Abstract
Activation of the cyclin-dependent kinase (Cdk1) cyclin B (CycB) complex (Cdk1:CycB) in mitosis brings about a remarkable extent of protein phosphorylation. Cdk1:CycB activation is switch-like, controlled by two auto-amplification loops—Cdk1:CycB activates its activating phosphatase, Cdc25, and inhibits its inhibiting kinase, Wee1. Recent experimental evidence suggests that parallel to Cdk1:CycB activation during mitosis, there is inhibition of its counteracting phosphatase activity. We argue that the downregulation of the phosphatase is not just a simple latch that suppresses futile cycles of phosphorylation/dephosphorylation during mitosis. Instead, we propose that phosphatase regulation creates coherent feed-forward loops and adds extra amplification loops to the Cdk1:CycB regulatory network, thus forming an integral part of the mitotic switch. These network motifs further strengthen the bistable characteristic of the mitotic switch, which is based on the antagonistic interaction of two groups of proteins: M-phase promoting factors (Cdk1:CycB, Cdc25, Greatwall and Endosulfine/Arpp19) and interphase promoting factors (Wee1, PP2A–B55 and a Greatwall counteracting phosphatase, probably PP1). The bistable character of the switch implies the existence of a CycB threshold for entry into mitosis. The end of G2 phase is determined by the point where CycB level crosses the CycB threshold for Cdk1 activation.
Keywords: mitotic switch, mitotic phosphatases, feedback loops, feed-forward loops, bistability
1. Introduction
Mitosis (or M phase in general) is brought about by abrupt activation of the M-phase promoting factor (MPF), initially discovered as a cytoplasmic activity that promoted oocyte maturation [1]. Early experiments detected high MPF activity during M phase in all eukaryotic cells examined by using an oocyte maturation assay [2]. Purification of MPF [3] revealed that it was composed of a dimer of the homologue of the fission yeast Cdc2 protein and B-type cyclins [4–6]. Cdc2 and its budding yeast homologue Cdc28, discovered by Nurse et al. [7] and Hartwell et al. [8], respectively, were shown to be serine–threonine protein kinases [9] that are conserved in all eukaryotes from yeasts to man, and whose activity fluctuates during the cell cycle [10,11]. The B-type cyclins (Cyclin B; CycB) were discovered in fertilized sea urchin eggs by virtue of their abrupt disappearance during mitosis, after steady accumulation during interphase [12,13]. Since association with cyclins turned out to be essential for Cdc2 protein kinase activity, Cdc2/Cdc28 became the founding member of the cyclin-dependent protein kinases and was renamed Cdk1. The Cdk1:CycB complex is considered to be the trigger of mitosis in all eukaryotic cells, because it can promote this state by phosphorylating many downstream mitotic proteins [14], which include other protein kinases such as aurora and polo. It is believed that this big increase in protein phosphorylation in M phase is responsible for bringing about all the changes associated with mitosis. Different studies have identified hundreds of mitotic phosphoproteins, many of them probably phosphorylated directly by Cdks [15–17]. However, there is still much more to understand about the relative importance of these phosphorylations and how these events are timed and coordinated to ensure ordered cell cycle progression.
As expected from its prominent role in triggering mitosis, the activation of Cdk1:CycB complexes is tightly regulated. CycB binding is necessary but not sufficient for Cdk1 activity, because the Cdk1:CycB dimers are not necessarily active. In interphase, the Cdk1 subunit of the dimer is phosphorylated and inactivated by protein kinases belonging to the Wee1 family [18]. The first member of these inhibitory kinases, Wee1, was discovered in fission yeast by Paul Nurse, who isolated mutant cells that advanced into mitosis at a reduced cell size [19,20]. Most organisms have duplicates of these inhibitory kinases (i.e. Wee1 and Mik1 in fission yeast, and Wee1 and Myt1 in Xenopus laevis) [21,22]. The Wee1-dependent inhibitory phosphorylations on Cdk1 are removed by the Cdc25 phosphatase, also discovered in fission yeast, which therefore acts as a Cdk1 activator [23]. Additionally, ingenious experiments with frog egg extracts showed that the Wee1-kinase and the Cdc25-phosphatase are also Cdk1:CycB substrates [24–26]. However, Cdk1-dependent phosphorylation of these regulatory enzymes has opposite effects on their activities; phosphorylation inhibits Wee1 but activates Cdc25 [27,28]. Therefore, the activity of Cdk1:CycB is regulated by a double-negative (Cdk1 ⊣ Wee1 ⊣ Cdk1) and a positive (Cdk1 → Cdc25 → Cdk1) feedback loop (top of figure 1), that is, Cdk1:CycB activates its activator and inhibits its inhibitor [29,30]. This regulatory network was unravelled by successful combination of yeast genetics and frog egg biochemistry and seems to be responsible for the activation of Cdk1:CycB at the G2/M transition. This feature is also well-conserved in the eukaryotic cell cycle control system [14], yet it raises a very important problem, which is how can the two stable states, interphase or mitosis, ever change? In interphase, Cdk1 activity is repressed by Wee1, whereas in mitosis, Wee1 activity is repressed by Cdk1. It is perhaps easier to understand that proteolysis of the cyclins leads to exit from mitosis and a return to interphase than it is to see how cells enter mitosis. In addition, there are numerous controls that affect entry into mitosis—the state of DNA replication, the integrity of the chromosomes, or the arrival of triggers like progesterone (in the case of frog oocytes) or 1-methyladenine (starfish oocytes) or fertilization (clam oocytes)—that still require full elucidation as to their modes of action.
Figure 1.
Influence diagram of mitotic regulators that make up the mitotic switch. Arrows represent activating interactions, and blunt-ended lines inhibition of activity of the target protein. Green- and red-labelled proteins and interactions are active in mitosis and interphase, respectively. Dashed lines represent proposed, but as yet unproven, interactions.
Nevertheless, the basic picture of the cell cycle control system, with cells accumulating mitotic cyclins during interphase, eventually leading to mitotic entry, followed by the abrupt degradation of CycB at the onset of anaphase, provides an appealingly simple picture of mitotic control in eukaryotes through the regulation of Cdk1:CycB activity [31]. During interphase (actually, only during S and G2 phases in somatic cells), mitotic cyclins are stable and their continuous synthesis leads to an increase in their abundance and hence the concentration of Cdk1: CycB dimers. Since Wee1 activity is high and Cdc25 activity is low, the dimers accumulate largely in the Cdk1-phosphorylated, inactive form. However, some Cdk1:CycB activity will be present, either because not all dimers get phosphorylated, or owing to a small amount of Cdc25 activity and/or because the phosphorylated form of Cdk1:CycB retains some residual activity. The activity will be proportional to the level of CycB. As cyclin levels rise, the proportion of active dimers relative to the total dimers remains unchanged but their absolute quantity increases [29]. Once Cdk1 activity reaches a critical level, required to significantly inactivate Wee1 and activate Cdc25, the inactive dimers start to be dephosphorylated and activated. This engages the positive and double-negative feedback loops, and leads to further Cdc25 activation and Wee1 inhibition, resulting in abrupt activation of Cdk1:CycB and transition from G2 to M phase. This abrupt activation of Cdk1 occurs only when the concentration of CycB exceeds a particular threshold, as demonstrated by Solomon et al. [32]. Active Cdk1:CycB eventually turns on CycB degradation by activating the APC/C (anaphase-promoting complex/cyclosome) ubiquitin-ligase, which labels CycB for degradation [33,34]. After proteasomal degradation of the CycBs, Cdk1 and APC/C lose their activities and the process repeats itself [35]. Although this model for Cdk1:CycB activation in the embryonic cell cycles is still essentially correct [29,30], it turns not to be the whole story of M-phase initiation. In the following, we describe recent discoveries about M-phase control in higher eukaryotes and speculate where the story might go further. Most of the recent discoveries have been made in Xenopus egg extracts, which is therefore our focus. However, some of the new features are being confirmed in other organisms, indicating that our proposals may have wider implications.
