Abstract
Checkpoints were originally identified as signalling pathways that delay mitosis in response to DNA damage or defects in chromosome replication, allowing time for DNA repair to occur. The ATR (ataxia- and rad-related) and ATM (ataxia-mutated) protein kinases are recruited to defective replication forks or to sites of DNA damage, and are thought to initiate the DNA damage response in all eukaryotes. In addition to delaying cell cycle progression, however, the S-phase checkpoint pathway also controls chromosome replication and DNA repair pathways in a highly complex fashion, in order to preserve genome integrity. Much of our understanding of this regulation has come from studies of yeasts, in which the best-characterized targets are the stimulation of ribonucleotide reductase activity by multiple mechanisms, and the inhibition of new initiation events at later origins of DNA replication. In addition, however, the S-phase checkpoint also plays a more enigmatic and apparently critical role in preserving the functional integrity of defective replication forks, by mechanisms that are still understood poorly. This review considers some of the key experiments that have led to our current understanding of this highly complex pathway.
Keywords: S-phase checkpoint, chromosome replication, genome integrity, DNA replication fork
1. Discovery of the S-phase checkpoint response
The S-phase checkpoint pathway was first characterized in the early 1990s, based on work with fission and budding yeasts [1–5]. These experiments were heavily influenced, however, by earlier studies of how mammalian cells regulate DNA replication in response to DNA damage. Treatment of human cells or Chinese hamster cells with X-rays was known to delay the entry into mitosis and also cause a very rapid decrease in the rate of DNA synthesis during chromosome replication. It was found in the mid-1970s that treating cells with the drug caffeine prevented both these effects and greatly sensitized cells to radiation [6,7], raising the possibility that the observed responses to radiation might normally help cells to survive DNA damage. Subsequent work suggested that radiation blocked the initiation of new replicons during chromosome replication [8], although this remained to be demonstrated more directly in later years [9]. The underlying mechanisms were not understood initially, and it was suggested that DNA damage changed the conformation of chromatin so as to block replication initiation, whereas caffeine might alter chromatin in a different way and somehow prevent this effect [8].
Several genetic diseases were known to be associated with increased sensitivity of cells to radiation, owing to defects in DNA repair. Importantly, however, it was reported in 1980 that the radiation sensitivity of cells from ataxia telangiectasia (AT) patients was associated with a defect in their ability to respond to DNA damage, rather than their ability to repair it [10]. Similar to HeLa cells treated with caffeine, AT cells do not reduce their rate of DNA synthesis upon irradiation, nor do they delay entry into mitosis. Viewed from a modern perspective, these features clearly indicate a defect in checkpoint signalling pathways. At the time, however, these data were interpreted in terms of AT cells having a chromatin conformation that prevented DNA damage from blocking replication initiation, analogous to the supposed effects of caffeine treatment [10].
The realization that such data reflected cellular pathways that have evolved to preserve chromosome integrity came at the end of the 1980s, when Weinert & Hartwell [11] reported that mutation of the budding yeast RAD9 gene caused irradiated cells to enter mitosis in the presence of DNA damage, rather than delaying cell cycle progression in G2 phase. This property of rad9 cells upon irradiation was analogous to human AT cells or to the behaviour of mammalian cells treated with caffeine, and its lethal consequences could be suppressed using a drug that depolymerizes microtubules and thus delays anaphase in the irradiated cells, restoring a period of time during which DNA damage could be repaired [11]. This led to the suggestion that eukaryotic cells have a variety of ‘checkpoint’ pathways that delay mitosis in response to DNA damage or defects in DNA synthesis, as well as ensuring the successful completion of key processes such as chromosome replication and mitosis [12].
Further evidence that a checkpoint pathway can act during S phase to protect cells from defects in chromosome replication came from work with fission yeast. It seemed clear by the late-1980s that activation of the Cdc2 protein kinase was a key determinant of the entry into mitosis in all eukaryotes, and dominant mutations in fission yeast Cdc2 caused premature entry into mitosis at a reduced cell size. Enoch & Nurse [2] found that cells expressing the dominant cdc2-3w allele were unable to delay mitosis when treated with the drug hydroxyurea that inhibits ribonucleotide reductase (RNR) and so blocks chromosome replication. This indicated that Cdc2 is the target of a checkpoint pathway that normally delays mitosis in response to defects in DNA replication. Multiple components of this pathway were identified in a screen for mutations that sensitized cells to hydroxyurea and caused mitotic entry in the absence of replication (‘hus’ mutants as they were hydroxyurea-sensitive), but that did not affect the timing of mitosis in a normal cell cycle [3]. Checkpoint genes with similar properties were also identified in two independent studies, which employed an analogous approach to that of Hartwell & Weinert by analysing fission yeast mutants that were sensitive to radiation [1,4]. Importantly, all three studies found that cells with mutations in the newly identified ‘checkpoint rad’ genes were much more sensitive to hydroxyurea than were cells with the cdc2-3w mutation. Moreover, Enoch et al. [3] used synchronized populations of fission yeast cells to show that checkpoint mutants and cdc2-3w were similarly defective in their ability to delay mitosis when replication was blocked, but the checkpoint mutants lost viability as soon as they entered chromosome replication in the presence of hydroxyurea, whereas cdc2-3w only lost viability when the cells entered mitosis subsequently. These data suggested that the products of the checkpoint rad genes do not simply regulate cell cycle progression, blocking inappropriate entry into mitosis in response to defects in DNA synthesis, but might also have a more direct role in regulating chromosome replication that is critical for the preservation of genome integrity. In an insightful passage that anticipated much of the following 20 years of research into the action of the S-phase checkpoint pathway, Enoch et al. [3] noted that the ‘possible functions for the hus and rad proteins might be maintaining the replication complex at the replication fork during S-phase arrest, preserving the structure of the replication bubble, repairing the damage caused by a poorly functioning polymerase or controlling the expression of genes involved in any of these processes’.
