This work identifies the nonprotein amino acid Nδ-acetylornithine and an acetyltransferase that synthesizes it in Arabidopsis thaliana, thus revealing a new methyl jasmonate–inducible defense response. It demonstrates that the genomic resources available for Arabidopsis make it relatively easy to move from a identifying a plant metabolite to the discovery of a previously unknown enzymatic activity.
Abstract
Since research on plant interactions with herbivores and pathogens is often constrained by the analysis of already known compounds, there is a need to identify new defense-related plant metabolites. The uncommon nonprotein amino acid Nδ-acetylornithine was discovered in a targeted search for Arabidopsis thaliana metabolites that are strongly induced by the phytohormone methyl jasmonate (MeJA). Stable isotope labeling experiments show that, after MeJA elicitation, Arg, Pro, and Glu are converted to Orn, which is acetylated by NATA1 to produce Nδ-acetylornithine. MeJA-induced Nδ-acetylornithine accumulation occurs in all tested Arabidopsis accessions, other Arabidopsis species, Capsella rubella, and Boechera stricta, but not in less closely related Brassicaceae. Both insect feeding and Pseudomonas syringae infection increase NATA1 expression and Nδ-acetylornithine accumulation. NATA1 transient expression in Nicotiana tabacum and the addition of Nδ-acetylornithine to an artificial diet both decrease Myzus persicae (green peach aphid) reproduction, suggesting a direct toxic or deterrent effect. However, since broad metabolic changes that are induced by MeJA in wild-type Arabidopsis are attenuated in a nata1 mutant strain, there may also be indirect effects on herbivores and pathogens. In the case of P. syringae, growth on a nata1 mutant is reduced compared with wild-type Arabidopsis, but growth in vitro is unaffected by Nδ-acetylornithine addition.
INTRODUCTION
Plants exhibit a wide array of defenses against herbivores and pathogens, ranging from the production of toxins and feeding deterrents (Agrawal, 1998; Kessler and Baldwin, 2002) to compensatory growth changes that allow tolerance of high damage levels (Agrawal, 2000; Stowe et al., 2000; Tiffin, 2000). Many, perhaps most, of the likely several hundred thousand secondary metabolites found in the plant kingdom contribute to defense against herbivores and pathogens (Dixon, 2001; Bino et al., 2004). Broadly distributed classes of secondary metabolites, which can be found as components of both constitutive and inducible plant defense responses, include phenolics, terpenes, alkaloids, and nonprotein amino acids (Howe and Jander, 2008).
In addition to the standard 20 proteinogenic amino acids, hundreds of other amino acids have been found in many different plant species (Fowden, 2001; Bell, 2003). Some of these, for example, Orn and homoserine, are essential in primary metabolism, whereas others are secondary metabolites that contribute to plant defense against herbivores and pathogens. For instance, canavanine, which is found in the seeds of many legumes, is toxic to herbivores because it competes with Arg in enzymatic reactions (Rosenthal, 2001). Similarly, azetidine-2-carboxylate, which is particularly abundant in Convallaria majalis (lily of the valley), can disrupt protein structures by binding to tRNA in place of Pro during protein synthesis (Peterson and Fowden, 1963; Norris and Fowden, 1972).
Another function for nonprotein amino acids may be to store nitrogen in a form that makes it metabolically unavailable to herbivores. About half of the 20 protein amino acids are essential dietary constituents because they cannot be synthesized by most animals. Degradation of Arg to Orn by tomato (Solanum lycopersicum) arginase significantly reduces Manduca sexta (tobacco hornworm) weight gain (Chen et al., 2005), showing that availability of a single essential amino acid can be growth-limiting for insects. Similarly, conversion of an essential amino acid into an uncommon nonprotein amino acid that herbivores cannot use would reduce the nutritive value of plant tissue.
The nonprotein amino acid Nδ-acetylornithine (Figure 1A) has been reported in Corydalis ochotensis (Manske, 1937), Asplenium nidus (Virtanen and Linko, 1955), Bistorta bistortoides (Lipson et al., 1996), some grasses (Fowden, 1958), and several legumes (Brown and Fowden, 1966; Zacharius, 1970; Kite and Ireland, 2002; Marona et al., 2003). Abundance of Nδ-acetylornithine in legume seeds (Brown and Fowden, 1966; Zacharius, 1970; Kite and Ireland, 2002) and B. bistortoides rhizomes (38% of free amino acids; Lipson et al., 1996) suggests a role in nitrogen storage. However, given that Nδ-acetylornithine has been found sporadically in ferns, monocots, and dicots (in the Aspleniaceae, Fabaceae, Papaveraceae, Poaceae, and Polygonaceae), there may be additional functions associated with this plant metabolite. Like most nonprotein amino acids found in plants, Nδ-acetylornithine has not been studied in a genetically tractable model species and almost nothing is known about the biosynthetic pathway(s).
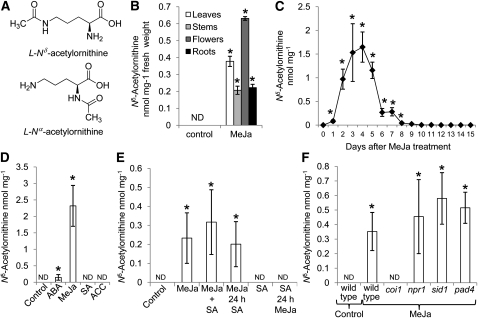
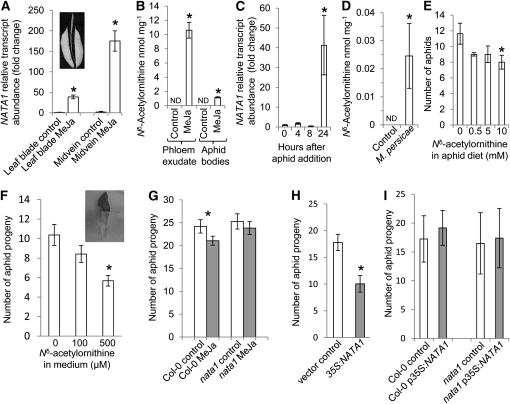
Figure 1.
Regulation of Nδ-Acetylornithine Production in Arabidopsis.
(A) Structures of l-Nδ-acetylornithine and l-Nα-acetylornithine.
(B) Accumulation of Nδ-acetylornithine in plant tissues 4 d after MeJA treatment. Mean ± se of n = 3. ND, not detected.
(C) Time course of Nδ-acetylornithine accumulation in rosette leaves after MeJA treatment. Mean ± se of n = 3.
(D) Nδ-acetylornithine accumulation 4 d after spraying with 450 μM MeJA, SA, ACC, or 100 μM ABA. Mean ± se of n = 3.
(E) Inhibition of Nδ-acetylornithine biosynthesis by SA. SA was added 24 h before, concomitant with MeJA, or 24 h later. Nδ-acetylornithine in rosette leaves was measured 4 d after the treatment. Mean ± se of n = 5.
(F) Nδ-acetylornithine in Arabidopsis defense signaling mutants, 4 d after MeJA treatment. Mean ± se of n = 4 or 5. *P < 0.05, t test relative to unelicited control plants or day 0 time point (C).
Research with Arabidopsis thaliana has identified diverse classes of secondary metabolites (D’Auria and Gershenzon, 2005), and, in particular, the defense-related glucosinolates have been studied extensively (Halkier and Gershenzon, 2006). However, very little is known about defensive nonprotein amino acids in Arabidopsis, and the majority of the estimated 5000 small molecules in a typical leaf remain unidentified (Bino et al., 2004). The plant hormone jasmonic acid (JA) and its more volatile form, methyl jasmonate (MeJA), induce numerous defense responses, including production of secondary metabolites, in Arabidopsis and other plants (Howe and Jander, 2008). Therefore, in an effort to identify and study previously unknown plant chemical defenses, we initiated a search for the MeJA-induced production of nonprotein amino acids in Arabidopsis. These experiments resulted in the identification of Nδ-acetylornithine, an Orn acetyltransferase that synthesizes this compound and likely defensive functions.
RESULTS
Induced Production of Nδ-Acetylornithine in Arabidopsis
Assays of phloem exudates from MeJA-induced Arabidopsis accession Columbia-0 (Col-0) showed accumulation of an unknown amino acid, which was determined to be Nδ-acetylornithine (Figure 1A) by mass spectrometry (MS) and NMR (see Supplemental Figure 1 online). Nδ-acetylornithine, which was not commercially available, was synthesized and shown to have the same properties as the purified Arabidopsis compound (see Supplemental Figures 1 and 2 online). The absolute configuration of Arabidopsis Nδ-acetylornithine was determined to be L by comparing the polarity of the purified Arabidopsis metabolite to synthesized l-Nδ-acetylornithine and a racemic d-l mixture. Nδ-acetylornithine, which has not been previously reported in Arabidopsis or other Brassicaceae, is distinct from Nα-acetylornithine (Figure 1A; see Supplemental Figure 2 online), an intermediate in plant Arg and Pro metabolism that is found in most, perhaps all, plants (Verslues and Sharma, 2010).
After treatment of with MeJA, Nδ-acetylornithine was detected in Arabidopsis leaves, stems, flowers, and roots (Figure 1B). Accumulation in rosette leaves peaked after 4 d and returned to undetectable levels by day 10 (Figure 1C), suggesting that there is also an Nδ-acetylornithine catabolic pathway in Arabidopsis. Other defense-related plant hormones were tested to determine whether they induce Nδ-acetylornithine accumulation. Nδ-acetylornithine abundance in leaves increased with increasing concentrations of exogenously added abscisic acid (ABA) (Figure 1D; see Supplemental Figure 3 online). By contrast, there was no detectable Nδ-acetylornithine after treatment with salicylic acid (SA) or the ethylene precursor 1-aminocyclopropane-1-carboxylate (ACC). SA inhibited the MeJA-induced production of Nδ-acetylornithine (Figure 1E), albeit only if it was applied 24 h before the MeJA. This is consistent with several prior studies showing antagonistic effects in plant defense induction and reduced effectiveness of MeJA elicitation once SA-mediated defenses are turned on (Thaler et al., 2002; Spoel et al., 2003; Mur et al., 2006). The nonexpresser of pr genes1 (npr1), salicylic acid induction deficient1 (sid1), and phytoalexin deficient4 (pad4) mutations, which result in defects in SA-related defense signaling, had no significant effect on Nδ-acetylornithine induction by MeJA (Figure 1F). Furthermore, these mutants did not have any detectable Nδ-acetylornithine in the absence of MeJA elicitation. By contrast, there was no MeJA-induced Nδ-acetylornithine accumulation in the coronatine insensitive1-1 (coi1-1) mutant (Figure 1F), indicating that induction requires a functional COI1 receptor, similar to most other JA-mediated responses in Arabidopsis (Chini et al., 2007; Thines et al., 2007).