2. Cdk1-counteracting phosphatases and greatwall
Because Cdk1:CycB is a protein kinase, it is generally assumed, if not demonstrated, that entry into mitosis is triggered by the phosphorylation of a certain set of proteins. Exit from mitosis must thus require dephosphorylation of these proteins by protein phosphatases, as it seems that few phosphoproteins are degraded at the end of mitosis. The question then arises, are these phosphatases regulated? Is entry into mitosis simply achieved by a tremendous, overwhelming burst of protein kinase activity, or are some of the phosphatases inactivated at the same time as the Cdk1:CycB is turned on? It has long been known that addition of the phosphatase inhibitor okadaic acid (OA) leads to M-phase entry [36], and this effect has been attributed to inhibition of phosphatases of the PP2A family [37]. This indicates that PP2A phosphatases are active in interphase and suggests that one or more of these phosphatases reverse the small amount of Cdk1-dependent phosphorylation that can take place in interphase [38]. Inhibition of Cdk1-counteracting phosphatases facilitates the phosphorylation of Cdk1 target proteins, which can then occur even at low Cdk1 activities. In addition to this effect, phosphatase inhibition also causes an activation of Cdk1 by affecting the feedback loops involving Wee1 and Cdc25. That is, because Wee1 and Cdc25 are also Cdk1 targets, inhibition of Cdk1-counteracting phosphatases can shift these proteins to their phosphorylated forms, which results in Cdc25 activation, Wee1 inhibition, and thus full activation of Cdk1: CycB dimers, even at low CycB levels. Indeed, OA eliminates the cyclin threshold of Cdk1 activation caused by inhibitory phosphorylations in Xenopus egg cell-free extracts and it fully activates any Cdk1 bound to CycB [29,32,39]. Despite these suggestive observations, however, research in mitotic phosphatases lagged behind that of mitotic kinases. The prevailing, though largely unexamined view used to be that phosphatases were neither terribly specific, nor regulated in interesting ways. Their effects were thus viewed as pleiotropic and their cell cycle-specific functions too difficult to dissect. Besides, the activity of Cdk1-counteracting phosphatases could, in principle, be constant throughout the cell cycle, and be overcome by the fluctuating Cdk1 activity in mitosis. Recent findings strongly challenge this view.
Mochida & Hunt [40] showed that phosphatase activity against a model Cdk1:CycB substrate fluctuated in the cell cycle of Xenopus egg extracts, being high in interphase and low in mitosis. It was shown that phosphatases such as PP2A can target specific substrates at particular times, depending on the binding of regulatory subunits [41]. Further work identified PP2A bound to its B55δ regulatory subunit as the specific and regulated Cdk1-counteracting phosphatase [42]. Depletion of B55δ from cycling Xenopus egg extracts advanced entry into mitosis and compromised exit from mitosis via CycB degradation. The mitotic advancement was caused by premature activation of Cdk1 and phosphorylation of mitotic substrates. By contrast, addition of extra, purified PP2A–B55δ complexes delayed and blocked Cdk1 activation and entry into mitosis, in a dosage-dependent manner, owing to Wee1-dependent phosphorylation of Cdk1 [42]. In a similar way to addition of OA, the simultaneous effects of promoting Cdk1 inhibitory phosphorylation, and dephosphorylation of Cdk1 substrates in interphase suggested that PP2A–B55δ acts on both downstream Cdk1 substrates and in the Cdk1 auto-activation loops (on Wee1 and Cdc25). Other recent studies have also implicated the B55α regulatory subunit of PP2A in the regulation of mitosis and as a Cdk-counteracting phosphatase in other experimental systems [43–45]. Therefore, in the following, we refer to the Cdk1-counteracting phosphatase as PP2A–B55, implying that several B55 isoforms can act as Cdk1-counteracting phosphatases in different cells or in different conditions.
Meanwhile, a novel mitotic kinase important for proper timing of G2–M transitions called Greatwall (GW) was discovered in Drosophila [46]. Subsequent work with Xenopus egg extracts revealed that GW is required for both establishment and maintenance of M phase [47,48]. This kinase was shown to be phosphorylated by Cdk1:CycB and its activity increased in mitosis [47]. Addition of the activated form of the kinase promoted M-phase entry in cycling extracts, while depletion prevented mitotic entry and Cdc25 activation. Depletion from CSF extracts (oocyte extracts blocked in metaphase of meiosis II by cytostatic factor, CSF) caused dephosphorylation of mitotic phosphoproteins and mitotic exit. GW depletion was accompanied by Wee1-dependent inactivation of Cdk1 without any cyclin degradation, suggesting that GW regulates the Cdk1 auto-amplification loop [47,48]. In the presence of OA, however, the M-phase extract was resistant to GW depletion, suggesting that GW inhibits an okadaic-sensitive phosphatase in M phase. Tantalizingly, addition of OA allowed mitotic entry in GW-depleted cycling extracts, also suggesting that GW had an inhibitory effect on an okadaic-sensitive phosphatase [48]. However, simultaneous depletion of GW and Wee1 from M-phase extracts still led to mitotic exit, even though Cdk1 activity remained high [49], suggesting that the activated phosphatase overcomes Cdk1:CycB activity.
These two initially independent lines of research on GW and PP2A–B55 finally converged, by the demonstration that GW downregulates PP2A–B55 activity [49,50]. Interestingly, GW inhibition of PP2A–B55 is not direct, but mediated by the low molecular-weight phosphatase inhibitors Endosulfine (ENSA) and Arpp19 (we refer to both as ENSA in the following). These proteins probably act as stoichiometric inhibitors, binding directly to the B55 subunit [51,52]. The level of ENSA is constant during early embryonic cycles in the frog, but it is extensively phosphorylated in mitosis, probably by several kinases. In particular, GW-dependent phosphorylation converts ENSA into a highly specific inhibitor of PP2A–B55. As expected from its function as a PP2A inhibitor, addition of constitutively active thio-phosphorylated ENSA promotes mitotic entry in cycling and interphase extracts, similar to OA, whereas its depletion blocks mitotic entry, like depletion of GW [51,52].
Thus, when Cdk1:CycB activity is low, its counteracting phosphatase is active, keeping mitotic substrates dephosphorylated. In contrast, in the mitotic state Cdk1:CycB downregulates its counteracting phosphatase by promoting the activities of both GW and ENSA, ensuring that while kinase activity is high, phosphatase activity is low. Therefore, it could be proposed that downregulation of the Cdk1-counteracting phosphatase activity in mitosis acts as a latch, by allowing downstream mitotic substrates to become fully phosphorylated thus preventing futile cycling. However, we argue that this is not the only consequence of downregulating Cdk1-counteracting phosphatases in mitosis, and that these findings have important consequences for the functioning of the mitotic control system.
