. Does the chronic fatigue syndrome involve the autonomic nervous system? Am. J. Med. 1997;102:357–364. doi: 10.1016/s0002-9343(97)00087-9. [DOI] [PubMed] [Google Scholar]
- 9.Bou-Holaigah I., Rowe P. C., Kan J., Calkins H. The relationship between neurally mediated hypotension and the chronic fatigue syndrome. JAMA, J. Am. Med. Assoc. 1995;274:961–967. [PubMed] [Google Scholar]
- 10.Stewart J. M., Gewitz M. H., Weldon A., Munoz J. Patterns of orthostatic intolerance: the orthostatic tachycardia syndrome and adolescent chronic fatigue. J. Pediatr. 1999;135:218–225. doi: 10.1016/s0022-3476(99)70025-9. [DOI] [PubMed] [Google Scholar]
- 11.Streeten D. H., Thomas D., Bell D. S. The roles of orthostatic hypotension, orthostatic tachycardia, and subnormal erythrocyte volume in the pathogenesis of the chronic fatigue syndrome. Am. J. Med. Sci. 2000;320:1–8. doi: 10.1097/00000441-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am. J. Med. Sci. 1999;317:75–77. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Garland E. M., Raj S. R., Black B. K., Harris P. A., Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology. 2007;69:790–798. doi: 10.1212/01.wnl.0000267663.05398.40. [DOI] [PubMed] [Google Scholar]
- 14.Low P. A., Sandroni P., Joyner M., Shen W. K. Postural tachycardia syndrome (POTS) J. Cardiovasc. Electrophysiol. 2009;20:352–358. doi: 10.1111/j.1540-8167.2008.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thieben M. J., Sandroni P., Sletten D. M., Benrud-Larson L. M., Fealey R. D., Vernino S., Lennon V. A., Shen W. K., Low P. A. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin. Proc. 2007;82:308–313. doi: 10.4065/82.3.308. [DOI] [PubMed] [Google Scholar]
- 16.Hoad A., Spickett G., Elliott J., Newton J. Postural orthostatic tachycardia syndrome is an under-recognized condition in chronic fatigue syndrome. Q. J. Med. 2008;101:961–965. doi: 10.1093/qjmed/hcn123. [DOI] [PubMed] [Google Scholar]
- 17.Jacob G., Shannon J. R., Black B., Biaggioni I., Mosqueda-Garcia R., Robertson R. M., Robertson D. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation. 1997;96:575–580. doi: 10.1161/01.cir.96.2.575. [DOI] [PubMed] [Google Scholar]
- 18.Jacob G., Costa F., Shannon J. R., Robertson R. M., Wathen M., Stein M., Biaggioni I., Ertl A., Black B., Robertson D. The neuropathic postural tachycardia syndrome. N. Engl. J. Med. 2000;343:1008–1014. doi: 10.1056/NEJM200010053431404. [DOI] [PubMed] [Google Scholar]
- 19.Jordan J., Shannon J. R., Diedrich A., Black B. K., Robertson D. Increased sympathetic activation in idiopathic orthostatic intolerance: role of systemic adrenoreceptor sensitivity. Hypertension. 2002;39:173–178. doi: 10.1161/hy1201.097202. [DOI] [PubMed] [Google Scholar]
- 20.Hurwitz B. E., Coryell V. T., Parker M., Martin P., Laperriere A., Klimas N. G., Sfakianakis G. N., Bilsker M. S. Chronic fatigue syndrome: illness severity, sedentary lifestyle, blood volume and evidence of diminished cardiac function. Clin. Sci. 2010;118:125–135. doi: 10.1042/CS20090055. [DOI] [PubMed] [Google Scholar]
- 21.Raj S. R., Biaggioni I., Yamhure P. C., Black B. K., Paranjape S. Y., Byrne D. W., Robertson D. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111:1574–1582. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan P. F., Pedersen N. L., Jacks A., Evengård B. Chronic fatigue in a population sample: definitions and heterogeneity. Psychol. Med. 2005;35:1337–1348. doi: 10.1017/S0033291705005210. [DOI] [PubMed] [Google Scholar]
- 23.Vercoulen J. H., Swanink C. M., Fennis J. F., Galama J. M., van der Meer J. W., Bleijenberg G. Dimensional assessment of chronic fatigue syndrome. J. Psychosom. Res. 1994;38:383–392. doi: 10.1016/0022-3999(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 24.Reeves W. C., Lloyd A., Vernon S. D., Klimas N., Jason L. A., Bleijenberg G., Evengard B., White P. D., Nisenbaum R., Unger E. R. Identification of ambiguities in the 1994 chronic fatigue syndrome research case definition and recommendations for resolution. BMC Health Serv. Res. 2003;3:25. doi: 10.1186/1472-6963-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hays R. D., Morales L. S. The RAND-36 measure of health-related quality of life. Ann. Med. 2001;33:350–357. doi: 10.3109/07853890109002089. [DOI] [PubMed] [Google Scholar]