Biosynthesis of Nδ-Acetylornithine from Other Amino Acids
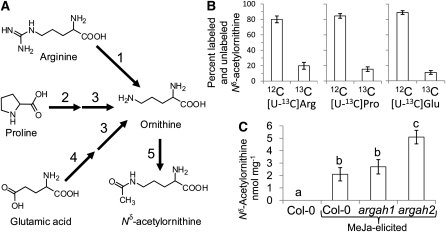
The structure of Nδ-acetylornithine suggested that it could be synthesized from Arg, Glu, or Pro, with Orn as an intermediate (Figure 2A; other pathways are also possible). If so, addition of [U-13C]Arg, [U-13C]Pro, or [U-13C]Glu to Arabidopsis leaves should result in the synthesis of [13C]Nδ-acetylornithine with a mass of +5 atomic mass units (see Supplemental Figure 4 online). As predicted, gas chromatography (GC)-MS analysis of Nδ-acetylornithine in MeJA-treated plants after the addition of 13C-labeled precursor amino acids showed significant incorporation from Arg, Pro, and Glu (Figure 2B). Although the labeling results showed a direct conversion of the three amino acids into Nδ-acetylornithine, the multiple pathways leading to Orn formation (Figure 2A) suggested that knockout of any one amino acid catabolic enzyme would not significantly reduce Nδ-acetylornithine accumulation. Consistent with this hypothesis, knockout mutations in either of the two Arabidopsis arginases (ARGININE AMINOHYDROLYASE1 [ARGAH1] and ARGAH2; Brownfield et al., 2008) did not decrease Nδ-acetylornithine abundance (Figure 2C). In fact, for as yet unknown reasons, the argah2 mutation increased MeJA-induced Nδ-acetylornithine levels.
Figure 2.
Biosynthesis of Nδ-Acetylornithine from Other Amino Acids.
(A) Possible pathways leading from Arg, Pro, and Glu to Nδ-acetylornithine. 1, Arginase (At2g39020 and At4g08900); 2, Pro dehydrogenase (At3g30775 and At5g38710); 3, Orn δ-aminotransferase (At5g46180); 4, pyrroline-5-carboxylate synthase (At2g39800 and At3g55610); 5, Orn Nδ-acetyltransferase (At2g39030).
(B) Incorporation of [U-13C]Arg, [U-13C]Pro, and [U-13C]Glu into Nδ-acetylornithine in MeJA-treated Arabidopsis leaves, expressed as the percentage of labeled [13C5]Nδ-acetylornithine and unlabeled [12C]Nδ-acetylornithine in rosette leaves. Mean ± se of n = 3.
(C) Nδ-acetylornithine accumulation in arginase mutants. Mean ± se of n = 5. Letters indicate significant differences, P < 0.05, analysis of variance, followed by Tukey’s HSD test.
Orn Acetyltransferase Mutant Isolation and Characterization
Given the incorporation of five carbon atoms from Arg, Pro, and Glu into Nδ-acetylornithine, we hypothesized that there is an Orn Nδ-acetyltransferase in Arabidopsis that converts Orn into Nδ-acetylornithine (Figure 2A). Analysis of publications reporting MeJA-regulated Arabidopsis gene expression showed elevated transcription of At2g39030, a member of the GNAT (Gcn5-related N-acetyltransferase) family of acetyltransferases (Vetting et al., 2005; Yan et al., 2007). Publicly available DNA microarray data also showed that At2g39030 is induced by JA, ABA, mechanical wounding, and a variety of biotic stresses, including Myzus persicae (green peach aphid) and Pseudomonas syringae (www.genevestigator.com; Zimmermann et al., 2004).
To determine whether At2g39030 encodes an Orn Nδ-acetyltransferase, we obtained the only available knockout line (GK-256F07; Rosso et al., 2003). Presence of a homozygous T-DNA insertion and lack of mRNA was confirmed by PCR and quantitative real-time RT-PCR (qRT-PCR), respectively. The mutant had no obvious visible defects and initiated flowering at the same time as wild-type Col-0. However, in contrast with the wild-type controls, no Nδ-acetylornithine was found in GK-256F07 leaves (Figure 3A), stems, flowers, and roots after MeJA treatment, indicating that At2g39030 encodes an Orn Nδ-acetyltransferase. We named the At2g39030 gene NATA1 (for N-Acetyltransferase Activity1) and the GK-256F07 mutant allele nata1-1. At2g39020, a gene that is directly adjacent to NATA1 in the Col-0 genome, encodes a predicted protein that is 78% identical to NATA1 at the amino acid sequence level and is also annotated as a putative GNAT. Amino acid analysis of the only available At2g39020 T-DNA insertion line (Salk_092319; Alonso et al., 2003) showed no change in the MeJA-induced accumulation of Nδ-acetylornithine (Figure 3A).
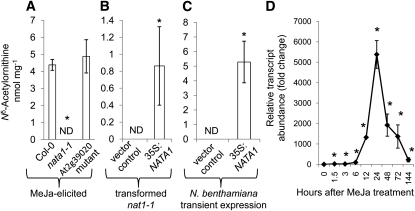
Figure 3.
Nδ-Acetylornithine Biosynthesis Requires NATA1 (At2g39030).
(A) Nδ-acetylornithine biosynthesis in nata1-1 and an At2g39020 T-DNA insertion mutant, 4 d after MeJA treatment. Mean ± se of n = 5. ND, not detected.
(B) Expression of p35S:NATA1 causes constitutive Nδ-acetylornithine production in nata1-1. Mean ± se of n = 3.
(C) p35S:NATA1 transient expression in N. benthamiana results in Nδ-acetylornithine production. Mean ± se of n = 5.
(D) NATA1 expression over time after MeJA treatment of wild-type Col-0. Mean ± se of n = 3 to 9. *P < 0.05, t test relative to control or 0 h time point.
The function of NATA1 in Nδ-acetylornithine biosynthesis was confirmed by complementing the nata1-1 mutation with a 35S:NATA1 construct, which caused constitutive production of Nδ-acetylornithine (Figure 3B). Although NATA1 overexpression decreased Orn and Pro levels and increased some other amino acids (see Supplemental Figure 5 online), there was no significant difference in the total free amino acid content between wild-type Col-0 and the 35S:NATA1 line. Nδ-acetylornithine also accumulated after transient NATA1 expression in Nicotiana benthamiana, which does not normally produce this amino acid (Figure 3C). By contrast, At2g39020 transient expression in N. benthamiana caused no detectable Nδ-acetylornithine accumulation. Together, these results confirmed that NATA1 functions in the biosynthesis of Nδ-acetylornithine in Arabidopsis.
NATA1 expression was induced by MeJA, with a significant, 20-fold increase observed within 1.5 h after treatment (Figure 3D) and a peak in transcript abundance after 24 h. Relative gene expression after 24 h in this experiment was quite high due to the very low, almost undetectable expression of NATA1 at the zero-hour time point. Over a period of several days after MeJA treatment, NATA1 expression declined back to basal levels. This gene expression pattern was consistent with the observed changes in Nδ-acetylornithine accumulation after MeJA treatment (Figure 1C), but with the increase in gene expression preceding metabolite accumulation.
P. syringae Induces Nδ-Acetylornithine Accumulation
The requirement of a functional COI1 gene for Nδ-acetylornithine accumulation (Figure 1F) suggested that infection with P. syringae, which produces the JA-Ile mimic coronatine, should also induce Nδ-acetylornithine production. Coronatine itself induced Nδ-acetylornithine accumulation in a concentration-dependent manner (Figure 4A). Spray inoculation with two virulent bacterial isolates, P. syringae pv tomato DC3000 (DC3000) and P. syringae pv maculicola 4326 (Psm4326), also induced Nδ-acetylornithine production (Figure 4A). However, there was much lower Nδ-acetylornithine accumulation after similar inoculation with coronatine-deficient P. syringae mutants (Brooks et al., 2004; Cui et al., 2005). Since coronatine inhibits stomatal closure, thereby facilitating successful P. syringae infection (Melotto et al., 2006), these experiments were done under high humidity conditions, which promote stomatal opening and allow similar entry and growth of coronatine mutant and wild-type P. syringae. In the flagellin-sensitive2 (fls2) mutant background, where the stomatal closure response to P. syringae is compromised and coronatine is not required for entry (Zeng and He, 2010), there was also reduced Nδ-acetylornithine induction by coronatine-mutant P. syringae (see Supplemental Figure 6 online). Inoculation of wild-type Col-0 with DC3000 avrRpt2 (Whalen et al., 1991) and DC3000 avrRpm1 (Century et al., 1995) did not cause Nδ-acetylornithine accumulation (Figure 4A). Two possible explanations for this lack of induction are enhanced Arabidopsis SA production in response to avirulent P. syringae and reduced coronatine production due to the slower growth of these strains.
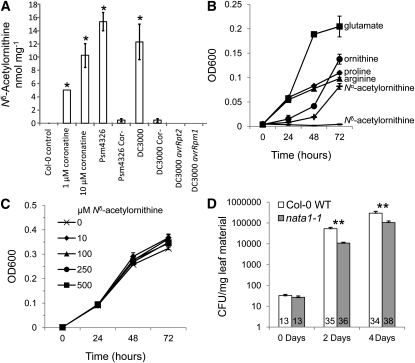
Figure 4.
Nδ-Acetylornithine Effects on P. syringae.
(A) Nδ-acetylornithine accumulation in wild-type Col-0 4 d after treatment with coronatine, coronatine-producing and nonproducing (Cor-) P. syringae strains Psm4326 and DC3000, and DC3000 carrying the avirulence genes avrRpt2 and avrRpm1. Mean ± se of n = 5, *P < 0.05, t test relative to control.
(B) Growth of P. syringae DC3000 in minimal maltose medium with 2 g L−1 of a single amino acid as the nitrogen source. Mean ± se of n = 3.
(C) Growth of P. syringae in minimal maltose medium with 0, 10, 100, 250, or 500 μM Nδ-acetylornithine and 2 g L−1 Glu as the nitrogen source. Mean ± se of n = 3.
(D) Growth of P. syringae DC3000 infiltrated into Col-0 wild-type and nata1-1 mutant leaves. CFU, colony-forming units. Numbers in bars indicate sample sizes ± se. **P < 0.01, t test.
P. syringae strain DC3000 was able to grow on minimal-maltose medium with Glu, Orn, Pro, Arg, or Nα-acetylornithine as the only nitrogen source (Figure 4B). However, there was no significant growth with Nδ-acetylornithine, showing that this amino acid cannot be used as a nitrogen source by P. syringae. When Nδ-acetylornithine was added to minimal medium containing Glu as a nitrogen source, there was no P. syringae growth inhibition (Figure 4C), indicating that Nδ-acetylornithine is not toxic or inhibitory. Further experiments were conducted in vivo to determine whether NATA1 activity affected bacterial growth in Arabidopsis. Compared with wild-type Col-0, P. syringae DC3000 grew significantly less well on nata-1 mutant plants (Figure 4D), suggesting that induction of NATA1 expression and Nδ-acetylornithine production by coronatine (Figure 4A) contributed to a successful bacterial infection.
Phloem Localization of Nδ-Acetylornithine
Previous research, including creation of a transgenic Arabidopsis line carrying a NATA1 promoter β-glucuronidase fusion, showed that this gene is expressed predominantly, though not exclusively, in phloem-associated tissue (Wenzel et al., 2008). On a weight-normalized basis, the NATA1 transcript was more abundant in the midveins of MeJA-treated plants than in the leaf blades (P < 0.05, t test; Figure 5A). Nδ-acetylornithine was also abundant in phloem exudates collected from MeJA-treated Arabidopsis rosettes (Figure 5B), where it constituted 12% of the free amino acids released from cut stems. Since aphid stylectomy experiments showed 49 mM total amino acid content in Arabidopsis phloem (Zhu et al., 2005), we estimated that there is 5.9 mM Nδ-acetylornithine in the phloem sap after MeJA elicitation. This concentration of Nδ-acetylornithine was higher than what we observed in assays of whole leaves and other Arabidopsis tissue (Figures 1B to 1F). MeJA elicitation in this experiment also caused a 24% decrease in exudation of the main phloem transport amino acids (Asn, Asp, Gln, and Glu; P < 0.01, t test; see Supplemental Figure 7 online), which together constitute ~70% of the total free amino acids in Arabidopsis phloem sap (Zhu et al., 2005).