3. Feed-forward loops and more feedback loops: switches or latches?
Because Cdk1:CycB promotes the inhibition of its counteracting phosphatase, its activity has a dual effect on the phosphorylation of mitotic substrates: it directly promotes their phosphorylation and indirectly inhibits their dephosphorylation, creating coherent feed-forward loops [53]. This ensures that the kinase-to-phosphatase ratio changes sharply between interphase and mitosis, helps to minimize unwanted futile cycles of phosphorylation/dephosphorylation of mitotic Cdk1 substrates and allows mitotic phosphoproteins to become phosphorylated to a great extent (assuming that there are no other phosphatases that can act on them, which may or may not be true). These coherent feed-forward loops also result in a nonlinear, ultrasensitive rise in the phosphorylation state of the substrates as a function of Cdk1 activity [39,54], in principle functioning as a latch by allowing efficient phosphorylation of downstream Cdk1 substrates. However, as Cdc25 and Wee1 are also Cdk1:CycB and PP2A–B55 substrates, they would also show a sigmoid response to increasing kinase levels, owing to the coherent feed-forward loops created by the regulation of PP2A–B55. This response is probably made even more switch-like by additional mechanisms such as multi-site phosphorylation, substrate competition and sequestration effects [27,28,55]. These sigmoid responses are very important for creating robust and efficient bistable switches, when involved in a positive (or double-negative) feedback loop [56], as explained below.
The indirect inhibition of PP2A–B55 by Cdk1: CycB also creates new feedback loops in the network. In particular, Cdk1:CycB and PP2A are involved in a deeply antagonistic relationship; not only do they reverse each other's reactions, but they also form double-negative feedback loops (figure 1). That is, Cdk1:CycB downregulates PP2A–B55 indirectly by activating GW and ENSA (Cdk1 → GW → ENSA ⊣ PP2A), while PP2A–B55 also indirectly inhibits Cdk1:CycB, by activating Wee1 (PP2A → Wee1 ⊣ Cdk1) and inhibiting Cdc25 (PP2A ⊣ Cdc25 → Cdk1). These double-negative feedback loops between Cdk1:CycB and PP2A–B55, together with the Cdc25–Cdk1-positive and Wee1–Cdk1 double-negative feedback loops, help create two characteristically different states in terms of kinase-to-phosphatase ratio. In the low kinase-to-phosphatase ratio state (interphase), PP2A-B55 and Wee1 activities are high and Cdk1, Cdc25, GW and ENSA activities are low. Thus, Cdk1 substrates remain dephosphorylated. In contrast, in the high kinase-to-phosphatase ratio states (M phase), the opposite is true: Cdk1, Cdc25, GW and ENSA activities are high; Wee1 and PP2A–B55 activities are low, and Cdk1 substrates are phosphorylated. The fact that the Cdk1:CycB-counteracting phosphatase PP2A–B55 is regulated in a cell cycle-dependent manner can explain the most recent biochemical data and provides a better description of mitotic control. Furthermore, the fact that it affects the feedback loops controlling Cdk1 activation makes this phosphatase and its regulation an integral component that shapes the mitotic switch, rather than being just a latch that allows full phosphorylation of downstream substrates. Thus, we propose that regulation of Cdk1-counteracting phosphatase activity in mitosis plays an important role in establishing the sharp separation between interphase and mitosis, because the phosphatase is involved in the feedback loops that regulate Cdk1:CycB activity. However, we suspect that the present model is still not complete and we discuss ways of possible further refinements.
4. Closing the feedback loops
To fully describe a network with reversible phosphorylation each phosphoprotein must be targeted by at least one kinase and one phosphatase. So far, we know that Wee1 and Cdc25 target Cdk1, while Cdk1:CycB and PP2A–B55 target Wee1 and Cdc25. However, we also know that Cdk1 phosphorylates GW and GW phosphorylates ENSA, but as the respective phosphatases have not yet been identified, this is at present a rather critical lacuna. In the following, we discuss some hypotheses about the nature and regulation of these phosphatases.
Since GW is a Cdk1 substrate, we suspect that it might be dephosphorylated, and thus inactivated, by the Cdk1-counteracting phosphatase PP2A–B55 (figure 1). If this were the case, GW and PP2A–B55 would be involved in yet another double-negative feedback loop, as GW inhibits PP2A–B55 through ENSA (GW → ENSA ⊣ PP2A) and PP2A–B55 counteracts GW activation by Cdk1 (PP2A ⊣ GW). This double-negative feedback loop could further sharpen the ultrasensitive phosphorylation of Cdk1 substrates, making the mitotic switch more robust [54].
Some evidence suggests that GW might be a PP2A–B55 substrate. Clues come from experiments showing that GW becomes phosphorylated at very low Cdk1 activity [48,51], suggesting that it has an inhibitory effect on its counteracting phosphatase, as explained below. For example, PP2A inhibition by microcystin or phosphorylated ENSA in Cdc25-depleted, interphase-arrested (cycloheximide blocked) frog egg extracts causes mitotic-like GW phosphorylation [51]. Under these conditions, CycB levels are low and the few Cdk1:CycB dimers are inhibited by Wee1-dependent phosphorylation. Since Cdk1 : CycB activity is low, if another phosphatase, not inhibited by microcystin or ENSA, were responsible for GW inhibition, then GW should remain dephosphorylated after PP2A–B55 inhibition, because such a phosphatase would still be active. A related observation is that activated (phosphorylated) GW induces mitosis in interphase extracts in the presence of the Cdk inhibitor roscovitine [48]. As in the previous argument, this suggests that active GW inhibits its own phosphatase, because otherwise, active GW should be quickly dephosphorylated and inactivated when placed into an extract where its activating kinase, Cdk1 is inhibited. This again points to PP2A–B55, because it is known that GW inhibits it through ENSA. However, other scenarios could help explain these results. For instance, GW could be phosphorylated by a kinase other than Cdk1 which becomes activated by PP2A inhibition, which seems unlikely. Another possibility is that GW is dephosphorylated by a phosphatase other than PP2A–B55 which is inhibited by Cdk1 and/or GW.
However, another key observation that suggests that PP2A dephosphorylates GW is the physical interaction of the two proteins [49]. Since GW indirectly regulates PP2A through ENSA, and there are no GW-phosphorylation sites on PP2A, the interaction could indicate that PP2A binds to phosphorylated GW in order to dephosphorylate it. However, it is possible that the relationship between PP2A–B55 and GW is still more complicated. Recent data suggest that GW binds to PP2A in interphase, where the phosphatase might prevent premature phosphorylation of GW [57]. Our proposal also raises the question of how PP2A–B55 can dephosphorylate GW at mitotic exit, as the phosphatase is expected to be inactivated by ENSA at this time. One possibility is that PP2A–B55 inhibition by ENSA is not complete, and since Cdk1 activity, and thereby GW phosphorylation drops at mitotic exit, autocatalytic activation of PP2A–B55 could be initiated at this time. Nonetheless, it is possible that phosphatases different from PP2A–B55 are in fact responsible for GW dephosphorylation or have an important role in its dephosphorylation. In fact, although it is clear that Cdk1 can phosphorylate GW, and that this phosphorylation evokes some activity, other modifying enzymes or kinases, and therefore phosphatases, may be involved in its regulation.