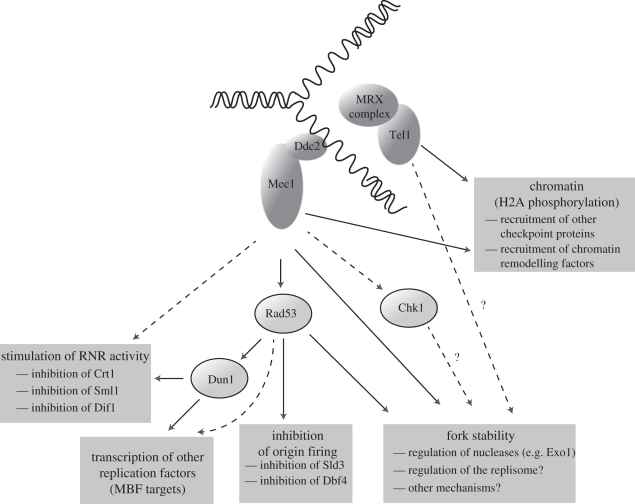
Multiple checkpoint genes were also identified in budding yeast around the same time via several screens. These included a further analysis of the collection of rad mutants [5,13], a screen for mutations in ‘mitotic entry checkpoint’ or ‘MEC’ genes that caused mitotic entry in cells with DNA damage resulting from inactivation of the Cdc13 protein [5,14], a screen for ‘spindle arrest defective’ or ‘sad’ mutants that failed to block anaphase when replication was blocked by treating cells with hydroxyurea [13], and a screen for mutations that caused sensitivity to the alkylating agent methyl methanesulphonate (MMS) as well as reduced meiotic recombination [15]. The RAD53/MEC2/SAD1 and MEC1/ESR1/SAD3 genes were of particular interest as they were required not only to block cell cycle progression in response to DNA damage in G2 phase, just like the other budding yeast checkpoint genes, but were also needed to block anaphase in hydroxyurea-treated cells. This suggested that the other checkpoint proteins might be required to sense DNA damage but not defective replication forks per se, whereas Rad53 and Mec1 might be needed in both situations to transduce the checkpoint signal. Moreover, both checkpoint kinases were found to be required to slow the rate of progression of DNA synthesis when yeast cells were treated with MMS [16], analogous to the failure of AT cells to inhibit replication upon irradiation. These data indicated that Mec1–Rad53 were central to an S-phase checkpoint response that regulates chromosome replication in budding yeast in response to DNA damage (figure 1).
Figure 1.
Regulation of chromosome replication by S-phase checkpoint response in budding yeast (see text for details). Dotted lines indicate minor functions, and question marks denote putative roles that are not well established. For the sake of simplicity, the regulation of mitosis by checkpoint kinases has been omitted.
Importantly, the RAD53 gene was found to encode a protein kinase that activates another related kinase, Dun1, which is needed to stimulate the transcription of RNR genes in response to DNA damage or defects in DNA replication [13]. This provided the first mechanistic insight into how checkpoint kinases are able to regulate chromosome replication in addition to controlling cell cycle progression, consistent with the earlier experiments with fission yeast. Later work showed that the sensitivity of rad53Δ or mec1Δ cells to hydroxyurea treatment could not be suppressed by blocking mitosis, and seemed to result from a failure to complete chromosome replication following transient depletion of nucleotides [17]. Moreover, budding yeast cells lacking Mec1 or Rad53 were similarly defective in delaying mitosis following transient treatment with hydroxyurea, but mec1Δ cells lost viability to a much greater degree than rad53Δ, indicating that Mec1 had other roles that were important for survival in response to defects in chromosome replication [17].
A key advance in understanding the molecular action of DNA structure checkpoint pathways came with the identification by positional cloning of the ATM gene (ataxia-mutated) that is defective in AT [18], as it was found to encode a protein related to fission yeast Rad3, budding yeast Mec1 and the newly identified Tel1 protein that was required to maintain telomere length in budding yeast. All these proteins are related to phosphoinositide 3-kinase (PI-3 kinase), but immune-precipitates of fission yeast Rad3 supported auto-phosphorylation and it is now clear that ATM/Rad3/Mec1/Tel1 are actually all protein kinases [19]. ATM is related most closely to Tel1, whereas Mec1 and Rad3 are more closely related to a further kinase in human cells known as ATR (ataxia- and rad-related), which was identified by virtue of its homology to fission yeast Rad3 [19]. Although Tel1 is less important than Mec1 for the survival of budding yeast cells that have been exposed to DNA damage, cells lacking both kinases were found to have an enhanced sensitivity to DNA damage and a greater defect in telomere preservation [20]. It now seems likely that almost all eukaryotes have checkpoint pathways based on orthologues of both ATM and ATR, which are targeted in different ways to defective DNA structures: ATM is principally recruited to double-strand breaks in DNA by the MRX complex (comprising the Mre11, Rad50 and Xrs2 proteins), whereas ATR is recruited to defective replication forks or processed double-strand breaks via single-strand DNA (ssDNA) that is exposed to a greater extent than at normal forks. The recruitment of ATR to ssDNA involves another subunit of the kinase, called ATRIP in vertebrates or Ddc2/Rad26 in yeasts, which associates with the single-strand binding protein RPA (replication protein A). Subsequently, it was found that Rad53 acts downstream of Mec1 in budding yeast [21], and work using fission yeast showed that the orthologue of Rad53 (known as Cds1) acts downstream of Rad3 in response to replication defects [22], whereas a further kinase called Chk1 is activated by Rad3 in response to DNA damage during G2 phase [23]. The other checkpoint proteins function as ‘adaptors’ that recognize the defective DNA structures that are the source of the checkpoint signal (including the junction between primers and ssDNA), and also as ‘mediators’ that help to activate the checkpoint kinases [24].