Figure 5.
NATA1 Expression and Nδ-Acetylornithine Affect Arabidopsis–M. persicae Interactions.
(A) Expression of NATA1 in dissected leaf blades and midveins (inset), with and without 24 h MeJA elicitation. Mean ± se of n = 4 or 5.
(B) Nδ-acetylornithine in petiole exudates and aphids feeding for 1 d from plants treated 4 d earlier with MeJA. Mean ± se of n = 3.
(C) NATA1 expression with 25 aphids feeding on one leaf. Fold induction, with 0 h set to 1. Mean ± se of n = 3 or 4.
(D) Nδ-acetylornithine accumulation in Col-0 wild type after 4 d of M. persicae feeding. Mean ± se of n = 3.
(E) Aphid reproduction on artificial diet containing 0 (control), 0.5, 5, or 10 mM Nδ-acetylornithine. Mean ± se of n = 8.
(F) M. persicae reproduction on detached nata1-1 leaves with petioles in a tube containing Nδ-acetylornithine (see inset). Number of aphid progeny after 4 d. Mean ± se of n = 22.
(G) M. persicae reproduction wild type and nata1-1 after MeJA treatment. Mean ± se of n = 19.
(H) M. persicae reproduction on N. tabacum transiently expressing p35S:NATA1. Number of aphid progeny after 7 d. Mean ± se of n = 25.
(I) M. persicae reproduction on Arabidopsis wild-type Col-0 and nata1 mutant transformed with p35S:NATA1. Number of aphid progeny after 7 d. Mean ± se of n = 25 to 27. ND, not detected. *P < 0.05, t test relative to control samples.
Nδ-Acetylornithine Effects on Insect Herbivory
Given the abundance of Nδ-acetylornithine in Arabidopsis phloem exudates (Figure 5B), we predicted that this amino acid would also be ingested by phloem-feeding M. persicae. This was confirmed through amino acid analysis of whole aphids, which contained Nδ-acetylornithine after short-term (24 h) feeding from MeJA-induced Col-0 but not after feeding from uninduced plants (Figure 5B). Longer infestation with M. persicae induced both NATA1 gene expression (Figure 5C) and Nδ-acetylornithine accumulation (Figure 5D).
To determine whether there was a direct toxic or deterrent effect on M. persicae, we added Nδ-acetylornithine to an artificial diet that also contained the 20 protein amino acids. This caused a significant reduction in M. persicae progeny production (Figure 5E) at Nδ-acetylornithine concentrations that are comparable to those that we have observed in phloem exudates. Similarly, exogenous addition of Nδ-acetylornithine to nata1-1 leaves via their petioles (Figure 5F) significantly reduced aphid reproduction. However, in this detached-leaf experiment, Nδ-acetylornithine addition may also affect aphid reproduction through the reduced abundance of three amino acids (Gly, Arg, and Thr) in the treated leaves relative to controls (see Supplemental Figure 8 online). As a percentage of total amino acid content, Nδ-acetylornithine was 5.9-fold more abundant in the honeydew of M. persicae than in the artificial diet from which they were feeding (0.5 mM Nδ-acetylornithine = 0.29% of total amino acids in the artificial diet; 1.7% ± 0.3% Nδ-acetylornithine in the honeydew; mean ± se of n = 3; P < 0.05, t test). This indicated that Nδ-acetylornithine was taken up and/or metabolized less efficiently than the 20 protein amino acids the aphid diet. Consistent with this observation, addition of Orn or Gln, but not Nδ-acetylornithine, as the only nitrogen source in artificial diet increased aphid progeny production relative to a Suc-only control diet (see Supplemental Figure 9 online).
Prior work showed that MeJA treatment significantly decreases M. persicae reproduction on Arabidopsis (Ellis et al., 2002). This effect was confirmed with our M. persicae strain on flower stalks of wild-type Col-0 (Figure 5G). However, on nata1-1 mutant plants, there was no significant reduction in M. persicae reproduction due to MeJA treatment, which suggested that NATA1 plays a role in defense against aphids. Transient expression of NATA1 from the 35S promoter in tobacco (Nicotiana tabacum) caused Nδ-acetylornithine accumulation and decreased M. persicae progeny production relative to vector-only controls (Figure 5H). However, stable transgenic expression of the same construct in wild-type Col-0 or nata1-1 mutant Arabidopsis did not have a measureable effect (Figure 5I). Since Nδ-acetylornithine inhibition of M. persicae reproduction was concentration dependent (Figures 5E and 5F), this difference may have resulted from lower accumulation of Nδ-acetylornithine in p35S:NATA1 Arabidopsis than in transiently expressing p35S:NATA1 N. tabacum or MeJA-elicited Arabidopsis (Figure 3).
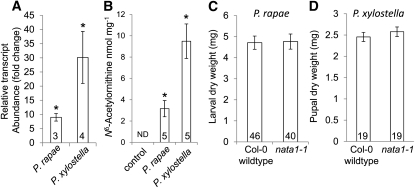
Feeding by two lepidopteran herbivores, Pieris rapae (white cabbage butterfly) and Plutella xylostella (diamondback moth), induced NATA1 transcription (Figure 6A) and Nδ-acetylornithine accumulation (Figure 6B). However, the nata1-1 mutation did not affect P. rapae (Figure 6C) or P. xylostella (Figure 6D) caterpillar growth.
Figure 6.
NATA1 Effects on P. rapae and P. xylostella Growth.
(A) NATA1 gene expression 3 d after one neonate caterpillar was placed on a 3-week-old Arabidopsis plant. Gene expression on control plants without caterpillars was set to 1.
(B) Nδ-acetylornithine accumulation 7 d after one neonate caterpillar was placed on a 3-week-old Arabidopsis plant.
(C) P. rapae dry weight 7 d after neonate larvae were placed on wild-type Col-0 or nata1-1.
(D) Pupal weight of P. xylostella that spent their entire larval growth on Col-0 or nata1-1. P values calculated with t tests. *P < 0.05, t test relative to controls. Numbers in bars indicate sample sizes ± se. ND, not detected.
Metabolic Changes Associated with the nata1-1 Mutation
Since glucosinolates have been implicated in Arabidopsis defense against both P. syringae and M. persicae (Bednarek et al., 2005; Kim and Jander, 2007; Clay et al., 2009; De Vos and Jander, 2009; Pfalz et al., 2009; Fan et al., 2011), we hypothesized that the nata1-1 mutation might alter glucosinolate accumulation. However, basal glucosinolate abundance was not changed in nata1-1 compared with wild-type Col-0 (see Supplemental Figure 10 online). Similar glucosinolate increases were also observed in nata1-1 and Col-0 after MeJA treatment, which indicated that the mutant is not compromised in this component of the Arabidopsis defense response.
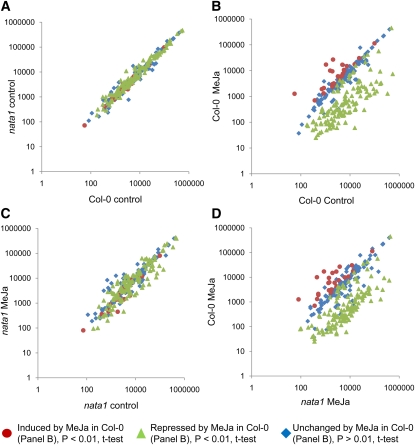
To identify other metabolic changes associated with NATA1 gene expression, we conducted GC-MS metabolite profiling of wild-type Col-0 and nata1-1, with and without MeJA treatment. Our assay detected 290 Arabidopsis metabolites, 73 of which were identified based on molecular standards (see Supplemental Data Set 1 online). In the absence of MeJA treatment, the metabolite abundance in Col-0 and nata1-1 was similar (Figure 7A; see Supplemental Data Set 1 online), as would be expected from the very low basal NATA1 expression (Figure 3D). Treating wild-type Col-0 with MeJA decreased the abundance of 133 metabolites, including free amino acids, sugars, and tricarboxylic acid cycle intermediates (triangles in Figure 7B; see Supplemental Data Set 1 online). There were 24 MeJA-induced metabolites detected in the MS assay (circles in Figure 7B), none of which had a known identity. By contrast, fewer metabolites were significantly altered (35 induced and 32 repressed; P < 0.01, t test) by MeJA treatment of the nata1-1 mutant (Figure 7C; see Supplemental Data Set 1 online). Agmatine, putrescine, and spermidine were induced by MeJA in the nata1-1 mutant but not in wild-type Col-0 (see Supplemental Figure 11 online). Most other metabolites that were altered by MeJA in Col-0 were affected to a lesser extent in nata1-1 (Figure 7D; see Supplemental Data Set 1 online). For instance, free Phe was reduced by 90% in Col-0 and 50% in nata1-1, and free Gln was reduced by 98% in Col-0 and 80% in nata1-1 by MeJA treatment. Since free amino acids as a source of nitrogen are of particular importance to aphid feeding (Wilson et al., 2010), these were measured in an independent assay (HPLC fluorescence detection) and were found to be significantly less repressed by MeJA in the nata1-1 mutant than in wild-type Col-0 (see Supplemental Figure 12 online). Although MeJA-induced metabolite changes were attenuated in the nata1-1 mutant, plant growth inhibition by MeJA was similar in nata1-1 and Col-0 (see Supplemental Figure 13 online), indicating that inhibition of primary metabolism can be separated from the growth-inhibitory effects of MeJA.
Figure 7.
Metabolite Changes Induced 4 d after MeJA Treatment of Wild-Type Col-0 and nata1-1 Mutant Plants.
(A) Comparison of metabolites in Col-0 and nata1-1 in the absence of MeJA treatment.
(B) Comparison of metabolites in Col-0 with and without MeJA treatment.
(C) Comparison of metabolites in nata1-1 with and without MeJA treatment.
(D) Comparison of MeJA-induced metabolites in Col-0 and nata1-1. Each data point represents the average of eight independent measurements. x and y axis units are arbitrary (mass spectrometer counts). Symbols represent the same group of metabolites in each figure, indicating those that are unaltered (diamonds), significantly induced (circles), or significantly repressed (triangles) by MeJA treatment of wild-type Col-0 in (B).
[See online article for color version of this figure.]