- 26.Mosqueda-Garcia R. Evaluation of autonomic failure. In: Robertson D., Biaggioni I., editors. Disorders of the Autonomic Nervous System. London: Harwood Academic Press; 1995. pp. 25–59. [Google Scholar]
- 27.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 28.Peltier A. C., Garland E., Raj S. R., Sato K., Black B., Song Y., Wang L., Biaggioni I., Diedrich A., Robertson D. Distal sudomotor findings in postural tachycardia syndrome. Clin. Auton. Res. 2010;20:93–99. doi: 10.1007/s10286-009-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacob G., Robertson D., Mosqueda-Garcia R., Ertl A. C., Robertson R. M., Biaggioni I. Hypovolemia in syncope and orthostatic intolerance role of the renin-angiotensin system. Am. J. Med. 1997;103:128–133. doi: 10.1016/s0002-9343(97)00133-2. [DOI] [PubMed] [Google Scholar]
- 30.Prins J. B., Bleijenberg G., Bazelmans E., Elving L. D., de Boo T. M., Severens J. L., van der Wilt G. J., Spinhoven P., van der Meer J. W. Cognitive behaviour therapy for chronic fatigue syndrome: a multicentre randomised controlled trial. Lancet. 2001;357:841–847. doi: 10.1016/S0140-6736(00)04198-2. [DOI] [PubMed] [Google Scholar]
- 31.Hickie I., Davenport T., Vernon S. D., Nisenbaum R., Reeves W. C., Hadzi-Pavlovic D., Lloyd A. Are chronic fatigue and chronic fatigue syndrome valid clinical entities across countries and health-care settings? Aust. N. Z. J. Psychiatry. 2009;43:25–35. doi: 10.1080/00048670802534432. [DOI] [PubMed] [Google Scholar]
- 32.Costigan A., Elliott C., McDonald C., Newton J. L. Orthostatic symptoms predict functional capacity in chronic fatigue syndrome: implications for management. Q. J. Med. 2010;103:589–595. doi: 10.1093/qjmed/hcq094. [DOI] [PubMed] [Google Scholar]
- 33.Wyller V. B., Barbieri R., Saul J. P. Blood pressure variability and closed-loop baroreflex assessment in adolescent chronic fatigue syndrome during supine rest and orthostatic stress. Eur. J. Appl. Physiol. 2010;111:497–507. doi: 10.1007/s00421-010-1670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boneva R. S., Decker M. J., Maloney E. M., Lin J. M., Jones J. F., Helgason H. G., Heim C. M., Rye D. B., Reeves W. C. Higher heart rate and reduced heart rate variability persist during sleep in chronic fatigue syndrome: a population-based study. Auton. Neurosci. 2007;137:94–101. doi: 10.1016/j.autneu.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Fu Q., Vangundy T. B., Shibata S., Auchus R. J., Williams G. H., Levine B. D. Menstrual cycle affects renal-adrenal and hemodynamic responses during prolonged standing in the postural orthostatic tachycardia syndrome. Hypertension. 2010;56:82–90. doi: 10.1161/HYPERTENSIONAHA.110.151787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wyller V. B., Evang J. A., Godang K., Solhjell K. K., Bollerslev J. Hormonal alterations in adolescent chronic fatigue syndrome. Acta Paediatr. 2010;99:770–773. doi: 10.1111/j.1651-2227.2010.01701.x. [DOI] [PubMed] [Google Scholar]
- 37.Mustafa H. I., Garland E. M., Biaggioni I., Black B. K., Dupont W. D., Robertson D., Raj S. R. Abnormalities of angiotensin regulation in postural tachycardia syndrome. Heart Rhythm. 2011;8:422–428. doi: 10.1016/j.hrthm.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu Q., Vangundy T. B., Galbreath M. M., Shibata S., Jain M., Hastings J. L., Bhella P. S., Levine B. D. Cardiac origins of the postural orthostatic tachycardia syndrome. J. Am. Coll. Cardiol. 2010;55:2858–2868. doi: 10.1016/j.jacc.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]