These data led to a model for checkpoint signalling in animal cells and fungi, by which orthologues of ATR/Mec1/Rad3 and ATM/Tel1 act as upstream ‘sensor’ kinases that are recruited and activated at sites of DNA damage by other checkpoint factors, leading to the subsequent activation of the downstream ‘effector’ kinases Rad53/Cds1 and Chk1. The situation in metazoan cells is slightly different from that in yeast, however, as the Chk2 orthologue of Rad53/Cds1 has swapped roles with Chk1 during the course of evolution, so that Chk2 acts downstream of ATM, largely in response to double-strand breaks, whereas Chk1 acts downstream of ATR, principally responding to defects at DNA replication forks. Checkpoint responses in plants also involve orthologues of ATR and ATM, but genome-sequencing projects have revealed the surprising fact that plants appear to lack orthologues of Chk1 or Rad53/Cds1/Chk2, as well as the adaptors and mediators that help activate the effector kinases in animal cells and fungi. It thus remains to be determined whether the checkpoint signals in plant cells are transmitted to their targets by new classes of effector kinases, or by effector kinases that have diverged very highly from their animal and fungal counterparts, or whether ATR and ATM are able to regulate the relevant targets directly in plant cells, without the need for downstream kinases.
2. The regulation of ribonucleotide reductase is a key feature of the S-phase checkpoint response
The activity of RNR is regulated in an exquisite fashion in eukaryotic cells and is an important determinant of genome integrity. It appears that cells make just enough deoxynucleotide triphosphates (dNTPs) during S phase for efficient chromosome replication, as too much or too little dNTPs can be mutagenic [25]. In addition, reduced dNTP levels slow fork progression and stimulate chromosome instability [26]. Recent data indicate that reduced dNTP levels make an important contribution to genome instability in the early development of cancer, in response to oncogene activation [27].
In all eukaryotes, it seems that ATP and dNTPs regulate RNR in an allosteric fashion [25]. Moreover, transcription of the genes encoding the various subunits of RNR has been shown from yeasts to humans to be stimulated during S phase and induced in response to DNA damage or defects in replication [28]. This regulation was first studied in budding yeast and led to the identification of the Dun1 protein kinase (expression of RNR genes was damage uninducible), which plays a key role in regulating RNR activity [29]. Dun1 is needed for transcription of RNR genes in response to replication defects or DNA damage, and Dun1 is activated by the related Rad53 checkpoint kinase as mentioned already. The RNR genes are part of a large set of genes that are normally induced during S phase and then repressed afterwards [30,31]. In response to replication defects, the S-phase checkpoint kinases preserve the S-phase transcriptional programme by blocking its repression, maintaining the expression of many genes that encode replication factors [30]. Although they are part of this programme, the RNR genes are also regulated independently by a repressor known as Crt1 (constitutive RNR3 transcription), which is inhibited by Dun1 in response to checkpoint activation, leading to greatly increased expression of RNR [32].
Subsequently, it was found that other proteins regulate RNR post-translationally, as part of the S-phase checkpoint pathway. The budding yeast MEC1 and RAD53 genes are essential for cell viability even in the absence of exogenous sources of replication stress or DNA damage. Mutation of a gene called SML1 (suppressor of Mec1 lethality) was found to suppress the inviability of mec1Δ or rad53Δ, as did increased expression of RNR genes [17,33]. The Sml1 protein encodes a direct inhibitor of RNR that must be degraded in each round of the cell cycle when budding yeast cells enter S phase, and that is also degraded in response to DNA damage and replication defects [33]. The regulation of Sml1 stability is rather complicated, as the Mec1–Rad53–Dun1 pathway is essential for the degradation of Sml1 in response to checkpoint activation, but Rad53 and particularly Dun1 are less important for downregulation of Sml1 in the normal cell cycle [26]. These data showed that the checkpoint kinases play an essential role in facilitating dNTP synthesis during S phase in budding yeast. Bypassing the essential role of Mec1 or Rad53 by deletion of SML1 or overexpression of RNR did not allow cells to survive DNA damage or hydroxyurea treatment in the absence of Rad53 or Mec1, indicating that the checkpoint kinases have other important roles too.
Later work showed that RNR is regulated in still more ways by the S-phase checkpoint pathway in yeast cells. The small subunit of RNR is normally hidden in the nucleus away from the largely cytoplasmic large subunit, until S-phase or checkpoint activation. The Dif1 protein (damage regulated import facilitator) takes the small subunit to the nucleus where it is tethered by another factor, and the Mec1–Rad53–Dun1 pathway phosphorylates Dif1 upon checkpoint activation and promotes its degradation [34,35]. It now seems that Sml1 and Dif1 have a common ancestry, and the fission yeast Spd1 protein (S-phase delayed) is a member of the same family of proteins that seems to play a role analogous to Sml1–Dif1 in regulating RNR activity during the cell cycle and in response to checkpoint activation [34–36].
Although it is clear that RNR genes are regulated at the transcriptional level in response to replication stress in mammalian cells as in yeast, metazoan orthologues of Sml1/Dif1/Spd1 have yet to be identified.
3. Checkpoint activation at defective replication forks inhibits initiation events at later origins
As discussed already, it was clear long before we understood the nature of checkpoints that irradiation of mammalian cells led to a very rapid inhibition of DNA synthesis. The response was too quick to be explained simply by a failure of G1-phase cells to enter S phase, and instead reflected a very rapid response during S phase, inhibiting new initiation events in parts of the genome where replication forks had yet to be established [9]. This phenomenon was defective in cells lacking the ATM kinase, which is needed for cells to respond properly to the DNA damage caused by irradiation. Similarly, later work with yeast showed that the Rad53 checkpoint kinase delays the firing of later origins of replication in response to defects in DNA synthesis at forks from early origins, in cells that have been treated with hydroxyurea [37,38]. As defective replication forks are a major potential source of chromosome instability, delaying the firing of new origins in response to replication defects will allow the cell to avoid making still more defective forks. Once the source of the damage or replication defect has been dealt with and the checkpoint has been inactivated, the previously silenced origins can give rise to new forks that will aid the completion of chromosome replication. Moreover, it seems likely that some of the factors that are needed at forks are present at limiting levels (dNTPs is one example), and this provides another rationale for a mechanism that prevents too many forks from being formed at the same time.