Nδ-Acetylornithine in Other Plant Species
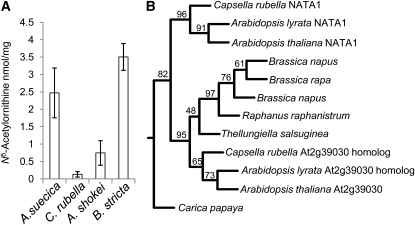
Amino acid analysis of 25 Arabidopsis accessions (see Supplemental Table 1 online) showed MeJA-induced production of Nδ-acetylornithine in every case. We also found MeJA-induced Nδ-acetylornithine accumulation in two other Arabidopsis species, as well as in Capsella rubella and Boechera stricta (Figure 8A). No Nδ-acetylornithine production was found in other tested Brassicaceae: Barbarea vulgaris, Brassica oleracea, Brassica rapa, Camelina sativa, Crucihimalaya lasiocarpa, Olimarabidopsis pumila, Sisymbrium irio, Thellungiella parvula, and Thellungiella salsuginea (see Supplemental Table 2 online). Analysis of publicly available sequence data (see Supplemental Data Set 2 online) and construction of a phylogenetic tree based on the predicted protein sequences showed likely At2g39020 homologs in several other Brassicaceae, but NATA1 homologs were found only in the genomes of C. rubella and Arabidopsis lyrata (Figure 8B). As in Arabidopsis, the NATA1 and At2g39020 homologs in the C. rubella and A. lyrata genomes were found to be tandem duplications, with the two genes ~80% identical in their predicted amino acid sequences.
Figure 8.
Presence of Nδ-Acetylornithine and NATA1-Like Proteins in the Brassicaceae.
(A) Nδ-acetylornithine accumulation after MeJA treatment of crucifer species closely related to Arabidopsis. Mean ± se of n = 3.
(B) Consensus phylogenetic tree of Brassicaceae NATA1 homologs produced with 1000 replicates. Carica papaya (Caricaceae) was used as an outgroup. Values at the branch points indicate bootstrap percentages. The alignment used to generate this tree is available as Supplemental Data Set 2 online.
Although Nδ-acetylornithine has been reported in some grasses (Fowden, 1958), we were unable to detect it in Zea mays, Oryza sativa, Brachypodium distachion, and Setaria viridis. Tested solanaceous species (Solanum tuberosum, Solanum lycopersicum, N. tabacum, and N. benthamiana) did not produce Nδ-acetylornithine, with or without MeJA treatment. Consistent with reports of Nδ-acetylornithine in the seeds of other legume species (Brown and Fowden, 1966; Zacharius, 1970; Kite and Ireland, 2002), Medicago truncatula cv Jemalong had 0.035 ± 0.003 nmol/gram Nδ-acetylornithine in the seeds. There was no detectable Nδ-acetylornithine in the leaves of this M. truncatula accession, with or without MeJA treatment.
DISCUSSION
By finding Nδ-acetylornithine and an acetyltransferase (NATA1, At2g39030) that synthesizes this nonprotein amino acid in Arabidopsis, we identified a new MeJA-inducible defense response. NATA1 likely contributes in more than one way to the induced M. persicae resistance that we and others have observed. Our experiments with Nδ-acetylornithine added to aphid artificial diet (Figure 5E) and Arabidopsis leaves (Figure 5F) suggest a direct toxic or deterrent effect. However, NATA1 likely also enhances aphid resistance by converting essential phloem amino acids into a form that the insects cannot utilize.
Different resource utilization by aphids, caterpillars, and P. syringae may account for the differential effects of the nata1-1 mutation on these organisms. Whereas aphids depend on nutrients from phloem sap, which contains nitrogen mostly in the form of free amino acids, caterpillars and P. syringae benefit from the much more abundant protein-bound amino acids and other forms of nitrogen in whole plant tissue. Arabidopsis foliage has 70 mg nitrogen/g dry weight (Lemaître et al., 2008), but only ~2% (1.5 mg nitrogen/g dry weight) is in the form of free amino acids (Lam et al., 2003; Lemaître et al., 2008). Therefore, changes in the abundance of free amino acids, such as those observed due to the nata1-1 mutation (see Supplemental Figure 12 online), would be have a bigger effect on aphids than on caterpillars and P. syringae.
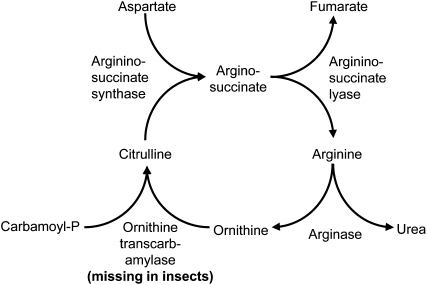
Metabolic differences between aphids and caterpillars may also influence the effects of Nδ-acetylornithine on insect herbivory. Insects lack Orn transcarbamylase (Figure 9) and are unable to synthesize Arg from Orn (Reddy and Campbell, 1977; Adams et al., 2000; Honeybee Genome Sequencing Consortium, 2006; Richards et al., 2008). If plant arginase already makes Arg unavailable for insects (Chen et al., 2005), then subsequent conversion of Orn to Nδ-acetylornithine might not provide any additional defensive benefit. Although aphids lack all of the enzymes of the urea cycle (Figure 9; Wilson et al., 2010), they have obligate endosymbiotic bacteria in the genus Buchnera that can produce Arg from Orn (Shigenobu et al., 2000). Therefore, conversion of Arg, Pro, and Glu to Nδ-acetylornithine (Figure 2B) would make nitrogen in phloem sap less available for aphids.
Figure 9.
The Urea Cycle Allows Synthesis of Arg from Orn.
This metabolic cycle is incomplete in insects due to lack of Orn transcarbamylase, thereby preventing synthesis of Arg from Orn. Aphids lack all enzymes of the urea cycle but contain endosymbiont Buchnera bacteria that are capable of Arg biosynthesis from Orn.
Observations of reduced M. persicae growth after MeJA treatment (Ellis et al., 2002) and improved growth on a coi1 mutant relative to the wild type (Mewis et al., 2005) show that the JA signaling pathway contributes to aphid defense in Arabidopsis. However, although caterpillar herbivory strongly induces JA-related genes, this is not the case for aphid feeding (Bidart-Bouzat and Kliebenstein, 2011). These differences in JA induction likely cause greater Nδ-acetylornithine accumulation in response to caterpillars (Figure 6B) than aphids (Figure 5D) and may represent active suppression of JA-regulated defenses by phloem-feeding insects (Zarate et al., 2007). ABA-regulated NATA1 expression also causes elevated Nδ-acetylornithine accumulation (Figure 1D). Publicly available microarray data show overlapping gene expression patterns induced by aphid feeding and ABA (www.genevestigator.com; Zimmermann et al., 2004), and heavier aphid infestation of Arabidopsis induces wilting that could lead to ABA-regulated gene expression changes.
Several prior studies show that coronatine promotes P. syringae growth on Arabidopsis and tomato (Feys et al., 1994; Kloek et al., 2001; Zhao et al., 2003; Cui et al., 2005; Laurie-Berry et al., 2006; Uppalapati et al., 2007, 2008, 2011; Fernández-Calvo et al., 2011). Although induction of JA-regulated gene expression by coronatine likely suppresses SA-mediated plant defenses, other molecular mechanisms that connect COI1 to increased P. syringae growth remain unknown. Our observation of improved P. syringae growth in the nata1-1 mutant background (Figure 4D) suggests that some of the observed NATA1-requiring metabolic changes (Figure 7) contribute to pathogen resistance. However, more direct effects on Orn-related metabolism also may be relevant for P. syringae virulence. WIN1 (At1g80600), a predicted Nα-acetylornithine transaminase, interacts directly with the P. syringae effector HopW1-1, and WIN1 overexpression prevents elicitation of Arabidopsis defenses by HopW1-1 and other P. syringae effectors (Lee et al., 2008).
In proceeding from the discovery of Nδ-acetylornithine, a previously unknown Arabidopsis metabolite, to the elucidation of a biosynthetic pathway and defense-related functions, we applied several of the excellent genetic and genomic resources that are available for Arabidopsis. Many recent studies of Arabidopsis defense mechanisms started with the identification of defense-related gene expression or mutants to identify new metabolites and signaling pathways. However, given the well-annotated Arabidopsis genome and extensive gene expression data sets, it now may be easier to move from identifying a novel plant metabolite to the discovery of a previously unknown enzymatic activity than vice versa. Our results, which have implications for research on both plant–pathogen and plant–herbivore interactions, provide new insight into the induction of biotic stress responses in plants.
METHODS
Plants and Growth Conditions
Seeds of Arabidopsis thaliana accessions (see Supplemental Table 1 online) and other Brassicaceae (see Supplemental Table 2 online) were obtained from the ABRC (www.Arabidopsis.org), T. Mitchell-Olds (Duke University, Durham, NC), and J. Jaworski (Danforth Center, St. Louis, MO). Seeds of other species were supplied by T. Brutnell (Boyce Thompson Institute, Ithaca, NY; Zea mays, Brachypodium distachion, and Setaria viridis), M. Kovach (Cornell University, Ithaca, NY; Oryza sativa), and N. Pumplin (Boyce Thompson Institute, Ithaca, NY; Medicago truncatula). Seeds of homozygous mutant argah1-1 (SALK_057987; Alonso et al., 2003) and argah2-1 (SAIL_181_C11; Sessions et al., 2002) were from C. Todd (University of Saskatchewan, Saskatoon, Canada; Flores et al., 2008). Homozygous fls2 mutant seeds were supplied by S. He (Michigan State University, East Lansing, MI; Zeng and He, 2010). Arabidopsis coi1 (Feys et al., 1994), npr1 (Cao et al., 1994), sid1 (Nawrath et al., 2002), and pad4 (Glazebrook et al., 1996) mutants were obtained from F. Ausubel (Massachusetts General Hospital, Boston, MA). The T-DNA insertion lines GK-256F07 (nata1-1; Rosso et al., 2003) and SALK_092319 (At2g39020 mutation; Alonso et al., 2003) were obtained from the ABRC. All plants were grown in Cornell mix (by weight 56% peat moss, 35% vermiculite, 4% lime, 4% Osmocoat slow-release fertilizer [Scotts], and 1% Unimix [Peters]) in 20 × 40-cm nursery flats in Conviron growth chambers with a photosynthetic photon flux density of 200 mmol m−2 s−1 and a 16-h photoperiod at 23°C with a 50% relative humidity.
Isolation and Purification of Nδ-Acetylornithine
Five-week-old Arabidopsis plants were sprayed with 0.45 mM MeJA, and ~620 g leaves were harvested, frozen with liquid nitrogen, and homogenized in 1.5 liters of 80% methanol. The extract was concentrated by evaporation and partitioned with dichloromethane. The aqueous fraction was passed through DEAE-Sephadex A-25 column to extract the glucosinolate fraction for a separate experiment. The eluate from the Sephadex A-25 was then passed through a packed column of Dowex-50WX8-200 ion-exchange resin. The column was washed repeatedly with water, and amino acids were eluted with 200 mL 4 n NH4OH followed by 100 mL water. Pooled eluate was lyophilized to leave 237 mg extract, which was then dissolved in 3 mL triethylamine (TEA) solution (2:2:1 of ethanol:water:TEA). The extract was then derivatized for 30 min in 1.26 mL phenylisothiocyanate (PITC) solution (980 μL ethanol, 70 μL water, 70 μL TEA, and 140 μL PITC). The reaction mixture was concentrated to dryness, taken up in 400 μL TEA solution, and concentrated to dryness again, leaving 680 mg residue. The extract was successively fractionated with Sephadex-LH-20 and RP18 packed columns and eluted with 95:5 water:methanol. The fraction containing the target compound, retention time = 29.90 min, was tracked by HPLC-DAD (Waters 2695 pump system and 996 diode array detector) using an RP18 column (Nova-Pak). HPLC Solvent A was 140 mm sodium acetate with 0.05% TEA (final pH adjusted to 6.33 with acetic acid). Solvent B was 60% acetonitrile. The gradient used was as follows: 0 to 10 min, 99% A; 10 to 35 min, linear gradient to 65% A; 35 to 40 min, 65% A; 40 to 42 min, linear gradient to 100% B; 42 to 44 min, 100% B. Further purification was achieved using a Lichrosphere column with water and acetonitrile as solvents to produce 1.1 mg of the target compound. For the HPLC runs (Lichrosphere column: 5 RP-18, ECAP 250, 4.6-mm inside diameter, 5-mm particle size) the mobile phases were A, water, and B, 90% acetonitrile, at a flow rate of 1.1 mL min−1 at 23°C. Column linear gradients for the samples were as follows: 0 to 1 min 75% A; 1 to 10 min, 60% A; 10 to 20 min 40% A; 20 to 30 min, 25% A; 30 to 35 min, 0% A; 35 to 40 min, 75% A.