The other unknown phosphatase in the mitotic control switch is the GW-counteracting phosphatase that dephosphorylates ENSA. Reversible phosphorylation/dephosphorylation of ENSA is well established; in Xenopus egg extracts, ENSA is phosphorylated during M phase, but not in interphase, while its amount remains constant throughout the cell cycle and the phosphorylated form does not seem to be specifically targeted for degradation [52]. Furthermore, thio-phosphorylated but not phosphorylated ENSA can stabilize M phase in CSF extracts or induce M phase in interphase extracts in the absence of GW [51]. This indicates that ENSA is rapidly dephosphorylated by an unidentified phosphatase in the absence of its kinase GW.
The activity of the GW-counteracting phosphatase acting on ENSA is crucial for the cell cycle control system, and we anticipate that perturbations to this activity could have effects very similar to perturbations to the Cdk1-counteracting phosphatase. For example, inhibition of this phosphatase in interphase could cause mitotic entry, similar to the addition of OA or removal of PP2A–B55 in interphase, because a small amount of background GW activity could be sufficient to activate ENSA. By the same logic, enhanced ENSA dephosphorylation in interphase should delay mitotic entry. In mitosis, increased activity of the ENSA phosphatase could cause premature mitotic exit owing to activation of PP2A–B55 and subsequent inhibition of Cdk1:CycB. In contrast, its depletion in M phase could delay or block mitotic exit because of compromised PP2A–B55 activation. Although cell cycle regulation of the ENSA-dephosphorylating phosphatase is not a necessary requirement for the control system, it looks like that this is the case. Preliminary experiments suggest that the ENSA phosphatase is active in mitosis and about twice as active in interphase (Mochida & Hunt 2011, unpublished data), and mathematical models suggest that this regulation could help make the mitotic switch more robust (Domingo-Sananes & Novak 2011, unpublished data).
The remaining question is the identity of this phosphatase that acts on ENSA, the GW-counteracting phosphatase. GW belongs to a different family of protein kinases from the Cdks, known as the AGC serine/threonine kinase family. Since GW phosphorylates completely different sites than Cdk1:CycB, it is unlikely that the same phosphatase dephosphorylates sites targeted by these kinases, potentially ruling out PP2A–B55 as a candidate. The characteristics of the ENSA phosphatase mentioned above suggest that PP1 could be responsible for ENSA dephosphorylation. This would explain recent data which showed that PP1 activity is required for dephosphorylation of mitotic proteins at mitotic exit [58].
If PP1 were indeed responsible for ENSA dephosphorylation, then even more feed-forward and feedback loops are created in the network, especially because PP1 is inhibited by Cdk1 [59]. In this case, Cdk1 downregulates PP2A–B55 via two redundant arms; activation of GW increases ENSA phosphorylation while inhibition of PP1 reduces ENSA dephosphorylation, which results in efficient ENSA phosphorylation and thus PP2A–B55 inhibition via a feed-forward loop at mitotic entry. Furthermore, if Cdk1 phosphorylates PP1, a tempting assumption is that PP2A–B55 acts as the Cdk1-counteracting phosphatase, which removes these inhibitory phosphates from PP1 (figure 1). This would create a positive feedback loop between PP2A–B55 and PP1, because PP2A activates PP1, while PP1 inhibits ENSA, thus activating PP2A (PP2A → PP1 ⊣ ENSA ⊣ PP2A). However, the identity of the GW-counteracting phosphatase(s) is still an open question that should be resolved, but we believe that it is likely to be regulated, as are all of the other players in our story.
5. All for one and one for all . . .
All the players of the mitotic control switch described above, along with proven and suggested links between them, are shown schematically in figure 1. The proposed network is beautifully symmetric in the interaction pattern of the mitotic regulators; all of the components and reactions are coherently regulated and every kinase is counteracted by a phosphatase. Even if the identities of some of the players involved remain to be confirmed, as well as the relationships between them, it conveys a very appealing picture of the organization of the network controlling the mitotic switch. The mitotic regulators can be divided into two major groups, which have an antagonistic relationship with each other (figure 2). One group contains Cdk1, Cdc25, GW and ENSA, which directly or indirectly increase the phosphorylation of downstream Cdk1 target proteins and thus promote M phase. Therefore, we refer to this group as the mitosis promoting factors (MPFs). The members of the other group, Wee1, PP2A-B55 and PP1, have an opposite effect; they directly or indirectly decrease the mitotic phosphorylations by Cdk1 and thus promote interphase. Therefore, we refer to this group as the interphase promoting factors (IPFs). The central nodes in the two groups are Cdk1 and PP2A–B55, which directly influence downstream Cdk1 targets. Besides having a negative effect on the other group, each member of a group promotes the activity of the other proteins in its own team via positive feedback loops similar to the ‘Three Musketeers’ (figure 2). This positive feedback is often direct, as for Cdk1 and Cdc25 (Cdk1 → Cdc25 → Cdk1). However, it can also be exerted through a long positive feedback that might contain an even number of inhibitory steps, as for Cdk1 and GW (Cdk1 → GW → ENSA ⊣ PP2A ⊣ Cdc25 → Cdk1). Similarly, for the IPF members, a relatively short positive feedback loop could connect PP2A–B55 and PP1, as described above (PP2A → PP1 ⊣ ENSA ⊣ PP2A), while there is a long positive feedback loop between PP2A–B55 and Wee1 (PP2A → Wee1 ⊣ Cdk1 → GW → ENSA ⊣ PP2A).
Figure 2.
The antagonistic and self-activating interaction between MPFs and IPFs. The protein molecules in the mitotic switch belong to one of the following two categories: mitosis promoting factors (MPFs) and interphase promoting factors (IPFs). Molecules in each group activate each other and inhibit the members of the other group represented by dashed lines. MPFs and IPFs have antagonistic effects on the phosphorylation of downstream substrates.
In this picture, self-activation and mutual inhibition between MPFs and IPFs create the two characteristically different stable states, M phase and interphase, which we can observe by the phosphorylation state of downstream Cdk1:CycB/PP2A–B55 substrates. When IPFs are active and MPFs are inactive, the kinase-to-phosphatase ratio (Cdk1:CycB/PP2A–B55) is low and the downstream substrates remain in a dephosphorylated state, characteristic of interphase. The other possible state occurs when MPFs are high and IPFs are low, leading to a high kinase-to-phosphatase ratio and phosphorylation of mitotic phosphoproteins, the hallmark of mitosis. Under normal circumstances, an intermediate situation where IPFs and MPFs are both partially active and most substrates only partially phosphorylated is unstable and almost impossible to maintain; as soon as one of the sides gains the upper hand, its self-activation and inhibition of the competing side always takes the system to one of the two stable states.