Very recent work with budding yeast has identified the main features of the mechanism by which the Rad53 kinase blocks the initiation step of replication [39–41]. Rad53 phosphorylates two factors that play a key role during the initiation of chromosome replication at each origin: the Dbf4 subunit of the Cdc7 kinase, and the Sld3 protein. Inhibiting these factors prevents the activation of the replicative DNA helicase at origins and so blocks the establishment of DNA replication forks.
It remains to be seen whether the mechanism by which checkpoint kinases control origin activation is similar in other eukaryotes. In mammalian cells, it seems that activation of the S-phase checkpoint blocks initiation in later regions of the genome but does not prevent new initiation events at sites that are very close to the defective forks [42,43]. This means that the spacing of origins in active regions actually decreases when fork progression is defective, as there is more time for initiation events at sites that would normally have been replicated passively. One possible explanation for these data would be that the checkpoint regulates the accessibility of initiation factors to chromatin loops that have yet to support replication, rather than blocking directly the interaction of such factors with the inactive replicative helicase at origins. Further work is needed before we will know whether this mechanism involves different targets to the response that blocks origin firing in budding yeast. At any rate, the end result is similar, and appears to be another conserved feature of the S-phase checkpoint, at least between animal cells and fungi.
4. Protecting replication forks: an ‘essential role’ of the S-phase checkpoint?
Checkpoint activation in response to replication defects blocks mitosis, and untimely entry into mitosis is one important reason why cells lacking the S-phase checkpoint would die under such circumstances. As discussed already, however, progression through mitosis is not enough to explain why checkpoint kinases are so important for cells to survive replication defects. Instead, it appears that the S-phase checkpoint is essential for chromosome replication to be completed following transient exposure of cells to agents such as hydroxyurea or DNA-alkylating agents, both in yeasts and human cells [17,44]. Although the best-characterized targets of the S-phase checkpoint pathway, such as the regulation of RNR, the transcription of genes encoding other replication factors and the inhibition of origin firing, are all likely to contribute to survival, several lines of evidence indicate that the checkpoint kinases might have a more direct role in preserving the functional integrity of DNA replication forks.
When assays were first developed to monitor the progression of individual replication forks through a budding yeast chromosome following DNA-alkylation, it became clear that DNA damage makes forks much slower, independently of the checkpoint response [45]. When cells lacking checkpoint kinases are treated with DNA-alkylating agents, cells complete the bulk of DNA synthesis more quickly [16], but this is due to the fact that all origins fire under such conditions [45]. Fork progression is still much slower than normal, and forks actually have a higher probability of failing to reach the end of each replicon in cells lacking Mec1 or Rad53 [45]. This means that some regions of the genome are replicated incompletely under such conditions, which guarantees lethality in the next round of cell division. Similarly, direct analysis of replication fork DNA in budding yeast cells treated with hydroxyurea showed that Rad53 is needed to prevent DNA damage or changes in the fork structure [46], which were interpreted as ‘replication fork collapse’.
The molecular details of the regulation underlying these phenomena are still under investigation, although several possibilities have emerged. One idea is that the checkpoint kinases prevent inappropriate and potentially dangerous recombination events from occurring at replication forks, despite the fact that recombination is crucial for fork progression to continue through at least some kinds of damaged DNA templates. Work with fission yeast has shown that the Rad60 regulator of recombination is excluded from the nucleus in response to checkpoint activation [47], and the Cds1 checkpoint kinase phosphorylates and apparently inhibits the Mus81 endonuclease that contributes to the resolution of joint DNA molecules during recombination. In addition, experiments with budding yeast have indicated that the resection of double-strand breaks is slower when the S-phase checkpoint is activated in cells treated with hydroxyurea [48]. This would provide a neat way of allowing ‘helpful’ recombination events to continue, using ssDNA already present at forks, while preventing harmful damage of forks by excessive action of nucleases on the ssDNA templates that are exposed to a greater extent at forks when replication is defective.
The Exo1 nuclease helps resect broken DNA ends after initial processing by other nucleases, and appears to be negatively regulated by checkpoint kinases in yeast and human cells [49–51]. Moreover, deletion of the EXO1 gene suppresses the defect in fork progression that is observed when budding yeast cells lacking Rad53 are treated with MMS [52]. The situation is complicated, however, as this improvement in fork progression requires the action of the Chk1 kinase, which does not normally play an important role in controlling fork progression when yeast cells are treated with DNA-alkylating agents. In addition, deletion of EXO1 does not suppress the defects in fork progression that are seen when mec1Δ cells are exposed to DNA damage [52]. These findings are reminiscent of a previous study that showed that the sensitivity of mec1Δ cells to MMS can be alleviated in part by removal of another nuclease called Sae2 [53], which normally helps us to resect double-strand DNA breaks. The mechanism of suppression in this case was found to be indirect, as removal of Sae2 leads to the persistence of double-strand breaks and so to increased signalling from Tel1, which helps us to activate Rad53 in the absence of Mec1 [53]. So, it remains to be seen whether the suppression of the MMS sensitivity of rad53Δ by removal of Exo1 is a direct consequence of preventing the potentially harmful effects of Exo1 at forks, or also involves a more indirect mechanism that activates another branch of the checkpoint pathway involving Chk1.
To make things more complicated, and in contrast to the negative regulation of Exo1 by checkpoint kinases, there is ample evidence that the checkpoint activates other cellular nucleases that are used to process damaged DNA. For example, Mec1 and Tel1 phosphorylate and activate the Sae2 nuclease as well as the Slx4 protein that serves as a scaffold for multiple cellular nucleases [54,55]. Clearly, we still need to learn much more about how the S-phase checkpoint response is able to balance the activity of cellular nucleases to preserve genome integrity at replication forks as well as stimulating DNA repair when necessary.
It also seems clear that chromatin is another important target of S-phase checkpoint kinases. Histone H2A (or the related H2AX) is phosphorylated by ATR/Mec1 and ATM/Tel1 in human cells and yeast [56,57], and serves to recruit other checkpoint proteins as well as chromatin-remodelling factors such as the Ino80 complex [58]. The mechanisms by which chromatin-remodelling factors help cells survive defects in chromosome replication are not understood, but some reconfiguration of chromatin around defective forks might be important to control access of other factors that are controlled by the S-phase checkpoint response.