The purified compound was analyzed by NMR. The (+ve)-ESI-MS spectrum showed an abundant molecular ion [M+PITC+Na]+ peak at 332, in addition to 175 [M+H-PITC]+ and 310 [M+H+PITC]+. In (–ve)-ESI-MS mode, the molecular ion was also detected with a peak [M+PITC-H]− at mass-to-charge ratio (m/z) 308 in addition to 173 [M-H-PITC]−. Therefore, a molecular weight of 174 was assigned to the isolated compound. From the 1HNMR, HMBC, and ESI-MS data, the isolated compound was assigned the structure of Nδ-acetylornithine. To further confirm the assigned structure, the isolated compound was compared with all possible acetylated products obtained after the treatment of l-Orn with glacial acetic acid and acetic acid anhydride after PITC derivatization. The isolated compound coeluted with Nδ-acetylornithine (retention time = 29.90 min.). Optical rotation measurements to determine whether Arabidopsis Nδ-acetylornithine has the L or D configuration were performed with a Polarimeter 241 (Perkin-Elmer) at 589 nm at 20°C using a sodium lamp.
Synthesis of Nδ-Acetylornithine
Nδ-Acetylornithine was synthesized from l-Orn and 4-nitrophenyl acetate (Leclerc and Benoiton, 1968). l-Orn monohydrochloride (0.5 g, 5.95 mmol) was dissolved in 5 mL water, and the pH was adjusted to 11 with 2 M NaOH. 4-Nitrophenyl acetate (1.07 g, 11.9 mmol) was then added and the mixture was stirred on ice. The mixture was stirred at room temperature for 4 h, concentrated to dryness with a Rotavap evaporator (Buchi), dissolved in 2 mL water, and partitioned repeatedly with ethyl acetate. The aqueous fraction containing the target compound was concentrated to dryness and redissolved in water followed by treatment with 0.1 M HCl to pH 4, until the mixture became colorless. Ethanol was added to the mixture and the precipitate was filtered with Buchner funnel to give a white precipitate (0.40 g). The precipitate was washed repeatedly with ethanol. The identity of synthesized Nδ-acetylornithine was confirmed by 1D- and 2D-NMR and ESI-MS. The corresponding ESI-MS spectral data are as follows: ESI-MS (negative, m/z, relative abundance): [M-H]− (m/z); [173] (100).
GC-MS (Varian 1200L; Agilent Technologies) analysis of N-methyl-N-(trimethylsilyl)trifluoroacetamide + 1% trichloromethylsilane derivatized synthetic Nδ-acetylornithine (retention time = 23.5 min [M+2TMS] and retention time = 23.95 min [M+1TMS]) was identical to the induced endogenous Arabidopsis Nδ-acetylornithine. Coelution of the synthetic and the naturally occurring compounds both on HPLC and GC further confirmed its identity. The 1H-NMR spectral data of the synthesized Nδ-acetylornithine were identical to those of earlier reports (Wohlfarth et al., 1993). The 1H-NMR (D2O) spectrum of Nδ-acetylornithine established the following: 1H-NMR (500 MHz, D2O) δ: 3.59 (1H, t, J = 6 Hz), 3.09 to 3.02 (2H, m), 1.82 (3H, s), 1.76 to 1.66 (2H, m), 1.49 to 1.36 (2H, m).The 13C-NMR extracted from the HMBC data revealed seven carbon atoms: 174.33, 174.08, 54.57, 38.63, 27.83, 24.26, and 22.02. GC-MS data of Nδ-acetylornithine with 2-TMS derivatives gave an EI spectrum with m/z (%) 318 [1.7, M+2TMSi], 201 (22, M–CO2TMSi), 112 (100, M-C2NH4O2-2TMSi), 75(76), and 73(55).
Amino Acid Assays
For analysis of leaf amino acids, 100 mg of plant tissue was frozen in liquid nitrogen in 2-mL microcentrifuge tubes and ground to fine powder with 3-mm steel beads using a Harbil model 5G-HD paint shaker. Ground tissue was taken up in 20 mM HCl (10 μL/mg of tissue), the extracts were centrifuged at 3800g for 20 min at 23°C, and the supernatant was saved for analysis. Phloem sap was collected from rosettes of 3-week-old Col-0 plants sprayed with water or 0.45 mM MeJA treatment. Roots were cut from the rosettes, leaving 1 mm of the shoot tissue, and put in 100 μL of 15 mM EDTA solution, pH 7.7, for 4 h in the dark with high humidity. Fifteen samples were pooled for each replicate of the experiment. For analysis of Nδ-acetylornithine uptake, nata1-1 leaf petioles were immersed in Nδ-acetylornithine solutions in a 1.5-mL microcentrifuge tube for 24 h prior to tissue harvest. The petiole and other portions of the leaf that were immersed in the Nδ-acetylornithine solution were not used for the amino acid analysis. For the measurement of Nδ-acetylornithine in aphid bodies, aphids were collected from Col-0 plants treated with water or MeJA and transferred into a 2 mL centrifuge tube containing three 3-mm steel balls. The aphids were homogenized and extracted with 20 mM HCl. For aphid honeydew collection, ~50 aphids were placed on artificial diet with and without Nδ-acetylornithine for 4 d, and honeydew was collected on aluminum foil. The aluminum foil was washed with 80% methanol to extract amino acids. The methanolic extract was concentrated to dryness a SpeedVac rotary evaporator and then redissolved in 40 μL 20 mM HCl.
Amino acids were derivatized with 6-aminoquinolyl-N-hydroxysuccinimidylcarbamate using an AccQ-Fluor reagent kit (Waters). For derivatization, 5 μL extracts were mixed with 35 μL borate buffer and the reaction was initiated by the addition of 10 μL 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate reagent, followed by immediate mixing and incubation for10 min at 55°C. Ten microliters of each sample were injected onto a Nova-Pak C18 column using a Waters 2695 pump system, and the data were recorded using Waters’ Empower Software. l-Norleucine was used as an internal standard. Eluted amino acid derivatives were detected using a Waters model 2475 fluorescence detector with an excitation wavelength of 250 nm and an emission wavelength of 395 nm. Solvent A (containing sodium acetate and TEA at pH 5.05) was purchased premixed from Waters; Solvent B was acetonitrile:water (60:40). The gradient used was 0 to 0.01 min, 100% A; 0.01 to 0.5 min, linear gradient to 3% B; 0.5 to 12 min, linear gradient to 5% B; 12 to 15 min, linear gradient to 8% B; 15 to 45 min, 35% B; 45 to 49 min, linear gradient to 35% B; 50 to 60 min, 100% B. Flow rate was 1.0 mL min−1.
Glucosinolate Assays
Leaves of 3-week old Col-0 wild-type nata1-1 Arabidopsis, with or without MeJA treatment, were collected and lyophilized. Extraction of plant tissue and preparation of desulphoglucosinolates was done as described previously (Kim et al., 2004; Barth and Jander, 2006). Desulphoglucosinolates were separated using a Waters 2695 HPLC and detected using a Waters 2996 photodiode array detector. For HPLC separation, the mobile phases were A, water, and B, 90% acetonitrile, with a flow rate of 1 mL/min at 23°C. Column linear gradients for samples were: 0 to 1 min, 98% A; 1 to 6 min 94% A; 6 to 8 min, 92% A; 8 to 16 min, 77% A; 16 to 20 min, 60% A; 20 to 25 min, 0% A; 25 to 27 min hold 0% A; 27 to 28 min, 98% A; 28 to 37 min, 98% A.
MeJA, SA, ACC, ABA, and Coronatine Induction
The leaves of 4-week-old Arabidopsis plants were sprayed with aqueous solution containing 0.01% (v/v) Tween 20, supplemented with each of 0.45 mM SA, 0.45 mM MeJA, 0.45 mM ACC, and 0.1 mM ABA, a combination of both SA and MeJA, 1 μM coronatine, or 10 μM coronatine. Control plants were treated with 0.01% Tween 20 only. Controls for MeJA treatment also contained 0.03% acetone. Plants were covered and harvested 4 d after elicitation (or at multiple times for a time course of induction) and immediately frozen in liquid nitrogen. Pulverized plants were extracted with 20 mM HCl, and amino acids were analyzed as described above.
For time-course experiments, 2-week-old Arabidopsis plants were treated with 0.45 mM MeJA, and leaves were harvested after 0 to 15 d. Leaves were frozen in liquid nitrogen and stored at −80°C before amino acid analysis as described above. For a separate experiment, the whole aerial parts were cut just above the root and weighed.
Stable Isotope Labeling Experiments
Petioles of a detached Col-0 leaves from 4-week-old plants were inserted into 1.5 mL microcentrifuge tubes containing 5 mM labeled amino acid ([13C5]Glu, [13C6]Arg, and [13C5]Pro) in water. In the case of Glu, 0.01% NaOH was used to aid solubility. Control leaves were treated with water only and allowed to stand for 12 h. After 12 h, leaves were sprayed with 0.45 mM MeJA. Leaf samples were covered and allowed to stand for 3 d. Three leaves receiving the same treatment were combined as one replicate for derivatization and GC-MS analysis of amino acids, as described above.
Amino acid analysis by GC-MS was performed as described previously (Joshi and Jander, 2009) with minor modifications. Single leaves harvested from Arabidopsis Col-0 wild type and nata1-1 mutant at the same developmental stages were used. Leaves were frozen in liquid nitrogen in 2-mL tubes and ground to fine powder with 3-mm steel beads using a Harbil model 5G-HD paint shaker. Ground tissue was taken up in 20 mM HCl (1 mL per leaf tissue), the extracts were centrifuged at 4000g for 20 min at 23°C, and the supernatant leaf acid extracts were applied to Dowex-50 columns (0.6 mL). These were eluted individually with 6 M NH4OH (1 mL). Fractions were combined, and solutions were concentrated to 200 μL with a rotary evaporator. These extracts were then completely dried under nitrogen flow, with heating at 70°C. Methoxyamine hydrochloride in anhydrous pyridine (50 μL, 15 mg mL−1) was added to the residue and the sample was heated at 30°C for 90 min. A volume of 90 μL N-methyl-N-(trimethylsilyl)trifluoroacetamide + 1% trimethylchlorosilane was then added, and the sample was heated for a further 30 min at 70°C. GC-MS analysis was performed using a Varian 1200L GC-MS with a DB-17 ms capillary column. Spectra of known amino acids were assigned by reference to an in-house spectral library and the NIST library.