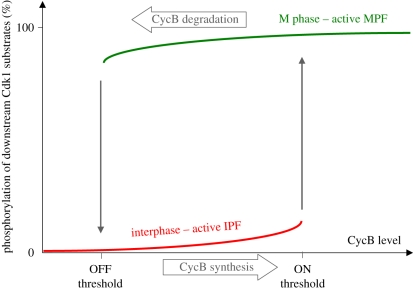
Which state is actually occupied by the mitotic control system is determined by an upstream regulator of Cdk1 activity, the CycB level. We can therefore visualize the system by plotting the phosphorylation level of a downstream Cdk1:CycB/PP2A–B55 substrate with respect to the CycB level (figure 3). When the substrate is mostly dephosphorylated, the system is in interphase (red) and when the substrate is phosphorylated, the system is in mitosis (green). Normally, at low CycB levels, IPFs win over MPFs and interphase is manifested. Increasing CycB strengthens MPFs and at a critical CycB threshold, which we call the ON-threshold, MPFs win against the IPFs, leading to an abrupt jump in phosphorylation of downstream substrates. Since the MPFs also support each other besides downregulating the IPFs, the M-phase state is self-stabilized. As a consequence of this self-stabilization, if CycB levels decrease once the control system is in M phase, MPFs stay active and the downstream targets remain phosphorylated. Dephosphorylation of the downstream targets requires that CycB levels drop below another threshold, the OFF-threshold, which is lower than the ON-threshold. At this stage, Cdk1 eventually loses its power struggle to keep the MPFs active and the IPFs win. The existence of these two thresholds has been demonstrated experimentally [60,61].
Figure 3.
Signal response curve of the mitotic switch. Phosphorylation state of downstream Cdk1–PP2A–B55 substrates are plotted as a function of cyclin B level (schematic). There are two stable states, interphase and mitosis, a CycB threshold for activation of MPFs and inhibition of IPFs (ON-threshold) and a different threshold for inactivation of MPFs and activation of IPFs (OFF-threshold). At these thresholds, the mitotic control system can jump from one stable state to the other. Cyclin synthesis drives the system from the left to the right on this diagram, while cyclin degradation pushes it in the reverse direction.
The horizontal movement of the mitotic control system in figure 3 depends on the relative rates of CycB synthesis and degradation. If the rate of synthesis exceeds the rate of degradation, the system moves to the right because the CycB level increases. If the system is in the interphase state, it will move along the lower branch until CycB levels surpass the ON-threshold, at which point it jumps to the mitosis state, leading to phosphorylation of the substrate. If CycB degradation is faster than its synthesis, the CycB level will decrease and the system moves to the left. Once the OFF-threshold is crossed, the system will move back to the interphase state. If synthesis and degradation are balanced, the system settles into one of the stable steady states. Which steady state is occupied depends primarily on the cyclin level. However, if the CycB level is between the two thresholds, then the final state depends on where the control system was before—its history.
The best example of transitions between the two states occurs during early embryonic cell cycles. Here, CycB synthesis is constant while degradation is abruptly increased at mitotic exit owing to Cdk1-dependent activation of APC/C [33]. Like some of the components of the mitotic switch shown in figure 1, APC/C is not a simple downstream target of Cdk1, because it feeds back to the control system by promoting CycB degradation. The crucial difference is that this regulation creates a negative feedback loop, which allows the system to cycle between interphase and M phase by controlling the availability of CycB. Therefore, in interphase, when CycB is low, the MPFs are low, IPFs are high, APC/C is inactive and cyclin synthesis is faster than its degradation, allowing cyclin accumulation and eventually entry into M phase and phosphorylation of downstream substrates. This results in APC/C activation, and thus a sharp drop in cyclin levels, because degradation wins over synthesis. When cyclin drops below the OFF-threshold, mitotic substrates are dephosphorylated, APC/C is inactivated and the cycle starts again.
In this revised view of the mitotic control network (figure 1), the discrete separation between interphase and mitosis, and the two CycB thresholds for mitotic entry and exit are system-level properties, which arise from the underlying interconnected network and create a robust switch. However, the high connectivity of the network also allows modifications of the components and their regulation to have significant effects on these system-level properties. For instance, weakening of any of the IPFs, or strengthening of the MPFs, reduces both CycB thresholds and makes the M-phase state preferable, even at low CycB levels. The opposite is also true; strengthening of IPFs or weakening of MPFs increases the CycB thresholds and destabilizes the mitotic state, making interphase preferable even at high CycB levels.
Because of these effects on the CycB thresholds, external intervention can change the CycB thresholds and allow the mitotic control system to change between interphase and mitosis at constant CycB. There is significant experimental evidence to support this idea. For instance, depletion of the MPFs Cdc25, GW and ENSA in interphase blocks the G2/M transition even at high CycB levels [48,52,62]. In contrast, depletion of any of these proteins in M phase can cause dephosphorylation of mitotic substrates and destabilization of M phase [47,62]. On the other hand, depletion or inhibition of the IPFs, PP2A–B55 and Wee1 in interphase can result in premature mitotic entry [42,47,62]. Inhibition of PP1 blocks exits from M phase [58], but strangely, depletion of B55δ only blocks mitotic exit when it is performed in the previous interphase but not in mitosis [42].
6. Commitment to enter into mitosis
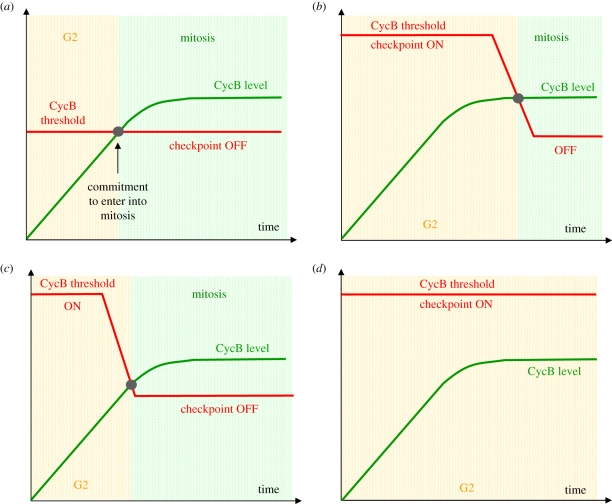
Figure 1 presents an up-to-date picture of the molecular interactions that constitute the mitotic switch, based on most recent experimental data from frog egg extracts and our speculations along these lines of evidence. In many cell types (e.g. Xenopus embryos, fission yeast), this mitotic switch determines the time of mitotic entry, i.e. the length of G2 phase. Since the mitotic switch represents a ‘trigger structure’, the question is what pulls the trigger? In other words, what are the rate-limiting components or processes for mitotic entry that determine the length of G2 phase? Mitotic entry happens when the CycB level meets the ON-threshold of the switch, much like a rendezvous of two people approaching each other from different directions. The threshold is determined by the strength of the regulatory interactions and feedback loops in the network in figure 1. These interactions are influenced by different surveillance mechanisms like DNA replication and/or cell size checkpoints, which can shift the position of the ON-threshold by affecting the abundance or activity of one or several components of the switch. Active checkpoints keep the ON-threshold at a high (physiologically unreachable) CycB level, making it impossible for the cell to enter M phase. The ON-threshold decreases during the process of checkpoint silencing, and once CycB and threshold meet, the cell commits to mitotic entry. Thus, there are several possible scenarios for when the mitotic switch can be flipped, schematically shown in figure 4. In these, we plot the CycB level and the value of the ON-threshold as a function of time. We start at CycB values close to zero, which correspond to early S phase, when mitotic cyclins start to accumulate. CycB can only reach a certain maximum steady-state level because of physiological limitations. For simplicity, we assume a mitotic block, i.e. CycB is not degraded after entry into mitosis.
Figure 4.