Another important concept to emerge from studies of the S-phase checkpoint in yeasts has been that the checkpoint kinases might directly regulate the replisome at defective forks, to stop it from collapsing. This idea came from chromatin immunoprecipitation experiments that monitored the association of various replisome components with forks in budding yeast cells treated with hydroxyurea. The replisome factors were observed around origins in control cells treated with hydroxyurea, but not in cells lacking Rad53 or Mec1 [59–64]. Collapse of the replisome in cells lacking checkpoint kinases might expose DNA at forks to nucleolytic attack, leading to the changes in DNA structure that have been observed in budding yeast cells lacking Rad53. Nevertheless, we recently developed more direct assays for monitoring replisome stability at defective replication forks isolated from extracts of budding yeast cells, and have found that replisome components still associate with each other and with chromosomal DNA in cells lacking Mec1 or Rad53. This indicates that replisome stability is actually independent of S-phase checkpoint kinases (G. De Piccoli & K. Labib, unpublished data). Moreover, studies of chromosome replication in extracts of Xenopus eggs indicated that checkpoint kinases are dispensable under some circumstances for replication to resume after transient inhibition [65], consistent with the idea that replisome stability is not a key target of the checkpoint response.
It is striking, however, that replication factors are highly enriched among the list of putative substrates identified in several large-scale screens for targets of checkpoint kinases in budding yeast and mammalian cells [66–68]. These data raise the possibility that checkpoint kinases might regulate the replisome in other ways, for example, by controlling its function rather than its stability. Consistent with this view, experiments with yeast and human cells have indicated that fork progression might be controlled by checkpoint kinases [69,70]. The relevant targets of this regulation remain to be determined, and our understanding of how the S-phase checkpoint allows cells to complete chromosome replication is still at a relatively early stage.
5. Future perspectives
There is still a lot to learn about how the S-phase checkpoint response preserves genome integrity during chromosome replication in eukaryotic cells. The best-characterized facets of the response in yeasts, such as stimulation of RNR and inhibition of origin activity, remain poorly understood in other species. The situation in plant cells is particularly intriguing, owing to the apparent absence of many checkpoint proteins, including the effector kinases that act downstream of Mec1 in other species.
Even in budding yeast, the mechanisms that underlie the critical role of the checkpoint in preserving the function of replication forks in response to replication stress are still very enigmatic. Nevertheless, many putative targets of the checkpoint kinases have been identified in both budding yeast and mammalian cells, and it seems very likely that the coming years will see considerable progress in our understanding of how cells cope with replication stress, and are able to ensure that they duplicate their chromosomes successfully in each round of the cell cycle.
Acknowledgements
We are grateful to Cancer Research UK that supports the work in our laboratory.
References
- 1.al Khodairy F., Carr A. M. 1992. DNA repair mutants defining G2 checkpoint pathways in Schizosaccharomyces pombe. EMBO J. 11, 1343–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enoch T., Nurse P. 1990. Mutation of fission yeast cell cycle control genes abolishes dependence of mitosis on DNA replication. Cell 60, 665–673 10.1016/0092-8674(90)90669-6 (doi:10.1016/0092-8674(90)90669-6) [DOI] [PubMed] [Google Scholar]
- 3.Enoch T., Carr A. M., Nurse P. 1992. Fission yeast genes involved in coupling mitosis to completion of dna-replication. Genes Dev. 6, 2035–2046 10.1101/gad.6.11.2035 (doi:10.1101/gad.6.11.2035) [DOI] [PubMed] [Google Scholar]
- 4.Rowley R., Subramani S., Young P. G. 1992. Checkpoint controls in Schizosaccharomyces pombe: rad1. EMBO J. 11, 1335–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinert T. A. 1992. Dual cell-cycle checkpoints sensitive to chromosome-replication and DNA damage in the budding yeast Saccharomyces cerevisiae. Radiat. Res. 132, 141–143 10.2307/3578518 (doi:10.2307/3578518) [DOI] [PubMed] [Google Scholar]
- 6.Tolmach L. J., Jones R. W., Busse P. M. 1977. The action of caffeine on X-irradiated HeLa cells. I. Delayed inhibition of DNA synthesis. Radiat. Res. 71, 653–665 10.2307/3574633 (doi:10.2307/3574633) [DOI] [PubMed] [Google Scholar]
- 7.Walters R. A., Gurley L. R., Tobey R. A. 1974. Effects of caffeine on radiation-induced phenomena associated with cell-cycle traverse of mammalian cells. Biophys. J. 14, 99–118 10.1016/S0006-3495(74)70002-9 (doi:10.1016/S0006-3495(74)70002-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Painter R. B. 1980. Effect of caffeine on DNA synthesis in irradiated and unirradiated mammalian cells. J. Mol. Biol. 143, 289–301 10.1016/0022-2836(80)90191-6 (doi:10.1016/0022-2836(80)90191-6) [DOI] [PubMed] [Google Scholar]
