Abstract
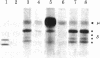
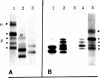
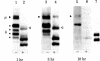
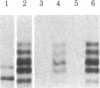
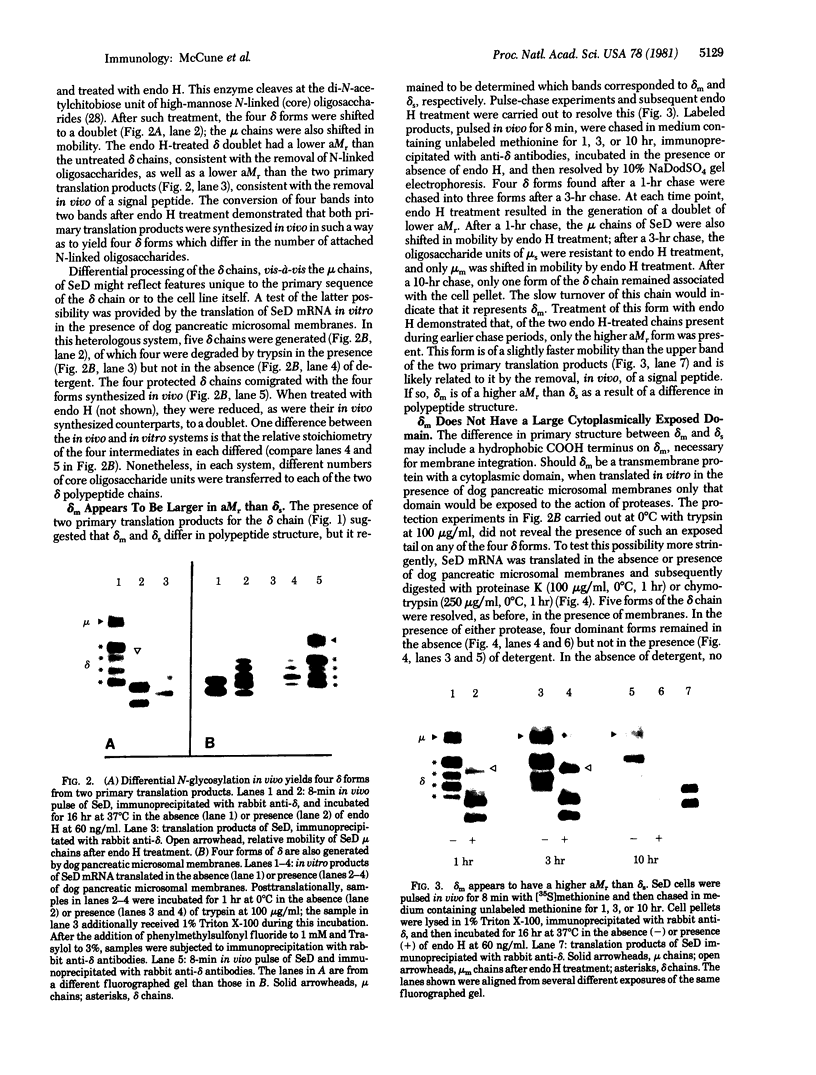
Structural differences between the heavy chain of membrane IgD (delta m) and the heavy chain of secreted IgD (delta s) were investigated by using a human lymphoblastoid cell line that expresses idiotypically identical IgM and IgD. In a wheat germ cell-free system, mRNA from this cell line was shown to encode two distinct delta chains that differed in molecular weight. When translated in vitro in the presence of dog pancreatic microsomal membranes or when synthesized in vivo, these two delta chains were processed to four discrete glycosylated forms, all of which shared idiotypic determinants, C region determinants, and light chain linkage. As shown by digestion with endo-beta-N-acetylglucosaminidase H, these four delta forms represent two delta polypeptide chains that are differentially N-glycosylated. Pulse-chase experiments demonstrated that, after endo-beta-N-acetylglucosaminidase H treatment, delta m has a higher molecular weight than delta s. After integration into dog pancreatic microsomal membranes in vitro, delta m was found not to have a large cytoplasmic domain exposed to proteolytic digestion. The finding that delta m and delta s differ in primary structure is analogous to previous work with the corresponding heavy chains of IgM (mu m and mu s) from the same cell line. Thus, this cell line produces four Ig heavy chains (mu m, mu s, delta m, and delta s), with the same idiotype. The observation of differential N-glycosylation, apparently unique for the delta class, is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney E. R., Parkhouse R. M. Candidate for immunoglobulin D present on murine B lymphocytes. Nature. 1974 Dec 13;252(5484):600–602. doi: 10.1038/252600a0. [DOI] [PubMed] [Google Scholar]
- Bargellesi A., Corte G., Cosulich E., Ferrarini M. Presence of serum IgD and IgD-containing plasma cells in the mouse. Eur J Immunol. 1979 Jun;9(6):490–492. doi: 10.1002/eji.1830090614. [DOI] [PubMed] [Google Scholar]
- Beintema J. J., Gaastra W., Scheffer A. J., Welling G. W. Carbohydrate in pancreatic ribonucleases. Eur J Biochem. 1976 Apr 1;63(2):441–448. doi: 10.1111/j.1432-1033.1976.tb10246.x. [DOI] [PubMed] [Google Scholar]
- Bielinska M., Boime I. Glycosylation of human chorionic gonadotropin in mRNA-dependent cell-free extracts: post-translational processing of an asparagine-linked mannose-rich oligosaccharide. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1208–1212. doi: 10.1073/pnas.76.3.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Corte G., Tonda P., Cosulich E., Milstein C. P., Bargellesi A., Ferrarini M. Characterization of IgD. I. Isolation of two molecular forms from human serum. Scand J Immunol. 1979;9(2):141–149. doi: 10.1111/j.1365-3083.1979.tb02716.x. [DOI] [PubMed] [Google Scholar]