The timing of mitosis and the length of G2 are determined by the Cdk1:CycB activation threshold and CycB synthesis rate. The red and green curves represent the change in the ON-threshold and CycB levels with time, respectively. For simplicity, we ignore APC/C-dependent CycB degradation which turns on at the end of mitosis. The intersection point of the two curves determines the commitment point for entering into mitosis. The value of the CycB threshold depends on the mitotic switch, which is influenced by DNA replication and cell size checkpoint mechanisms. Several scenarios are possible. (a) The cyclin threshold remains constant throughout interphase because checkpoints are inactive or already satisfied. CycB increases gradually in interphase and its rate of accumulation (the slope of the green line) determines the timing of mitotic entry. This situation is probably realized in embryonic cell cycles. (b) The cyclin threshold drops when checkpoints are turned off, but this occurs after CycB has reached its maximum level. In this case, the silencing of the checkpoint is rate-limiting for mitotic entry. This situation probably occurs in cells recovering from some types of checkpoint arrest. (c) Checkpoints are silenced while the CycB level is still increasing. Here, both the rate of CycB accumulation and checkpoint silencing determine the timing of mitotic entry. This is most likely the case in many somatic and free-living cells. Cases (a) and (b) can be seen as the two extremes of this picture. (d) This picture represents a stable interphase arrest owing to permanent activation of checkpoints. The cyclin threshold remains higher than the maximum physiological CycB level so that there is no mitotic entry. Eventual silencing of the checkpoint would result in case (b).
Figure 4a depicts a situation where checkpoint mechanisms are absent or turned off early in the cycle. The ON-threshold remains fixed, and the time to enter mitosis depends only on when CycB reaches this threshold value. Thus, the length of G2 depends on the slope of the increase in CycB, its rate of accumulation. This situation probably occurs in most early embryos, where the ‘trigger structure’ only provides a time-delay for the switch and the rate-limiting process is CycB synthesis. However, experimental modification can change the threshold and lead to early or late mitotic entry. Inhibition of PP2A, for instance weakens the IPFs and lowers the ON-threshold, thus advancing entry, while supplementing this phosphatase increases the threshold and delays or even blocks entry into mitosis [39,42,49].
Figure 4b depicts the possibility that CycB reaches its maximum level before the checkpoints turn off. In this case, the silencing of the surveillance mechanisms becomes the rate-limiting step. Note that once the checkpoints are disabled, CycB accumulation can again become the rate-limiting step, restoring the situation shown in figure 4a. An intermediate situation is also possible if checkpoint silencing takes place while CycB is still increasing (figure 4c). In this case, both processes determine the length of G2: if checkpoint silencing is delayed or fails, mitotic entry is also delayed (or blocked as in figure 4d), but if silencing happens earlier, CycB accumulation will determine the length of G2.
Which of these situations is realized will most likely depend on the particular cell type, organism and on the environmental context in which the cell lives. Also, more complicated scenarios not described here are possible. Despite the conservation of many features of the network controlling the mitotic switch from yeasts to man [43,63,64], the relative contributions and perhaps even the identities of individual components might vary, and some components may yet be discovered. Regardless of these differences, it is important to keep in mind that the timing of mitotic entry is a property of the whole network, determined by all the components of the network of figure 1 and the rate of CycB accumulation. Thus, as we have seen, it is difficult to decide which parts are more important in an absolute sense. Understanding the similarities and differences between different organisms and cell types will require careful quantitative measurements and provide interesting topics for further research.
Acknowledgements
The authors are grateful to Dr Daniel Fisher for invaluable discussions and Bernhard Schmierer for critically reading the manuscript. This work has been supported by BBSRC (OCISB) and by the European Community's Seventh Framework Programmes (UniCellSys: 201142 and MitoSys: 241548). M.R.D.-S. was supported by a Clarendon Fund Scholarship.
References
- 1.Masui Y., Markert C. L. 1971. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool. 177, 129–145 10.1002/jez.1401770202 (doi:10.1002/jez.1401770202) [DOI] [PubMed] [Google Scholar]
- 2.Doree M., Labbe J. C., Picard A. 1989. M phase-promoting factor: its identification as the M phase-specific H1 histone kinase and its activation by dephosphorylation. J. Cell Sci. Suppl. 12, 39–51 [DOI] [PubMed] [Google Scholar]
- 3.Lohka M. J., Hayes M. K., Maller J. L. 1988. Purification of maturation-promoting factor, an intracellular regulator of early mitotic events. Proc. Natl Acad. Sci. USA 85, 3009–3013 10.1073/pnas.85.9.3009 (doi:10.1073/pnas.85.9.3009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunphy W. G., Brizuela L., Beach D., Newport J. 1988. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell 54, 423–431 10.1016/0092-8674(88)90205-X (doi:10.1016/0092-8674(88)90205-X) [DOI] [PubMed] [Google Scholar]
- 5.Gautier J., Minshull J., Lohka M., Glotzer M., Hunt T., Maller J. L. 1990. Cyclin is a component of maturation-promoting factor from Xenopus. Cell 60, 487–494 10.1016/0092-8674(90)90599-A (doi:10.1016/0092-8674(90)90599-A) [DOI] [PubMed] [Google Scholar]
- 6.Gautier J., Norbury C., Lohka M., Nurse P., Maller J. 1988. Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+. Cell 54, 433–439 10.1016/0092-8674(88)90206-1 (doi:10.1016/0092-8674(88)90206-1) [DOI] [PubMed] [Google Scholar]
- 7.Nurse P., Thuriaux P., Nasmyth K. 1976. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet. 146, 167–178 10.1007/BF00268085 (doi:10.1007/BF00268085) [DOI] [PubMed] [Google Scholar]
- 8.Hartwell L. H., Culotti J., Pringle J. R., Reid B. J. 1974. Genetic control of the cell division cycle in yeast. Science 183, 46–51 10.1126/science.183.4120.46 (doi:10.1126/science.183.4120.46) [DOI] [PubMed] [Google Scholar]
- 9.Simanis V., Nurse P. 1986. The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell 45, 261–268 10.1016/0092-8674(86)90390-9 (doi:10.1016/0092-8674(86)90390-9) [DOI] [PubMed] [Google Scholar]