- 9.Larner J. M., Lee H., Hamlin J. L. 1994. Radiation effects on DNA synthesis in a defined chromosomal replicon. Mol. Cell Biol. 14, 1901–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Painter R. B., Young B. R. 1980. Radiosensitivity in ataxia-telangiectasia: a new explanation. Proc. Natl Acad. Sci. USA 77, 7315–7317 10.1073/pnas.77.12.7315 (doi:10.1073/pnas.77.12.7315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinert T. A., Hartwell L. H. 1988. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science 241, 317–322 10.1126/science.3291120 (doi:10.1126/science.3291120) [DOI] [PubMed] [Google Scholar]
- 12.Hartwell L. H., Weinert T. A. 1989. Checkpoints: controls that ensure the order of cell cycle events. Science 246, 629–634 10.1126/science.2683079 (doi:10.1126/science.2683079) [DOI] [PubMed] [Google Scholar]
- 13.Allen J. B., Zhou Z., Siede W., Friedberg E. C., Elledge S. J. 1994. The SAD1/RAD53 protein kinase controls multiple checkpoints and DNA damage-induced transcription in yeast. Genes Dev. 8, 2401–2415 10.1101/gad.8.20.2401 (doi:10.1101/gad.8.20.2401) [DOI] [PubMed] [Google Scholar]
- 14.Weinert T. A., Kiser G. L., Hartwell L. H. 1994. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev. 8, 652–665 10.1101/gad.8.6.652 (doi:10.1101/gad.8.6.652) [DOI] [PubMed] [Google Scholar]
- 15.Kato R., Ogawa H. 1994. An essential gene, ESR1, is required for mitotic cell growth, DNA repair and meiotic recombination in Saccharomyces cerevisiae. Nucleic Acids Res. 22, 3104–3112 10.1093/nar/22.15.3104 (doi:10.1093/nar/22.15.3104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paulovich A. G., Hartwell L. H. 1995. A checkpoint regulates the rate of progression through S phase in S. cerevisiae in response to DNA damage. Cell 82, 841–847 10.1016/0092-8674(95)90481-6 (doi:10.1016/0092-8674(95)90481-6) [DOI] [PubMed] [Google Scholar]
- 17.Desany B. A., Alcasabas A. A., Bachant J. B., Elledge S. J. 1998. Recovery from DNA replicational stress is the essential function of the S-phase checkpoint pathway. Genes Dev. 12, 2956–2970 10.1101/gad.12.18.2956 (doi:10.1101/gad.12.18.2956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savitsky K., et al. 1995. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268, 1749–1753 10.1126/science.7792600 (doi:10.1126/science.7792600) [DOI] [PubMed] [Google Scholar]
- 19.Bentley N. J., et al. 1996. The Schizosaccharomyces pombe rad3 checkpoint gene. EMBO J. 15, 6641–6651 [PMC free article] [PubMed] [Google Scholar]
- 20.Morrow D. M., Tagle D. A., Shiloh Y., Collins F. S., Hieter P. 1995. TEL1, an S. cerevisiae homolog of the human gene mutated in ataxia telangiectasia, is functionally related to the yeast checkpoint gene MEC1. Cell 82, 831–840 10.1016/0092-8674(95)90480-8 (doi:10.1016/0092-8674(95)90480-8) [DOI] [PubMed] [Google Scholar]
- 21.Sanchez Y., Desany B. A., Jones W. J., Liu Q., Wang B., Elledge S. J. 1996. Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways. Science 271, 357–360 10.1126/science.271.5247.357 (doi:10.1126/science.271.5247.357) [DOI] [PubMed] [Google Scholar]
- 22.Lindsay H. D., Griffiths D. J. F., Edwards R. J., Christensen P. U., Murray J. M., Osman F., Walworth N., Carr A. M. 1998. S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe. Genes Dev. 12, 382–395 10.1101/gad.12.3.382 (doi:10.1101/gad.12.3.382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walworth N. C., Bernards R. 1996. rad-dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science 271, 353–356 10.1126/science.271.5247.353 (doi:10.1126/science.271.5247.353) [DOI] [PubMed] [Google Scholar]
- 24.Harper J. W., Elledge S. J. 2007. The DNA damage response: ten years after. Mol. Cell 28, 739–745 10.1016/j.molcel.2007.11.015 (doi:10.1016/j.molcel.2007.11.015) [DOI] [PubMed] [Google Scholar]
- 25.Chabes A., Georgieva B., Domkin V., Zhao X., Rothstein R., Thelander L. 2003. Survival of DNA damage in yeast directly depends on increased dNTP levels allowed by relaxed feedback inhibition of ribonucleotide reductase. Cell 112, 391–401 10.1016/S0092-8674(03)00075-8 (doi:10.1016/S0092-8674(03)00075-8) [DOI] [PubMed] [Google Scholar]
- 26.Zhao X., Chabes A., Domkin V., Thelander L., Rothstein R. 2001. The ribonucleotide reductase inhibitor Sml1 is a new target of the Mec1/Rad53 kinase cascade during growth and in response to DNA damage. EMBO J. 20, 3544–3553 10.1093/emboj/20.13.3544 (doi:10.1093/emboj/20.13.3544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bester A. C., et al. 2011. Nucleotide deficiency promotes genomic instability in early stages of cancer development. Cell 145, 435–446 10.1016/j.cell.2011.03.044 (doi:10.1016/j.cell.2011.03.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elledge S. J., Zhou Z., Allen J. B., Navas T. A. 1993. DNA-damage and cell-cycle regulation of ribonucleotide reductase. Bioessays 15, 333–339 10.1002/bies.950150507 (doi:10.1002/bies.950150507) [DOI] [PubMed] [Google Scholar]
- 29.Zhou Z., Elledge S. J. 1993. DUN1 encodes a protein kinase that controls the DNA damage response in yeast. Cell 75, 1119–1127 10.1016/0092-8674(93)90321-G (doi:10.1016/0092-8674 (93)90321-G) [DOI] [PubMed] [Google Scholar]
- 30.Bruin D. 2009. All eukaryotes: before turning off G1-S transcription, please check your DNA. Cell Cycle 8, 214–217 10.4161/cc.8.2.7412 (doi:10.4161/cc.8.2.7412) [DOI] [PubMed] [Google Scholar]
- 31.Elledge S. J., Zhou Z., Allen J. B. 1992. Ribonucleotide reductase: regulation, regulation, regulation. Trends Biochem. Sci. 17, 119–123 10.1016/0968-0004(92)90249-9 (doi:10.1016/0968-0004(92)90249-9) [DOI] [PubMed] [Google Scholar]