- Corte G., Viale G., Cosulich E., Bargellesi A., Ferrarini M. Characterization of IgD. II. Molecular forms of IgD in human B cells. Scand J Immunol. 1979;10(3):275–280. doi: 10.1111/j.1365-3083.1979.tb01350.x. [DOI] [PubMed] [Google Scholar]
- Fu S. M., Winchester R. J., Feizi T., Walzer P. D., Kunkel H. G. Idiotypic specificity of surface immunoglobulin and the maturation of leukemic bone-marrow-derived lymphocytes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4487–4490. doi: 10.1073/pnas.71.11.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding J. W., Herzenberg L. A. Biosynthesis of lymphocyte surface IgD in the mouse. J Immunol. 1980 Jun;124(6):2540–2547. [PubMed] [Google Scholar]
- Goding J. W. Structural studies of murine lymphocyte surface IgD. J Immunol. 1980 May;124(5):2082–2088. [PubMed] [Google Scholar]
- Hurley J. N., Fu S. M., Kunkel H. G., McKenna G., Scharff M. D. Lymphoblastoid cell lines from patients with chronic lymphocytic leukemia: identification of tumor origin by idiotypic analysis. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5706–5710. doi: 10.1073/pnas.75.11.5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. C., Putnam F. W. Primary structure of the Fc region of human immunoglobulin D: implications for evolutionary origin and biological function. Proc Natl Acad Sci U S A. 1981 Jan;78(1):504–508. doi: 10.1073/pnas.78.1.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. P., Tucker P. W., Mushinski J. F., Blattner F. R. Mapping of heavy chain genes for mouse immunoglobulins M and D. Science. 1980 Sep 19;209(4463):1348–1353. doi: 10.1126/science.6774414. [DOI] [PubMed] [Google Scholar]
- McCune J. M., Fu S. M., Blobel G., Kunkel H. G. Biogenesis of membrane-bound and secreted immunoglobulins. II. Two forms of the human alpha chain translated in vitro and processed in vivo as distinct polypeptide chains. J Exp Med. 1981 Jun 1;153(6):1684–1689. doi: 10.1084/jem.153.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Fu S. M., Kunkel H. G. J chain biosynthesis in pre-B cells and other possible precursor B cells. J Exp Med. 1981 Jul 1;154(1):138–145. doi: 10.1084/jem.154.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Lingappa V. R., Fu S. M., Blobel G., Kunkel H. G. Biogenesis of membrane-bound and secreted immunoglobulins. I. Two distinct translation products of human mu-chain, with identical N-termini and different C-termini. J Exp Med. 1980 Aug 1;152(2):463–468. doi: 10.1084/jem.152.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher U., Uhr J. W. Cell surface immunoglobulin. XVI. Polypeptide chain structure of mouse IgM and IgD-like molecule. J Immunol. 1976 Feb;116(2):409–415. [PubMed] [Google Scholar]
- Melcher U., Vitetta E. S., McWilliams M., Lamm M. E., Phillips-Quagliata J. M., Uhr J. W. Cell surface immunoglobulin. X. Identification of an IgD-like molecule on the surface of murine splenocytes. J Exp Med. 1974 Nov 1;140(5):1427–1431. doi: 10.1084/jem.140.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M. F., Pollock R. R. Murine cell surface immunoglobulin: two forms of delta-heavy chain. J Immunol. 1979 Sep;123(3):1155–1161. [PubMed] [Google Scholar]