- 10.Lee M. G., Nurse P. 1987. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature 327, 31–35 10.1038/327031a0 (doi:10.1038/327031a0) [DOI] [PubMed] [Google Scholar]
- 11.Moreno S., Hayles J., Nurse P. 1989. Regulation of p34cdc2 protein kinase during mitosis. Cell 58, 361–372 10.1016/0092-8674(89)90850-7 (doi:10.1016/0092-8674(89)90850-7) [DOI] [PubMed] [Google Scholar]
- 12.Evans T., Rosenthal E. T., Youngblom J., Distel D., Hunt T. 1983. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 33, 389–396 10.1016/0092-8674(83)90420-8 (doi:10.1016/0092-8674(83)90420-8) [DOI] [PubMed] [Google Scholar]
- 13.Pines J., Hunt T. 1987. Molecular cloning and characterization of the mRNA for cyclin from sea urchin eggs. EMBO J. 6, 2987–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nurse P. 1990. Universal control mechanism regulating onset of M-phase. Nature 344, 503–508 10.1038/344503a0 (doi:10.1038/344503a0) [DOI] [PubMed] [Google Scholar]
- 15.Dephoure N., Zhou C., Villen J., Beausoleil S. A., Bakalarski C. E., Elledge S. J., Gygi S. P. 2008. A quantitative atlas of mitotic phosphorylation. Proc. Natl Acad. Sci. USA 105, 10 762–10 767 10.1073/pnas.0805139105 (doi:10.1073/pnas.0805139105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Errico A., Deshmukh K., Tanaka Y., Pozniakovsky A., Hunt T. 2010. Identification of substrates for cyclin dependent kinases. Adv. Enzyme Regul. 50, 375–399 10.1016/j.advenzreg.2009.12.001 (doi:10.1016/j.advenzreg.2009.12.001) [DOI] [PubMed] [Google Scholar]
- 17.Holt L. J., Tuch B. B., Villen J., Johnson A. D., Gygi S. P., Morgan D. O. 2009. Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science 325, 1682–1686 10.1126/science.1172867 (doi:10.1126/science.1172867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gould K. L., Nurse P. 1989. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature 342, 39–45 10.1038/342039a0 (doi:10.1038/342039a0) [DOI] [PubMed] [Google Scholar]
- 19.Nurse P. 1975. Genetic control of cell size at cell division in yeast. Nature 256, 547–551 10.1038/256547a0 (doi:10.1038/256547a0) [DOI] [PubMed] [Google Scholar]
- 20.Thuriaux P., Nurse P., Carter B. 1978. Mutants altered in the control co-ordinating cell division with cell growth in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet. 161, 215–220 [DOI] [PubMed] [Google Scholar]
- 21.Lundgren K., Walworth N., Booher R., Dembski M., Kirschner M., Beach D. 1991. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell 64, 1111–1122 10.1016/0092-8674(91)90266-2 (doi:10.1016/0092-8674(91)90266-2) [DOI] [PubMed] [Google Scholar]
- 22.Mueller P. R., Coleman T. R., Kumagai A., Dunphy W. G. 1995. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science 270, 86–90 10.1126/science.270.5233.86 (doi:10.1126/science.270.5233.86) [DOI] [PubMed] [Google Scholar]
- 23.Russell P., Nurse P. 1986. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell 45, 145–153 10.1016/0092-8674(86)90546-5 (doi:10.1016/0092-8674(86)90546-5) [DOI] [PubMed] [Google Scholar]
- 24.Kumagai A., Dunphy W. G. 1992. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell 70, 139–151 10.1016/0092-8674(92)90540-S (doi:10.1016/0092-8674(92)90540-S) [DOI] [PubMed] [Google Scholar]
- 25.Tang Z., Coleman T. R., Dunphy W. G. 1993. Two distinct mechanisms for negative regulation of the Wee1 protein kinase. EMBO J. 12, 3427–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izumi T., Maller J. L. 1993. Elimination of cdc2 phosphorylation sites in the cdc25 phosphatase blocks initiation of M-phase. Mol. Biol. Cell. 4, 1337–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S. Y., Ferrell J. E., Jr 2007. Substrate competition as a source of ultrasensitivity in the inactivation of Wee1. Cell 128, 1133–1145 10.1016/j.cell.2007.01.039 (doi:10.1016/j.cell.2007.01.039) [DOI] [PubMed] [Google Scholar]
- 28.Trunnell N. B., Poon A. C., Kim S. Y., Ferrell J. E., Jr 2011. Ultrasensitivity in the regulation of Cdc25C by Cdk1. Mol. Cell 41, 263–274 10.1016/j.molcel.2011.01.012 (doi:10.1016/j.molcel.2011.01.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novak B., Tyson J. J. 1993. Numerical analysis of a comprehensive model of M-phase control in Xenopus oocyte extracts and intact embryos. J. Cell. Sci. 106, 1153–1168 [DOI] [PubMed] [Google Scholar]
- 30.Pomerening J. R., Kim S. Y., Ferrell J. E., Jr 2005. Systems-level dissection of the cell-cycle oscillator: bypassing positive feedback produces damped oscillations. Cell 122, 565–578 10.1016/j.cell.2005.06.016 (doi:10.1016/j.cell.2005.06.016) [DOI] [PubMed] [Google Scholar]
- 31.Nurse P. 1991. The Florey Lecture, 1990 How is the cell division cycle regulated? Phil. Trans. R. Soc. Lond. B 332, 271–276 10.1098/rstb.1991.0055 (doi:10.1098/rstb.1991.0055) [DOI] [PubMed] [Google Scholar]
- 32.Solomon M. J., Glotzer M., Lee T. H., Philippe M., Kirschner M. W. 1990. Cyclin activation of p34cdc2. Cell 63, 1013–1024 10.1016/0092-8674(90)90504-8 (doi:10.1016/0092-8674(90)90504-8) [DOI] [PubMed] [Google Scholar]
- 33.King R. W., Peters J. M., Tugendreich S., Rolfe M., Hieter P., Kirschner M. W. 1995. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 81, 279–288 10.1016/0092-8674(95)90338-0 (doi:10.1016/0092-8674(95)90338-0) [DOI] [PubMed] [Google Scholar]
- 34.Sudakin V., Ganoth D., Dahan A., Heller H., Hershko J., Luca F. C., Ruderman J. V., Mershko P. 1995. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol. Biol. Cell. 6, 185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray A. W., Kirschner M. W. 1989. Cyclin synthesis drives the early embryonic cell cycle. Nature 339, 275–280 10.1038/339275a0 (doi:10.1038/339275a0) [DOI] [PubMed] [Google Scholar]
- 36.Picard A., Capony J. P., Brautigan D. L., Doree M. 1989. Involvement of protein phosphatases 1 and 2A in the control of M phase-promoting factor activity in starfish. J. Cell. Biol. 109, 3347–3354 10.1083/jcb.109.6.3347 (doi:10.1083/jcb.109.6.3347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maton G., Lorca T., Girault J. A., Ozon R., Jessus C. 2005. Differential regulation of Cdc2 and Aurora-A in Xenopus oocytes: a crucial role of phosphatase 2A. J. Cell. Sci. 118, 2485–2494 10.1242/jcs.02370 (doi:10.1242/jcs.02370) [DOI] [PubMed] [Google Scholar]
- 38.Lorca T., Fesquet D., Zindy F., Le Bouffant F., Cerruti M., Brechot C., Devauchelle G., Dorée M. 1991. An okadaic acid-sensitive phosphatase negatively controls the cyclin degradation pathway in amphibian eggs. Mol. Cell. Biol. 11, 1171–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krasinska L., Domingo-Sananes M. R., Kapuy O., Parisis N., Harker B., Moorhead G., Rossignol M., Novak B., Fisher D. In press Protein phosphatase 2A controls the order and dynamics of cell cycle transitions. Mol. Cell. [DOI] [PubMed] [Google Scholar]