- 32.Huang M., Zhou Z., Elledge S. J. 1998. The DNA replication and damage checkpoint pathways induce transcription by inhibition of the Crt1 repressor. Cell 94, 595–605 10.1016/S0092-8674(00)81601-3 (doi:10.1016/S0092-8674(00)81601-3) [DOI] [PubMed] [Google Scholar]
- 33.Zhao X., Muller E. G., Rothstein R. 1998. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol. Cell 2, 329–340 10.1016/S1097-2765(00)80277-4 (doi:10.1016/S1097-2765(00)80277-4) [DOI] [PubMed] [Google Scholar]
- 34.Lee Y. D., Wang J., Stubbe J., Elledge S. J. 2008. Dif1 is a DNA-damage-regulated facilitator of nuclear import for ribonucleotide reductase. Mol. Cell 32, 70–80 10.1016/j.molcel.2008.08.018 (doi:10.1016/j.molcel.2008.08.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu X., Huang M. 2008. Dif1 controls subcellular localization of ribonucleotide reductase by mediating nuclear import of the R2 subunit. Mol. Cell. Biol. 28, 7156–7167 10.1128/MCB.01388-08 (doi:10.1128/MCB.01388-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nestoras K., et al. 2010. Regulation of ribonucleotide reductase by Spd1 involves multiple mechanisms. Genes Dev. 24, 1145–1159 10.1101/gad.561910 (doi:10.1101/gad.561910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santocanale C., Diffley J. F. X. 1998. A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature 395, 615–618 10.1038/27001 (doi:10.1038/27001) [DOI] [PubMed] [Google Scholar]
- 38.Shirahige K., Hori Y., Shiraishi K., Yamashita M., Takahashi K., Obuse C., Tsurimoto T., Yoshikawa H. 1998. Regulation of DNA-replication origins during cell-cycle progression. Nature 395, 618–621 10.1038/27007 (doi:10.1038/27007) [DOI] [PubMed] [Google Scholar]
- 39.Duch A., Palou G., Jonsson Z. O., Palou R., Calvo E., Wohlschlegel J., Quintana D. G. 2011. A Dbf4 mutant contributes to bypassing the Rad53-mediated block of origins of replication in response to genotoxic stress. J. Biol. Chem. 286, 2486–2491 10.1074/jbc.M110.190843 (doi:10.1074/jbc.M110.190843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Mosqueda J., Maas N. L., Jonsson Z. O., DeFazio-Eli L. G., Wohlschlegel J., Toczyski D. P. 2010. Damage-induced phosphorylation of Sld3 is important to block late origin firing. Nature 467, 479–483 10.1038/nature09377 (doi:10.1038/nature09377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zegerman P., Diffley J. F. 2010. Checkpoint-dependent inhibition of DNA replication initiation by Sld3 and Dbf4 phosphorylation. Nature 467, 474–478 10.1038/nature09373 (doi:10.1038/nature09373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ge X. Q., Jackson D. A., Blow J. J. 2007. Dormant origins licensed by excess Mcm2-7 are required for human cells to survive replicative stress. Genes Dev. 21, 3331–3341 10.1101/gad.457807 (doi:10.1101/gad.457807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ge X. Q., Blow J. J. 2010. Chk1 inhibits replication factory activation but allows dormant origin firing in existing factories. J. Cell Biol. 191, 1285–1297 10.1083/jcb.201007074 (doi:10.1083/jcb.201007074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toledo L. I., et al. 2011. A cell-based screen identifies ATR inhibitors with synthetic lethal properties for cancer-associated mutations. Nat. Struct. Mol. Biol. 18, 721–727 10.1038/nsmb.2076 (doi:10.1038/nsmb.2076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tercero J. A., Diffley J. F. 2001. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature 412, 553–557 10.1038/35087607 (doi:10.1038/35087607) [DOI] [PubMed] [Google Scholar]
- 46.Lopes M., Cotta-Ramusino C., Pellicioli A., Liberi G., Plevani P., Muzi-Falconi M., Newlon C. S., Foiani M. 2001. The checkpoint response stabilizes stalled DNA replication forks. Nature 412, 599–602 10.1038/35087613 (doi:10.1038/35087613) [DOI] [PubMed] [Google Scholar]
- 47.Boddy M. N., Shanahan P., McDonald W. H., Lopez-Girona A., Noguchi E., Yates J. R., III, Russell P. 2003. Replication checkpoint kinase Cds1 regulates recombinational repair protein Rad60. Mol. Cell. Biol. 23, 5939–5946 10.1128/MCB.23.16.5939-5946.2003 (doi:10.1128/MCB.23.16.5939-5946.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alabert C., Bianco J. N., Pasero P. 2009. Differential regulation of homologous recombination at DNA breaks and replication forks by the Mrc1 branch of the S-phase checkpoint. EMBO J. 28, 1131–1141 10.1038/emboj.2009.75 (doi:10.1038/emboj.2009.75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bolderson E., Tomimatsu N., Richard D. J., Boucher D., Kumar R., Pandita T. K., Burma S., Khanna K. K. 2010. Phosphorylation of Exo1 modulates homologous recombination repair of DNA double-strand breaks. Nucleic Acids Res. 38, 1821–1831 10.1093/nar/gkp1164 (doi:10.1093/nar/gkp1164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morin I., Ngo H.-P., Greenall A., Zubko M. K., Morrice N., Lydall D. 2008. Checkpoint-dependent phosphorylation of Exo1 modulates the DNA damage response. EMBO J. 27, 2400–2410 10.1038/emboj.2008.171 (doi:10.1038/emboj.2008.171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El-Shemerly M., Hess D., Pyakurel A. K., Moselhy S., Ferrari S. 2008. ATR-dependent pathways control hEXO1 stability in response to stalled forks. Nucleic Acids Res. 36, 511–519 10.1093/nar/gkm1052 (doi:10.1093/nar/gkm1052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Segurado M., Diffley J. F. 2008. Separate roles for the DNA damage checkpoint protein kinases in stabilizing DNA replication forks. Genes Dev. 22, 1816–1827 10.1101/gad.477208 (doi:10.1101/gad.477208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Usui T., Ogawa H., Petrini J. H. 2001. A DNA damage response pathway controlled by Tel1 and the Mre11 complex. Mol. Cell 7, 1255–1266 10.1016/S1097-2765(01)00270-2 (doi:10.1016/S1097-2765(01)00270-2) [DOI] [PubMed] [Google Scholar]