Confirmation of T-DNA Insertions and Expression of Transgenes
Homozygous T-DNA insertions in NATA1 (GK-256F07; Rosso et al., 2003) and At2g39020 (Salk_092319; Alonso et al., 2003) were confirmed by PCR using a previously outlined strategy using three primers to amplify both the insertion junction and the genomic DNA without an insertion (http://signal.salk.edu/tdnaprimers.2.html) and primers described in Supplemental Table 3 online. PCR reactions were run using Taq DNA polymerase (Fisher) under the following conditions: 95°C for 5 min, 40 cycles of 95°C for 1 min, 55°C for 1 min and 72°C for 1 min, followed by 72°C for 10 min.
For transgenic expression, NATA1 and At2g39020 were cloned behind the cauliflower mosaic virus 35S promoter in the T-DNA binary vector pMDC32 (Curtis and Grossniklaus, 2003) using the Gateway recombination system (Invitrogen), and inserts were confirmed by DNA sequencing. Stable Arabidopsis p35S:NATA1 transgenics were made by dipping transformation with Agrobacterium tumefaciens strain GV3101 (Clough and Bent, 1998). Seeds collected from transformed Arabidopsis plants were selected on agar containing 40 mg mL−1 hygromycin, and transgene expression was confirmed by qRT-PCR. The same T-DNA plasmids in GV3010 were used for transient expression of NATA1 and At2g39020 in tobacco (Nicotiana benthamiana and Nicotiana tabacum) by Agrobacterium infiltration (Voinnet et al., 1998). Agrobacterium was cultured overnight at 30°C in Luria-Bertani (LB) broth supplemented with 50 μg mL−1 rifampicin, 25 μg mL−1 gentamycin, and 100 μg mL−1 kanamycin. Bacterial cultures were centrifuged at 8000g, washed three times with sterile water, and resuspended in sterile water to 0.2 × 108 colony-forming units/mL. Cultures were mixed with Agrobacterium (0.1 × 108/mL) carrying T-DNA constructs expressing the turnip crinkle virus capsid protein (P38; Thomas et al., 2003) to reduce expression silencing. N. benthamiana or N. tabacum leaves (four leaves per plant) were infiltrated with 1 mL bacterial solution using a 1 mL syringe. Excess bacterial solution was wiped off with paper towel. Three days after infiltration, leaf plugs (8-mm diameter) were collected to confirm expression of the transgenes by qRT-PCR.
qRT-PCR
Total RNA was extracted from frozen tissue samples using the SV Total RNA Isolation system with on-column DNase treatment (Promega). RNA integrity was verified using a 1.2% formaldehyde agarose gel (Sambrook et al., 1989). Transcript abundance of NATA1 was analyzed with qRT-PCR, using eEF1-α (elongation factor 1-α, At5g60390) as an internal standard. eEF1-α was identified from publicly available microarray data as constitutively expressed before and after herbivory, and stable expression was verified across samples using qRT-PCR. After RNA extraction and DNase treatment, 1 μg of total RNA was reverse transcribed with SMART MMLV reverse transcriptase (Clonetech) using oligo-dT12-18 as a primer. Gene-specific primers used for qRT-PCR were designed using Primer-Blast (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) with the following criteria: temperature of 60°C, PCR amplicon lengths of 90 to 150 bp, primer sequences with lengths of 18 to 24 nucleotides with an optimum at 21 nucleotides, and GC contents of 40 to 60%. Primers for amplifying NATA1 and eEF1-α are described in Supplemental Table 3 online. Reactions were performed using 5 μL of the SYBR Green PCR master mix (Applied Biosystems), with 800 nM of primer, in the 7900HT instrument (Applied Biosystems). The PCR was initiated by incubation at 95°C for 10 min to activate the enzyme. Then the following cycle was repeated 40 times: 95°C for 15 s, 60°C for 15 s, and 72°C for 15 s. The CT values were quantified and analyzed according to the standard curve method.
GC-MS Metabolomics
For metabolomic analysis, 3-week-old wild-type and nata1-1 mutant plants treated with water or 0.45 mM MeJA. After 4 d, 6.5-mm disks was taken from the central part of the first and second adult leaves of each plant. The two disks were combined and stored into a 2-mL microcentrifuge tubes put on ice. One milliliter of prechilled extraction buffer (acetonitrile:isopropanol:water [3:3:2]) and one metal ball were added to the sample. Plant tissue was macerated briefly for 30 s using a Harbil model 5G-HD paint shaker. Extracts were centrifuged at 4000g for 2 min at 4°C. Supernatants from each sample (400 μL) were transferred into new 2-mL tubes and stored at −80°C until automated derivatization and GC-TOF-MS analysis at the UC Davis Genome Center Metabolomics Facility (http://metabolomics-core.ucdavis.edu/; Fiehn et al., 2005). Metabolite identity was determined by comparing retention time and mass to the UC Davis Genome Center Metabolomics Facility metabolites database (http://fiehnlab.ucdavis.edu/Metabolite-Library-2007/; Fiehn et al., 2005). This library contains reference spectra for 713 known metabolites, generated by the analysis of purified reference compounds. Metabolites not contained within this library are listed as unknown or unidentified metabolites. After analysis, samples were subject to quality control by further analysis of samples with internal standard values >3 σ from the experimental mean within a given experiment, suggesting faulty derivatization or GC-TOF analysis of that specific sample.
Bacterial Bioassays
Pseudomonas syringae strains were supplied by H.G. Kang (Boyce Thompson Institute, Ithaca, NY; DC3000 AvrRpt2 and DC3000 AvrRpm1), C. Danna (Massachusetts General Hospital, Boston, MA; Psm4326 and Psm4326 Cor-), and N. Clay (Yale University, New Haven, CT; PstDC3000 and PstDC3000 Cor-). For assays of Nδ-acetylornithine production in Arabidopsis, P. syringae was cultured at 30°C in LB broth. Bacteria were centrifuged and resuspended in water at an OD600 of 0.2. Bacterial suspensions containing 0.01% Tween 20 were sprayed on Arabidopsis plants. Control plants are sprayed with water containing 0.01% Tween 20 only. After 4 d, infected leaves were collected for amino acid analysis as described above.
For in vitro growth experiments, P. syringae strain DC3000 was cultured at 30°C in modified mannitol-glutamate medium (Kean et al., 1970; per liter: 10 g mannitol, 2 g L−1 Glu, 0.5 g KH2PO4, 0.2 g NaCl, 0.2 g MgSO4-7H2O; pH adjusted to 7.0 with 3 n NaOH prior to autoclaving). To test growth with other amino acids as nitrogen sources, Glu was replaced successively with 2 g L−1 Arg, Pro, Orn, Nα-acetylornithine, and Nδ-acetylornithine. Cultures were inoculated by diluting a fresh overnight culture of P. syringae 1:100 (50 μL into 5 mL). OD600 was measured at 0, 14, 48, and 72 h after treatment. For growth inhibition experiments, cultures were grown in minimal medium with 2 g L−1 Glu and 0, 10, 100, 250, or 500 μM Nδ-acetylornithine.
For in planta growth experiments, P. syringae PstDC3000 was cultured at 30°C in LB broth supplemented with 50 μg mL−1 rifampicin, centrifuged, resuspended in sterile water, and diluted to a concentration of 105 colony-forming units/mL. Col-0 wild-type and nata1-1 leaves were infiltrated with 0.2 mL bacterial solution using a 1-mL syringe. Excess bacterial solution was wiped off with paper towel. Leaf plugs (8-mm diameter) were collected at 0, 2, and 4 d after infiltration. Leaf discs were floated in 1 mL sterile water with 0.01% Tween 20 on a shaker for ~2 h. Ten-microliter samples of a bacterial dilution series were spotted on an LB agar plate containing 50 μg mL−1 rifampicin and incubated at 30°C. Bacterial colonies were counted after 2 d, and the concentration of bacteria in the leaf plugs was calculated.
Caterpillar Bioassays
The Pieris rapae were from a colony maintained by the Jander laboratory, which is descended from 20 adult insects collected in the wild on the Cornell University campus in July, 2008. Plutella xylostella eggs were obtained from Benzon Research. Neonate caterpillars were confined on the leaves of 3-week-old wild-type and nata1-1 plants using mesh-covered cups. P. rapae were allowed to feed on plants for 7 d before harvesting. P. xylostella were harvested after pupation. Caterpillars and pupae were lyophilized and dry weight was determined using a precision balance (Sartorius). Mutant and wild-type leaves infested by caterpillar were also collected for amino acid analysis as described above.
Aphid Bioassays
Artificial diet for Myzus persicae fecundity assays consisted of Suc (440 mM) and 20 amino acids (Ala, 10 mM; Arg, 16 mM; Asn, 20 mM; Asp, 10 mM; Cys, 3.3 mM; Glu, 10 mM; Gln, 10 mM; Gly, 10 mM; His, 10 mM; Ile, 6 mM; Leu, 6 mM; Lys, 10 mM; Met, 5 mM; Phe, 3 mM; Pro,7 mM; Ser, 10 mM; Thr, 12 mM; Trp, 4 mM; Tyr, 2 mM; Val, 7 mM). Zero, 0.1, 0.5, 5, or 10 mM Nδ-acetylornithine was added to the diet. For experiments with a single amino acid as the nitrogen source, Orn, Glu, and Nδ-acetylornithine were added at 10 mM concentration to 440 mM Suc. One wingless adult aphid was placed in a 30-mL plastic cup that was covered with a Parafilm sachet containing 100 μL of the liquid diet. Aphid nymphs were counted after 4 d.
M. persicae reproduction was compared on Arabidopsis Col-0 wild type and nata1-1, with and without MeJA treatment. Plants were covered after MeJA application for 48 h. The cover was then removed 48 h prior to the caging aphids on Arabidopsis flower stalks or rosette leaves. Plant trays with and without MeJA were separated and covered to prevent volatiles induced by MeJA from eliciting defenses in control plants. Aphid progeny production was recorded after 7 d. For N. tabacum experiments, aphids were caged on individual leaves in the area where Agrobacterium constructs had been infiltrated 3 d previously, and progeny production was assessed 7 d later.
To assess effects of exogenously added Nδ-acetylornithine, petioles from mature leaves of 4-week-old nata1-1 plants were inserted in 1.5-mL microcentrifuge tubes containing 0, 0.1, or 0.5 mM Nδ-acetylornithine in water. After 24 h, one aphid was placed on each individual leaf, and aphid progeny production was recorded after 4 d.
