Intracellular Na+/H+ antiporters (NHXs) play important roles in maintaining homeostasis of Na+ and K+. In plants, NHXs direct the movement of Na+ or K+ across the tonoplast and into the vacuole (or other organelles) by catalyzing the exchange of Na+ and/or K+ for H+. The electrochemical H+ gradients maintaining H+ for such exchange are generated by vacuolar H+-ATPase and H+-PPase. Arabidopsis thaliana encodes six intracellular NHXs that may be divided into two groups: NHX1-NHX4, which are localized to the vacuolar membrane, and NHX5-NHX6, associated with the endosome. Bassil et al. (2011a) recently characterized NHX5 and NHX6 through single and double knockout mutant analysis and showed that these two NHXs mediate vesicular trafficking and play an important role in mediating plant response to salt stress. Now, the same group (Bassil et al., pages 3482–3497) used reverse genetics to explore how NHX1 and NHX2 function at the tonoplast impacts plant growth and development, showing that these NHXs together control cell expansion in vegetative tissues and male reproductive organs and are required for normal flower development.
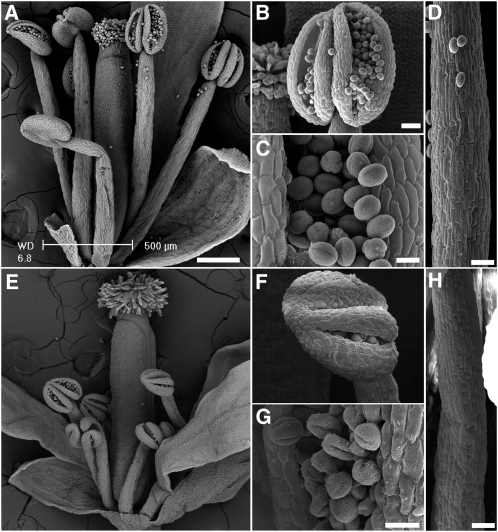
The authors found that nhx1 nhx2 double mutant plants were significantly smaller than the wild type, and this was associated with a decrease in cell size rather than defects in tissue organization. The single knockout mutants displayed only a mild reduction in growth (nhx1) or no obvious phenotype (nhx2) relative to the wild type, suggesting that NHX1 and NHX2 function in a partially redundant manner to control cell expansion. The double mutant plants also displayed abnormalities in male reproductive organs, in that the stamens lacked both filament elongation and anther dehiscence (see figure). In wild-type Arabidopsis, expression of NHX1 was abundant in most flower organs but was low in nonelongated filaments and immature anthers and high in elongated filaments, whereas NHX2 was highly expressed in anthers and pollen.
Scanning electron micrographs comparing the anatomy of wild-type and nhx1 nhx2 flowers. Flowers and organs of the wild type ([A] to [D]) and nhx1 nhx2 ([E] to [H]). Whole flowers ([A] and [E]), dehiscent anthers ([B] and [F]), pollen grains ([C] and [G]), and filaments ([D] and [H]). Bars = 200 μm (A) and (E), 50 μm in (B) and (F), 10 μm in (C) and (G), and 50 μm in (D) and (H). (Reprinted from Figure 4 of Bassil et al. [2011b].)
Given the roles of NHX-type transporters in Na+ and K+ homeostasis, the authors examined the response to salt treatment and found that a moderate amount of Na+ in the media improved growth of nhx1 nhx2 compared with the mutant grown on control medium, whereas an equimolar amount of K+ led to a significant decrease in growth, the latter producing tiny, tightly curled plants. Together, the data indicated that nhx1 nhx2 plants were compromised in their ability to accumulate K+ in favor of Na+ and were not able to adjust their K+ content when challenged with Na+, as seen in wild-type plants. Further analyses using ratiometric dyes and imaging-based techniques showed that nhx1 nhx2 plants had both lower vacuolar pH and lower K+ concentration compared with the wild type.
A typical mesophytic plant can be well over 90% water, and inorganic ion and consequent osmotic-dependent water relations dominate the process of growth at a cellular level as well as myriad turgor-related physiological processes. The work of Bassil et al. shows how NHX function influences a number of turgor-related processes that rely on the generation of low osmotic potential in the vacuole and how this is related to plant growth and development. In particular, the data suggest that NHX1 and NHX2 function in maintenance of K+ homeostasis in the vacuole, especially in rapidly expanding cells of flowers and male reproductive organs. The results provide a clear and thorough demonstration of how the “cash” in the savings account of a plant cell (the protons pumped into the central vacuole) is “spent” to accumulate cations and thus drive cell growth. The double mutant phenotypes described provide an excellent basis for learning some of the fundamental ways in which plants work. Students of plant physiology might be encouraged to examine the various leaf and floral phenotypes of the mutants presented in the article and consider what processes might be impaired in the mutant and what treatments might be applied to wild-type plants to (1) mimic the mutant phenotype and (2) test the hypothesis that cation accumulation in the central vacuole drives water uptake leading to cell expansion and growth. In addition, the floral organ phenotypes of the mutant plants provide an informative demonstration of the roles played by cell expansion and turgor in a number of processes related to fertilization, with relevance to several horticultural crops.
References
- Bassil E., Ohto M.A., Esumi T., Tajima H., Zhu Z., Cagnac O., Belmonte M., Peleg Z., Yamaguchi T., Blumwald E. (2011a). The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell 23: 224–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil E., Tajima H., Liang Y.-C., Ohto M., Ushijima K., Nakano R., Esumi T., Coku A., Belmonte M., Blumwald E. (2011b). The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 23: 3482–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]