- Moore K. W., Rogers J., Hunkapiller T., Early P., Nottenburg C., Weissman I., Bazin H., Wall R., Hood L. E. Expression of IgD may use both DNA rearrangement and RNA splicing mechanisms. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1800–1804. doi: 10.1073/pnas.78.3.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLUMMER T. H., Jr, HIRS C. H. ON THE STRUCTURE OF BOVINE PANCREATIC RIBONUCLEASE B. ISOLATION OF A GLYCOPEPTIDE. J Biol Chem. 1964 Aug;239:2530–2538. [PubMed] [Google Scholar]
- Parkhouse R. M., Lifter J., Choi Y. S. Chemical characterisation of the Fab and Fc fragments from surface immunoglobulin. Nature. 1980 Mar 20;284(5753):280–281. doi: 10.1038/284280a0. [DOI] [PubMed] [Google Scholar]
- Pearson T., Galfrè G., Ziegler A., Milstein C. A myeloma hybrid producing antibody specific for an allotypic determinant on "IgD-like" molecules of the mouse. Eur J Immunol. 1977 Oct;7(10):684–690. doi: 10.1002/eji.1830071006. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pernis B., Brouet J. C., Seligmann M. IgD and IgM on the membrane of lymphoid cells in macroglobulinemia. Evidence for identity of membrane IgD and IgM antibody activity in a case with anti-IgG receptors. Eur J Immunol. 1974 Nov;4(11):776–778. doi: 10.1002/eji.1830041114. [DOI] [PubMed] [Google Scholar]
- Pernis B. Lymphocyt membrane IgD. Immunol Rev. 1977;37:210–218. doi: 10.1111/j.1600-065x.1977.tb00251.x. [DOI] [PubMed] [Google Scholar]
- ROWE D. S., FAHEY J. L. A NEW CLASS OF HUMAN IMMUNOGLOBULINS. II. NORMAL SERUM IGD. J Exp Med. 1965 Jan 1;121:185–199. doi: 10.1084/jem.121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Rowe D. S., Hug K., Forni L., Pernis B. Immunoglobulin D as a lymphocyte receptor. J Exp Med. 1973 Oct 1;138(4):965–972. doi: 10.1084/jem.138.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salsano F., Froland S. S., Natvig J. B., Michaelsen T. E. Same idiotype of B-lymphocyte membrane IgD and IgM. Formal evidence for monoclonality of chronic lymphocytic leukemia cells. Scand J Immunol. 1974;3(6):841–846. doi: 10.1111/j.1365-3083.1974.tb01321.x. [DOI] [PubMed] [Google Scholar]
- Shields D., Blobel G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc Natl Acad Sci U S A. 1977 May;74(5):2059–2063. doi: 10.1073/pnas.74.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer P. A., Singer H. H., Williamson A. R. Different species of messenger RNA encode receptor and secretory IgM mu chains differing at their carboxy termini. Nature. 1980 May 29;285(5763):294–300. doi: 10.1038/285294a0. [DOI] [PubMed] [Google Scholar]
- Sitia R., Corte G., Ferrarini M., Bargellesi A. Lymphocyte membrane immunoglobulins: similarities between human IgD and mouse IgD-like molecules. Eur J Immunol. 1977 Aug;7(8):503–507. doi: 10.1002/eji.1830070802. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]