Sequence Alignment and Phylogenetic Tree Generation
Arabidopsis NATA1 homologs in the Brassicaceae (Arabidopsis lyrata, Brassica rapa, Brassica napus, Raphanus raphanistrum, and Capsella rubella) were obtained by comparison to genomic and EST data in GenBank. The closest NATA1 homolog of Carica papaya in the Caricaeae, a related family in the Brassicales, was used as an outgroup. Multiple sequence alignment of translated protein sequences was performed with ClustalW (Larkin et al., 2007). Programs in the PHYLIP version 3.6 software package (Felsenstein, 2005) were used to create a phylogenetic tree. The SEQBOOT function was used to make 1000 bootstrapped data sets. PROMLK was used to construct rooted phylogenetic trees, using the parameters search for the best tree, Jones-Taylor-Thornton probability model, one category of substitution rates, constant rate variation among sites, no global rearrangement, no weighted sites, and randomization of input sequences. A rooted consensus tree was made with CONSENSE using the majority rule (extended) parameter.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: At2g39030 (NATA1), At2g39020, At4g08900 (ARGAH1), At4g08870 (ARGAH2), At3g30775 (ProDH1), At5g38710 (ProDH2), At2g39800 (P5CS1), At3g55610 (P5CS2), At5g46180 (OAT), At1g80600 (WIN1), At5g46330 (FLS2), At4g39030 (SID1), At1g64280 (NPR1), and At2g39940 (COI1). National Center for Biotechnology Information accession numbers for the sequences used for the phylogenetic analysis are as follows: Arabidopsis (NP_565898 and NP_181435), A. lyrata (XP_002879780 and XP_002879781), B. rapa (EE421453 and EX097874), B. napus (EE409067), R. raphanistrum (FD557329), and C. papaya (EX280263). C. rubella sequences were kindly supplied by Y. Guo and D. Weigel in advance of publication (C. rubella raw sequence data can be found at http://www.ncbi.nlm.nih.gov/bioproject/13878).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Identification of Nδ-Acetylornithine by MS and NMR.
Supplemental Figure 2. Nδ-Acetylornithine Is Distinct from the Common Plant Metabolite Nα-Acetylornithine.
Supplemental Figure 3. Nδ-Acetylornithine Accumulation in Arabidopsis Foliage after Elicitation with Abscisic Acid.
Supplemental Figure 4. Pathways for Incorporation of [U-13C]Arg, [U-13C]Pro, and [U-13C]Glu into Nδ-Acetylornithine.
Supplemental Figure 5. Accumulation of Free Amino Acids in Foliage of nata-1 Mutant and nata1-1 p35S:NATA1 Plants.
Supplemental Figure 6. Nδ-Acetylornithine Accumulation in fls2 Mutant Arabidopsis after P. syringae Treatment.
Supplemental Figure 7. Abundance of Transport Amino Acids in Phloem Exudates after MeJA Treatment.
Supplemental Figure 8. Free Amino Acids in nata1-1 Mutant Leaves with and without Added Nδ-Acetylornithine.
Supplemental Figure 9. Aphid Reproduction on an Artificial Diet with a Single Amino Acid as the Nitrogen Source.
Supplemental Figure 10. Glucosinolate Accumulation in Col-0 and nata1-1, with and without MeJA Treatment.
Supplemental Figure 11. Polyamine Accumulation in Col-0 and nata1-1, with and without MeJA Treatment.
Supplemental Figure 12. Free Amino Acids in Control and MeJA-Induced Col-0 Wild Type and nata1-1 Mutants.
Supplemental Figure 13. Growth of Col-0 Wild Type and nata1-1, with and without MeJA Treatment.
Supplemental Table 1. Arabidopsis Isolates That Show MeJA-Induced Production of Nδ-Acetylornithine.
Supplemental Table 2. Crucifers That Were Tested for the Presence of Nδ-Acetylornithine.
Supplemental Table 3. PCR Primers Used in This Study.
Supplemental Data Set 1. Metabolite Profiling of Col-0 Wild-Type and nata1-1 Mutant Leaves, with and without MeJA Treatment.
Supplemental Data Set 2. ClustalW Sequence Alignment of NATA1 Homologs in the Brassicaceae.
Acknowledgments
We thank T. Mitchell-Olds, N. Pumplin, J. Jaworski, T. Brutnell, M. Kovach, C. Todd, H. G. Kang, C. Danna, S. He, F. Ausubel, and N. Clay for seeds and bacterial strains, Y. Guo and D. Weigel for providing C. rubella genome sequence data (U.S. Department of Energy Joint Genome Institute, supported by the Office of Science of the U.S. Department of Energy under Contract DE-AC02-05CH11231), F. Schroeder for assistance in identifying Nδ-acetylornithine, and H.G. Kang for advice with P. syringae assays. This research was funded through National Science Foundation Grant IOS-0718733 and National Institutes of Health Grant 3R37GM048707-17S1 to G.J. as well as National Science Foundation Grants IOS-1021861 and DBI-0820580 to D.J.K.
AUTHOR CONTRIBUTIONS
A.M.A. and G.J. designed the research. A.M.A., C.L.S., M.D.V., J.H.K., V.J., B.L., C.J., J.D., and G.J. performed research. A.M.A., C.L.C., M.D.V., J.H.K., V.J., D.J.K., and G.J. analyzed data. A.M.A. and G.J. wrote the article.
References
- Adams M.D., et al. (2000). The genome sequence of Drosophila melanogaster. Science 287: 2185–2195 [DOI] [PubMed] [Google Scholar]
- Agrawal A.A. (1998). Induced responses to herbivory and increased plant performance. Science 279: 1201–1202 [DOI] [PubMed] [Google Scholar]
- Agrawal A.A. (2000). Overcompensation of plants in response to herbivory and the by-product benefits of mutualism. Trends Plant Sci. 5: 309–313 [DOI] [PubMed] [Google Scholar]
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Barth C., Jander G. (2006). Arabidopsis myrosinases TGG1 and TGG2 have redundant function in glucosinolate breakdown and insect defense. Plant J. 46: 549–562 [DOI] [PubMed] [Google Scholar]
- Bednarek P., Schneider B., Svatos A., Oldham N.J., Hahlbrock K. (2005). Structural complexity, differential response to infection, and tissue specificity of indolic and phenylpropanoid secondary metabolism in Arabidopsis roots. Plant Physiol. 138: 1058–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E.A. (2003). Nonprotein amino acids of plants: significance in medicine, nutrition, and agriculture. J. Agric. Food Chem. 51: 2854–2865 [DOI] [PubMed] [Google Scholar]
- Bidart-Bouzat M.G., Kliebenstein D. (May 31, 2011). An ecological genomic approach challenging the paradigm of differential plant responses to specialist versus generalist insect herbivores. Oecologia http://dx.doi.org/10.1007/s00442-011-2015-z [DOI] [PubMed] [Google Scholar]
- Bino R.J., et al. (2004). Potential of metabolomics as a functional genomics tool. Trends Plant Sci. 9: 418–425 [DOI] [PubMed] [Google Scholar]
- Brooks D.M., Hernández-Guzmán G., Kloek A.P., Alarcón-Chaidez F., Sreedharan A., Rangaswamy V., Peñaloza-Vázquez A., Bender C.L., Kunkel B.N. (2004). Identification and characterization of a well-defined series of coronatine biosynthetic mutants of Pseudomonas syringae pv. tomato DC3000. Mol. Plant Microbe Interact. 17: 162–174 [DOI] [PubMed] [Google Scholar]
- Brown D.H., Fowden L. (1966). Characterization of δ-acetyl-L-ornithine isolated from Onobrychis vicifolia Scop. Phytochemistry 5: 881–886 [Google Scholar]
- Brownfield D.L., Todd C.D., Deyholos M.K. (2008). Analysis of Arabidopsis arginase gene transcription patterns indicates specific biological functions for recently diverged paralogs. Plant Mol. Biol. 67: 429–440 [DOI] [PubMed] [Google Scholar]
- Cao H., Bowling S.A., Gordon A.S., Dong X. (1994). Characterization of an Arabidopsis mutant that Is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6: 1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Century K.S., Holub E.B., Staskawicz B.J. (1995). NDR1, a locus of Arabidopsis thaliana that is required for disease resistance to both a bacterial and a fungal pathogen. Proc. Natl. Acad. Sci. USA 92: 6597–6601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Wilkerson C.G., Kuchar J.A., Phinney B.S., Howe G.A. (2005). Jasmonate-inducible plant enzymes degrade essential amino acids in the herbivore midgut. Proc. Natl. Acad. Sci. USA 102: 19237–19242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini A., Fonseca S., Fernández G., Adie B., Chico J.M., Lorenzo O., García-Casado G., López-Vidriero I., Lozano F.M., Ponce M.R., Micol J.L., Solano R. (2007). The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671 [DOI] [PubMed] [Google Scholar]
- Clay N.K., Adio A.M., Denoux C., Jander G., Ausubel F.M. (2009). Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323: 95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cui J., Bahrami A.K., Pringle E.G., Hernandez-Guzman G., Bender C.L., Pierce N.E., Ausubel F.M. (2005). Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proc. Natl. Acad. Sci. USA 102: 1791–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis M.D., Grossniklaus U. (2003). A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Auria J.C., Gershenzon J. (2005). The secondary metabolism of Arabidopsis thaliana: Growing like a weed. Curr. Opin. Plant Biol. 8: 308–316 [DOI] [PubMed] [Google Scholar]
- De Vos M., Jander G. (2009). Myzus persicae (green peach aphid) salivary components induce defence responses in Arabidopsis thaliana. Plant Cell Environ. 32: 1548–1560 [DOI] [PubMed] [Google Scholar]
- Dixon R.A. (2001). Natural products and plant disease resistance. Nature 411: 843–847 [DOI] [PubMed] [Google Scholar]
- Ellis C., Karafyllidis I., Turner J.G. (2002). Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. Mol. Plant Microbe Interact. 15: 1025–1030 [DOI] [PubMed] [Google Scholar]
- Fan J., Crooks C., Creissen G., Hill L., Fairhurst S., Doerner P., Lamb C. (2011). Pseudomonas sax genes overcome aliphatic isothiocyanate-mediated non-host resistance in Arabidopsis. Science 331: 1185–1188 [DOI] [PubMed] [Google Scholar]
- Felsenstein J. (2005). PHYLIP (Phylogeny Inference Package) Version 3.6. (Seattle, WA: University of Washington; ). [Google Scholar]
- Fernández-Calvo P., et al. (2011). The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 23: 701–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys B., Benedetti C.E., Penfold C.N., Turner J.G. (1994). Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6: 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiehn O., Wohlgemuth G., Scholz M. (2005). Setup and annotation of metabolomics experiments by integrating biological and mass spectrometric metadata. Lect. Not. Comp. Sci. 3615: 224–239 [Google Scholar]
- Flores T., Todd C.D., Tovar-Mendez A., Dhanoa P.K., Correa-Aragunde N., Hoyos M.E., Brownfield D.M., Mullen R.T., Lamattina L., Polacco J.C. (2008). Arginase-negative mutants of Arabidopsis exhibit increased nitric oxide signaling in root development. Plant Physiol. 147: 1936–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden L. (1958). δ-Acetylornithine: A constituent of some common grasses. Nature 182: 406–407 [DOI] [PubMed] [Google Scholar]
- Fowden L. (2001). Plant amino acid research in retrospect: From Chinball to Singh. Amino Acids 20: 217–224 [DOI] [PubMed] [Google Scholar]
- Glazebrook J., Rogers E.E., Ausubel F.M. (1996). Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics 143: 973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkier B.A., Gershenzon J. (2006). Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 57: 303–333 [DOI] [PubMed] [Google Scholar]