- 54.Baroni E., Viscardi V., Cartagena-Lirola H., Lucchini G., Longhese M. P. 2004. The functions of budding yeast Sae2 in the DNA damage response require Mec1- and Tel1-dependent phosphorylation. Mol. Cell. Biol. 24, 4151–4165 10.1128/MCB.24.10.4151-4165.2004 (doi:10.1128/MCB.24.10.4151-4165.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flott S., Alabert C., Toh G. W., Toth R., Sugawara N., Campbell D. G., Haber J. E., Pasero P., Rouse J. 2007. Phosphorylation of Slx4 by Mec1 and Tel1 regulates the single-strand annealing mode of DNA repair in budding yeast. Mol. Cell. Biol. 27, 6433–6445 10.1128/MCB.00135-07 (doi:10.1128/MCB.00135-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Downs J. A., Lowndes N. F., Jackson S. P. 2000. A role for Saccharomyces cerevisiae histone H2A in DNA repair. Nature 408, 1001–1004 10.1038/35050000 (doi:10.1038/35050000) [DOI] [PubMed] [Google Scholar]
- 57.Rogakou E. P., Pilch D. R., Orr A. H., Ivanova V. S., Bonner W. M. 1998. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273, 5858–5868 10.1074/jbc.273.10.5858 (doi:10.1074/jbc.273.10.5858) [DOI] [PubMed] [Google Scholar]
- 58.van Attikum H., Gasser S. M. 2009. Crosstalk between histone modifications during the DNA damage response. Trends Cell Biol. 19, 207–217 10.1016/j.tcb.2009.03.001 (doi:10.1016/j.tcb.2009.03.001) [DOI] [PubMed] [Google Scholar]
- 59.Cobb J. A., Bjergbaek L., Shimada K., Frei C., Gasser S. M. 2003. DNA polymerase stabilization at stalled replication forks requires Mec1 and the RecQ helicase Sgs1. EMBO J. 22, 4325–4336 10.1093/emboj/cdg391 (doi:10.1093/emboj/cdg391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katou Y., Kanoh Y., Bando M., Noguchi H., Tanaka H., Ashikari T., Sugimoto K., Shirahige K. 2003. S-phase checkpoint proteins Tof1 and Mrc1 form a stable replication-pausing complex. Nature 424, 1078–1083 10.1038/nature01900 (doi:10.1038/nature01900) [DOI] [PubMed] [Google Scholar]
- 61.Lucca C., Vanoli F., Cotta-Ramusino C., Pellicioli A., Liberi G., Haber J., Foiani M. 2004. Checkpoint-mediated control of replisome-fork association and signalling in response to replication pausing. Oncogene 23, 1206–1213 10.1038/sj.onc.1207199 (doi:10.1038/sj.onc.1207199) [DOI] [PubMed] [Google Scholar]
- 62.Cobb J. A., Schleker T., Rojas V., Bjergbaek L., Tercero J. A., Gasser S. M. 2005. Replisome instability, fork collapse, and gross chromosomal rearrangements arise synergistically from Mec1 kinase and RecQ helicase mutations. Genes Dev. 19, 3055–3069 10.1101/gad.361805 (doi:10.1101/gad.361805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cotta-Ramusino C., Fachinetti D., Lucca C., Doksani Y., Lopes M., Sogo J., Foiani M. 2005. Exo1 processes stalled replication forks and counteracts fork reversal in checkpoint-defective cells. Mol. Cell 17, 153–159 10.1016/j.molcel.2004.11.032 (doi:10.1016/j.molcel.2004.11.032) [DOI] [PubMed] [Google Scholar]
- 64.Raveendranathan M., Chattopadhyay S., Bolon Y.-T., Haworth J., Clarke D. J., Bielinsky A.-K. 2006. Genome-wide replication profiles of S-phase checkpoint mutants reveal fragile sites in yeast. EMBO J. 25, 3627–3639 10.1038/sj.emboj.7601251 (doi:10.1038/sj.emboj.7601251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luciani M. G., Oehlmann M., Blow J. J. 2004. Characterization of a novel ATR-dependent, Chk1-independent, intra-S-phase checkpoint that suppresses initiation of replication in Xenopus. J. Cell Sci. 117, 6019–6030 10.1242/jcs.01400 (doi:10.1242/jcs.01400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen S. H., Albuquerque C. P., Liang J., Suhandynata R. T., Zhou H. 2010. A proteome-wide analysis of kinase-substrate network in the DNA damage response. J. Biol. Chem. 285, 12 803–12 812 10.1074/jbc.M110.106989 (doi:10.1074/jbc.M110.106989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsuoka S., et al. 2007. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316, 1160–1166 10.1126/science.1140321 (doi:10.1126/science.1140321) [DOI] [PubMed] [Google Scholar]
- 68.Smolka M. B., Albuquerque C. P., Chen S. H., Zhou H. 2007. Proteome-wide identification of in vivo targets of DNA damage checkpoint kinases. Proc. Natl Acad. Sci. USA 104, 10 364–10 369 10.1073/pnas.0701622104 (doi:10.1073/pnas.0701622104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seiler J. A., Conti C., Syed A., Aladjem M. I., Pommier Y. 2007. The intra-S-phase checkpoint affects both DNA replication initiation and elongation: single-cell and -DNA fiber analyses. Mol. Cell. Biol. 27, 5806–5818 10.1128/MCB.02278-06 (doi:10.1128/MCB.02278-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szyjka S. J., Aparicio J. G., Viggiani C. J., Knott S., Xu W., Tavare S., Aparicio O. M. 2008. Rad53 regulates replication fork restart after DNA damage in Saccharomyces cerevisiae. Genes Dev. 22, 1906–1920 10.1101/gad.1660408 (doi:10.1101/gad.1660408) [DOI] [PMC free article] [PubMed] [Google Scholar]