- 40.Mochida S., Hunt T. 2007. Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature 449, 336–340 10.1038/nature06121 (doi:10.1038/nature06121) [DOI] [PubMed] [Google Scholar]
- 41.Virshup D. M., Shenolikar S. 2009. From promiscuity to precision: protein phosphatases get a makeover. Mol. Cell. 33, 537–545 10.1016/j.molcel.2009.02.015 (doi:10.1016/j.molcel.2009.02.015) [DOI] [PubMed] [Google Scholar]
- 42.Mochida S., Ikeo S., Gannon J., Hunt T. 2009. Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 28, 2777–2785 10.1038/emboj.2009.238 (doi:10.1038/emboj.2009.238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manchado E., et al. 2010. Targeting mitotic exit leads to tumor regression in vivo: modulation by Cdk1, Mastl, and the PP2A/B55α, δ phosphatase. Cancer Cell 18, 641–654 10.1016/j.ccr.2010.10.028 (doi:10.1016/j.ccr.2010.10.028) [DOI] [PubMed] [Google Scholar]
- 44.Schmitz M. H., et al. 2010. Live-cell imaging RNAi screen identifies PP2A-B55α and importin-β1 as key mitotic exit regulators in human cells. Nat. Cell. Biol. 12, 886–893 10.1038/ncb2092 (doi:10.1038/ncb2092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z., Mui M. Z., Chan F., Roopchand D. E., Marcellus R. C., Blanchette P., Li S., Berghuis A. M., Branton P. E. 2011. Genetic analysis of B55α/Cdc55 protein phosphatase 2A subunits: association with the adenovirus E4orf4 protein. J. Virol. 85, 286–295 10.1128/JVI.01381-10 (doi:10.1128/JVI.01381-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu J., Fleming S. L., Williams B., Williams E. V., Li Z., Somma P., Rieder C. L., Goldberg M. L. 2004. Greatwall kinase: a nuclear protein required for proper chromosome condensation and mitotic progression in Drosophila. J. Cell. Biol. 164, 487–492 10.1083/jcb.200310059 (doi:10.1083/jcb.200310059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu J., Zhao Y., Li Z., Galas S., Goldberg M. L. 2006. Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol. Cell 22, 83–91 10.1016/j.molcel.2006.02.022 (doi:10.1016/j.molcel.2006.02.022) [DOI] [PubMed] [Google Scholar]
- 48.Zhao Y., Haccard O., Wang R., Yu J., Kuang J., Jessus C., Goldberg M. L. 2008. Roles of Greatwall kinase in the regulation of cdc25 phosphatase. Mol. Biol. Cell. 19, 1317–1327 10.1091/mbc.E07-11-1099 (doi:10.1091/mbc.E07-11-1099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vigneron S., Brioudes E., Burgess A., Labbe J. C., Lorca T., Castro A. 2009. Greatwall maintains mitosis through regulation of PP2A. EMBO J. 28, 2786–2793 10.1038/emboj.2009.228 (doi:10.1038/emboj.2009.228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castilho P. V., Williams B. C., Mochida S., Zhao Y., Goldberg M. L. 2009. The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55δ, a phosphatase directed against CDK phosphosites. Mol. Biol. Cell. 20, 4777–4789 10.1091/mbc.E09-07-0643 (doi:10.1091/mbc.E09-07-0643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gharbi-Ayachi A., Labbe J. C., Burgess A., Vigneron S., Strub J. M., Brioudes E., Van-Dorsselaer A., Castro A., Lorca T. 2010. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 330, 1673–1677 10.1126/science.1197048 (doi:10.1126/science.1197048) [DOI] [PubMed] [Google Scholar]
- 52.Mochida S., Maslen S. L., Skehel M., Hunt T. 2010. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science 330, 1670–1673 10.1126/science.1195689 (doi:10.1126/science.1195689) [DOI] [PubMed] [Google Scholar]
- 53.Alon U. 2007. Network motifs: theory and experimental approaches. Nat. Rev. Genet. 8, 450–461 10.1038/nrg2102 (doi:10.1038/nrg2102) [DOI] [PubMed] [Google Scholar]
- 54.Novak B., Kapuy O., Domingo-Sananes M. R., Tyson J. J. 2010. Regulated protein kinases and phosphatases in cell cycle decisions. Curr. Opin. Cell. Biol. 22, 801–808 10.1016/j.ceb.2010.07.001 (doi:10.1016/j.ceb.2010.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kapuy O., Barik D., Sananes M. R., Tyson J. J., Novak B. 2009. Bistability by multiple phosphorylation of regulatory proteins. Prog. Biophys. Mol. Biol. 100, 47–56 10.1016/j.pbiomolbio.2009.06.004 (doi:10.1016/j.pbiomolbio.2009.06.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tyson J. J., Chen K. C., Novak B. 2003. Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr. Opin. Cell. Biol. 15, 221–231 10.1016/S0955-0674(03)00017-6 (doi:10.1016/S0955-0674(03)00017-6) [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto T. M., Blake-Hodek K., Williams B. C., Lewellyn A. L., Goldberg M. L., Maller J. L. 2011. Regulation of Greatwall kinase during Xenopus oocyte maturation. Mol. Biol. Cell. 22, 2157–2164 10.1091/mbc.E11-01-0008 (doi:10.1091/mbc.E11-01-0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu J. Q., Guo J. Y., Tang W., Yang C. S., Freel C. D., Chen C., Nairn A. C., Kornbluth S. 2009. PP1-mediated dephosphorylation of phosphoproteins at mitotic exit is controlled by inhibitor-1 and PP1 phosphorylation. Nat. Cell. Biol. 11, 644–651 10.1038/ncb1871 (doi:10.1038/ncb1871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dohadwala M., da Cruz e Silva E. F., Hall F. L., Williams R. T., Carbonaro-Hall D. A., Nairn A. C., Greengard P., Berndt N. 1994. Phosphorylation and inactivation of protein phosphatase 1 by cyclin-dependent kinases. Proc. Natl Acad. Sci. USA 91, 6408–6412 10.1073/pnas.91.14.6408 (doi:10.1073/pnas.91.14.6408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pomerening J. R., Sontag E. D., Ferrell J. E., Jr 2003. Building a cell cycle oscillator: hysteresis and bistability in the activation of Cdc2. Nat. Cell. Biol. 5, 346–351 10.1038/ncb954 (doi:10.1038/ncb954) [DOI] [PubMed] [Google Scholar]
- 61.Sha W., Moore J., Chen K., Lassaletta A. D., Yi C. S., Tyson J. J., Sible J. C. 2003. Hysteresis drives cell-cycle transitions in Xenopus laevis egg extracts. Proc. Natl Acad. Sci. USA 100, 975–980 10.1073/pnas.0235349100 (doi:10.1073/pnas.0235349100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lorca T., Bernis C., Vigneron S., Burgess A., Brioudes E., Labbe J. C., Castro A. 2010. Constant regulation of both the MPF amplification loop and the Greatwall-PP2A pathway is required for metaphase II arrest and correct entry into the first embryonic cell cycle. J. Cell. Sci. 123, 2281–2291 10.1242/jcs.064527 (doi:10.1242/jcs.064527) [DOI] [PubMed] [Google Scholar]
- 63.Burgess A., Vigneron S., Brioudes E., Labbe J. C., Lorca T., Castro A. 2010. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc. Natl Acad. Sci. USA 107, 12 564–12 569 10.1073/pnas.0914191107 (doi:10.1073/pnas.0914191107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Voets E., Wolthuis R. M. 2010. MASTL is the human orthologue of Greatwall kinase that facilitates mitotic entry, anaphase and cytokinesis. Cell Cycle 9, 3591–3601 10.4161/cc.9.17.12832 (doi:10.4161/cc.9.17.12832) [DOI] [PubMed] [Google Scholar]