- Honeybee Genome Sequencing Consortium (2006). Insights into social insects from the genome of the honeybee Apis mellifera. Nature 443: 931–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe G.A., Jander G. (2008). Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 59: 41–66 [DOI] [PubMed] [Google Scholar]
- Joshi V., Jander G. (2009). Arabidopsis methionine gamma-lyase is regulated according to isoleucine biosynthesis needs but plays a subordinate role to threonine deaminase. Plant Physiol. 151: 367–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean P.J., Kerr A., New P.B. (1970). Crown gall of stone fruit: II, Identification and nomenclature of Agrobacterium isolates. Aust. J. Biol. Sci. 23: 585–595 [Google Scholar]
- Kessler A., Baldwin I.T. (2002). Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 53: 299–328 [DOI] [PubMed] [Google Scholar]
- Kim J.H., Durrett T.P., Last R.L., Jander G. (2004). Characterization of the Arabidopsis TU8 glucosinolate mutation, an allele of TERMINAL FLOWER2. Plant Mol. Biol. 54: 671–682 [DOI] [PubMed] [Google Scholar]
- Kim J.H., Jander G. (2007). Myzus persicae (green peach aphid) feeding on Arabidopsis induces the formation of a deterrent indole glucosinolate. Plant J. 49: 1008–1019 [DOI] [PubMed] [Google Scholar]
- Kite G.C., Ireland H. (2002). Non-protein amino acids of Bocoa (Leguminosae; Papilionoideae). Phytochemistry 59: 163–168 [DOI] [PubMed] [Google Scholar]
- Kloek A.P., Verbsky M.L., Sharma S.B., Schoelz J.E., Vogel J., Klessig D.F., Kunkel B.N. (2001). Resistance to Pseudomonas syringae conferred by an Arabidopsis thaliana coronatine-insensitive (coi1) mutation occurs through two distinct mechanisms. Plant J. 26: 509–522 [DOI] [PubMed] [Google Scholar]
- Lam H.M., Wong P., Chan H.K., Yam K.M., Chen L., Chow C.M., Coruzzi G.M. (2003). Overexpression of the ASN1 gene enhances nitrogen status in seeds of Arabidopsis. Plant Physiol. 132: 926–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin M.A., et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948 [DOI] [PubMed] [Google Scholar]
- Laurie-Berry N., Joardar V., Street I.H., Kunkel B.N. (2006). The Arabidopsis thaliana JASMONATE INSENSITIVE 1 gene is required for suppression of salicylic acid-dependent defenses during infection by Pseudomonas syringae. Mol. Plant Microbe Interact. 19: 789–800 [DOI] [PubMed] [Google Scholar]
- Leclerc J., Benoiton L. (1968). On the selectivity of acylation of unprotected diamino acids. Can. J. Chem. 46: 1047–1051 [Google Scholar]
- Lee M.W., Jelenska J., Greenberg J.T. (2008). Arabidopsis proteins important for modulating defense responses to Pseudomonas syringae that secrete HopW1-1. Plant J. 54: 452–465 [DOI] [PubMed] [Google Scholar]
- Lemaître T., Gaufichon L., Boutet-Mercey S., Christ A., Masclaux-Daubresse C. (2008). Enzymatic and metabolic diagnostic of nitrogen deficiency in Arabidopsis thaliana Wassileskija accession. Plant Cell Physiol. 49: 1056–1065 [DOI] [PubMed] [Google Scholar]
- Lipson D.L., Raab T.K., Monson R.K. (1996). δ-Acetylornithine as a major nitrogen storage compound in Bistorta bistortoides. Phytochemistry 41: 29–30 [Google Scholar]
- Manske R.H.F. (1937). The natural occurence of acetyl-ornithine. Can. J. Res. 15B: 84–87 [Google Scholar]
- Marona H.R.N., Schenkel E.P., Bergonoci J.I. (2003). Phytotoxic activity of Ateleia glazioviana Baill. extracts on lettuce seeds. Acta Farm. Bonaerense 22: 17–20 [Google Scholar]
- Melotto M., Underwood W., Koczan J., Nomura K., He S.Y. (2006). Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980 [DOI] [PubMed] [Google Scholar]
- Mewis I., Appel H.M., Hom A., Raina R., Schultz J.C. (2005). Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol. 138: 1149–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur L.A., Kenton P., Atzorn R., Miersch O., Wasternack C. (2006). The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol. 140: 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C., Heck S., Parinthawong N., Métraux J.P. (2002). EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14: 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris R.D., Fowden L. (1972). Substrate discrimination by prolyl-tRNA synthetase from various higher plants. Phytochemistry 11: 2921–2935 [Google Scholar]
- Peterson P.J., Fowden L. (1963). Different specificities of proline-activating enzymes from some plant species. Nature 200: 148–151 [Google Scholar]
- Pfalz M., Vogel H., Kroymann J. (2009). The gene controlling the indole glucosinolate modifier1 quantitative trait locus alters indole glucosinolate structures and aphid resistance in Arabidopsis. Plant Cell 21: 985–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S.R., Campbell J.W. (1977). Enzymic basis for the nutritional requirement of arginine in insects. Experientia 33: 160–161 [DOI] [PubMed] [Google Scholar]
- Richards S., et al. ; Tribolium Genome Sequencing Consortium (2008). The genome of the model beetle and pest Tribolium castaneum. Nature 452: 949–955 [DOI] [PubMed] [Google Scholar]
- Rosenthal G.A. (2001). L-Canavanine: A higher plant insecticidal allelochemical. Amino Acids 21: 319–330 [DOI] [PubMed] [Google Scholar]
- Rosso M.G., Li Y., Strizhov N., Reiss B., Dekker K., Weisshaar B. (2003). An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol. Biol. 53: 247–259 [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. (1989). Molecular Cloning: A Laboratory Manual, 2nd ed (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; ). [Google Scholar]
- Sessions A., et al. (2002). A high-throughput Arabidopsis reverse genetics system. Plant Cell 14: 2985–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenobu S., Watanabe H., Hattori M., Sakaki Y., Ishikawa H. (2000). Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 407: 81–86 [DOI] [PubMed] [Google Scholar]
- Spoel S.H., et al. (2003). NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15: 760–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe K.A., Marquis R.J., Hochwender C.G., Simms E.L. (2000). The evolutionary ecology of tolerance to consumer damage. Annu. Rev. Ecol. Syst. 31: 565–595 [Google Scholar]
- Thaler J.S., Karban R., Ullman D.E., Boege K., Bostock R.M. (2002). Cross-talk between jasmonate and salicylate plant defense pathways: Effects on several plant parasites. Oecologia 131: 227–235 [DOI] [PubMed] [Google Scholar]
- Thines B., Katsir L., Melotto M., Niu Y., Mandaokar A., Liu G., Nomura K., He S.Y., Howe G.A., Browse J. (2007). JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448: 661–665 [DOI] [PubMed] [Google Scholar]
- Thomas C.L., Leh V., Lederer C., Maule A.J. (2003). Turnip crinkle virus coat protein mediates suppression of RNA silencing in Nicotiana benthamiana. Virology 306: 33–41 [DOI] [PubMed] [Google Scholar]
- Tiffin P. (2000). Mechanisms of tolerance to herbivore damage: What do we know? Evol. Ecol. 14: 523–536 [Google Scholar]
- Uppalapati S.R., Ishiga Y., Ryu C.M., Ishiga T., Wang K., Noël L.D., Parker J.E., Mysore K.S. (2011). SGT1 contributes to coronatine signaling and Pseudomonas syringae pv. tomato disease symptom development in tomato and Arabidopsis. New Phytol. 189: 83–93 [DOI] [PubMed] [Google Scholar]
- Uppalapati S.R., Ishiga Y., Wangdi T., Kunkel B.N., Anand A., Mysore K.S., Bender C.L. (2007). The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with Pseudomonas syringae pv. tomato DC3000. Mol. Plant Microbe Interact. 20: 955–965 [DOI] [PubMed] [Google Scholar]
- Uppalapati S.R., Ishiga Y., Wangdi T., Urbanczyk-Wochniak E., Ishiga T., Mysore K.S., Bender C.L. (2008). Pathogenicity of Pseudomonas syringae pv. tomato on tomato seedlings: phenotypic and gene expression analyses of the virulence function of coronatine. Mol. Plant Microbe Interact. 21: 383–395 [DOI] [PubMed] [Google Scholar]
- Verslues P.E., Sharma S. (2010). Proline metabolism and its implications for plant-environment interaction. In The Arabidopsis Book 8: e0140, doi/10.1199/tab.0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetting M.W., de Carvalho L.P., Roderick S.L., Blanchard J.S. (2005). A novel dimeric structure of the RimL Nalpha-acetyltransferase from Salmonella typhimurium. J. Biol. Chem. 280: 22108–22114 [DOI] [PubMed] [Google Scholar]
- Virtanen A.I., Linko P. (1955). The occurrence of free ornithine and it N-acetyl derivative in plants. Acta Chem. Scand. 9: 531–554 [Google Scholar]
- Voinnet O., Vain P., Angell S., Baulcombe D.C. (1998). Systemic spread of sequence-specific transgene RNA degradation in plants is initiated by localized introduction of ectopic promoterless DNA. Cell 95: 177–187 [DOI] [PubMed] [Google Scholar]
- Wenzel C.L., Hester Q., Mattsson J. (2008). Identification of genes expressed in vascular tissues using NPA-induced vascular overgrowth in Arabidopsis. Plant Cell Physiol. 49: 457–468 [DOI] [PubMed] [Google Scholar]
- Whalen M.C., Innes R.W., Bent A.F., Staskawicz B.J. (1991). Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidopsis and soybean. Plant Cell 3: 49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A.C., Ashton P.D., Calevro F., Charles H., Colella S., Febvay G., Jander G., Kushlan P.F., Macdonald S.J., Schwartz J.F., Thomas G.H., Douglas A.E. (2010). Genomic insight into the amino acid relations of the pea aphid, Acyrthosiphon pisum, with its symbiotic bacterium Buchnera aphidicola. Insect Mol. Biol. 19(Suppl. 2): 249–258 [DOI] [PubMed] [Google Scholar]
- Wohlfarth A., Severin J., Galinski E.A. (1993). Identification of N-delta-acetylornithine as a novel osmolyte in some gram-positive halophilic eubacteria. Appl. Microbiol. Biotechnol. 39: 568–573 [Google Scholar]
- Yan Y., Stolz S., Chételat A., Reymond P., Pagni M., Dubugnon L., Farmer E.E. (2007). A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19: 2470–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R.M. (1970). Composition of the nitrogenous components of the bush bean seed (Phaseolus vulgaris) including isolation of δ-N-acetylornithine. Phytochemistry 9: 2047–2051 [Google Scholar]
- Zarate S.I., Kempema L.A., Walling L.L. (2007). Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 143: 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W., He S.Y. (2010). A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol. 153: 1188–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Thilmony R., Bender C.L., Schaller A., He S.Y., Howe G.A. (2003). Virulence systems of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. Plant J. 36: 485–499 [DOI] [PubMed] [Google Scholar]
- Zhu X., Shaw P.N., Pritchard J., Newbury J., Hunt E.J., Barrett D.A. (2005). Amino acid analysis by micellar electrokinetic chromatography with laser-induced fluorescence detection: application to nanolitre-volume biological samples from Arabidopsis thaliana and Myzus persicae. Electrophoresis 26: 911–919 [DOI] [PubMed] [Google Scholar]
- Zimmermann P., Hirsch-Hoffmann M., Hennig L., Gruissem W. (2004). GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 136: 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]