Abstract
To evaluate the thermo-responsive poly(N-isopropylacrylamide) (PNiPAAm) polymer as an adjuvant, we synthesized PNiPAAm through free radical polymerization and characterized it both in vitro and in vivo. The polymer when mixed with collagen type II (CII) induced antigen-specific autoimmunity and arthritis. Mice immunized with PNiPAAm–CII developed significant levels of CII-specific IgG response comprising major IgG subclasses. Antigen-specific cellular recall response was also enhanced in these mice, while negligible level of IFN-γ was detected in splenocyte cultures, in vitro. PNiPAAm–CII-immunized arthritic mouse paws showed massive infiltration of immune cells and extensive damage to cartilage and bone. As determined by immunostaining, most of the CII protein retained its native configuration after injecting it with PNiPAAm in naive mice. Physical adsorption of CII and the high-molecular-weight form of moderately hydrophobic PNiPAAm induced a significant anti-CII antibody response. Similar to CII, mice immunized with PNiPAAm and ovalbumin (PNiPAAm–Ova) induced significant anti-ovalbumin antibody response. Comparable levels of serum IFN-γ, IL-1β and IL-17 were observed in ovalbumin-immunized mice with complete Freund, incomplete Freund (CFA and IFA) or PNiPAAm adjuvants. However, serum IL-4 levels were significantly higher in PNiPAAm–Ova and CFA–Ova groups compared with the IFA–Ova group. Thus, we show for the first time, biocompatible and biodegradable thermo-responsive PNiPAAm can be used as an adjuvant in several immunological applications as well as in better understanding of the autoimmune responses against self-proteins.
Keywords: thermo-responsive polymer, biocompatibility, collagen, arthritis, autoimmunity
1. Introduction
Rheumatoid arthritis (RA) is an autoimmune disease mediated by the concerted action of the innate and acquired immune system in which inflammation is associated with progressive destruction of the extracellular matrices of bone and cartilage. To dissect disease pathways and genes modulating arthritis development, several animal models are used [1,2]. Most of the induced models of arthritis (both chronic and acute) use an adjuvant either at the time of administration of protein antigen or later for inducing/enhancing disease development. The most commonly used adjuvants contain bacterial derivatives with or without mineral oil, but they often tend to strongly deviate the ensuing immune response [3,4], thereby precluding our understanding of the actual immune response to self-proteins. Furthermore, several local and systemic toxicities have been associated with these adjuvants and thus have restrictions for common use. In this context, a number of natural and synthetic molecules have been analysed for their adjuvant properties [5] and the mechanism(s) of action of these diverse compounds vary, as does their induction of immune responses. Hence, searching for ideal adjuvants that are biocompatible, modifiable but do not strongly deviate the immune system could be useful to study autoimmunity and autoimmune diseases.
Stimulus-responsive or smart polymers have evolved as an important class of polymeric materials having several important biomedical and industrial applications [6–8]. These polymers undergo sharp reversible phase transition in response to small changes in environmental stimuli such as pH, temperature, ionic strength, electric field or light [6]. Thermo-responsive polymers are one of the classes of responsive polymers that respond to change in temperature and undergo reversible phase change at above and below their critical temperature [9]. Among them, poly(N-isopropylacrylamide) (PNiPAAm) is one of the typical examples that has been widely studied for various biotechnological applications. PNiPAAm has a lower critical solution temperature (LCST) of precipitation around 32.5°C in water and changes reversibly from hydrophilic below this temperature to hydrophobic above it [10]. The reversible phase transition of PNiPAAm can be used for colloid suspension formation together with an antigen or any other biomolecule as the temperature is increased above the LCST, thus providing the possibility of their use as an adjuvant in combination with a specific antigen.
Although the nature of the autoantigens in RA is not yet clarified, collagen type II (CII), which is present abundantly in the articular cartilage, the site of inflammatory attack in arthritis, is a potential candidate autoantigen. Immunization of mice with CII and an adjuvant induces arthritis that resembles human RA, the so-called collagen-induced arthritis (CIA) model. CIA is the widely used animal model for not only dissecting pathogenic mechanisms and identifying gene targets but also for testing potential drug candidates modifying arthritis disease process. Here, we synthesized the thermo-responsive PNiPAAm and characterized it both in vitro as well as in vivo. PNiPAAm was tested for its adjuvant properties with CII and ovalbumin and compared with Freund's adjuvant(s).
2. Material and methods
2.1. Mice
Founders of B10.RIII/Rhd mice were from Prof. Jan Klein (Professor Emeritus, Tübingen University, Tübingen, Germany). Breeding pairs of C57BL/6NJ mice were from Jackson Laboratories (Bar Harbor, ME, USA). Rhd indicates that this classic inbred mouse strain has been maintained in our laboratory for more than two decades. Age-matched 8–12-week-old mice of both genders were used for experiments. All the animals were kept in a climate-controlled environment having 12 L : 12 D cycles in polystyrene cages containing wood shavings under specific pathogen-free conditions, fed standard rodent chow and water ad libitum. Local (Stockholm, Sweden) animal welfare authorities approved the animal experiments (permit numbers: N310-07, M107-07 and N66-10).
2.2. Antigens and antibodies
CII was prepared from nasal bovine cartilage by pepsin digestion and further purified as described earlier [11]. Monoclonal antibodies CB20 (against native conformation of collagen II) [12] and GB8 (against denatured form of collagen) [13] were generated, characterized, affinity purified and biotinylated for further use. Ovalbumin protein grade II was obtained from Sigma-Aldrich (St Louis, MO, USA).
2.3. Poly(N-isopropylacrylamide) synthesis
N-isopropylacrylamide (NiPAAm) was purchased from Acros Organics (Schwerte, Germany). Ammonium persulphate (APS) and N,N,N′,N′-tetramethylethylenediamine (TEMED) were from Sisco Research Laboratories Pvt Ltd (Mumbai, India). PNiPAAm was synthesized through free radical polymerization by using APS and TEMED. For synthesis of PNiPAAm (1%, w/v), 100 mg of NiPAAm was dissolved in 10 ml of degassed water. Initially, the solution was bubbled with N2 gas for 20 min and free radical polymerization was initiated by adding 15 mg of APS and 19.5 µl of TEMED. The reaction vial was immediately filled with nitrogen and tightly sealed. The reaction was stopped after 18 h by salt-induced thermal precipitation. The thermo-precipitation (40°C) and redissolution (4°C) were done two times to ensure complete removal of unreacted monomers and the polymer was freeze-dried for further use. The PNiPAAm with 70 and 120 kDa molecular weight was prepared through termination of free radical polymerization reaction at two different time points, 8 and 12 h, respectively.
2.4. Lower critical solution temperature determination
The LCST or cloud point of synthesized PNiPAAm (0.1%, w/v) was determined by an abrupt change in the absorbance of the polymer solution at 450 nm, using a HEλIOS α spectrophotometer (Thermo Electron Corporation, Cambridge, UK) [14]. The temperature of the solution was maintained using cryostat Julabo F34 (Julabo Labortechnik GmbH, Seelbach, Germany) for 10 min, followed by a gradual increase of 1°C to observe the sudden change in solution phase, which represents the average value of LCST.
2.5. Molecular weight determination
The molecular weight of PNiPAAm was determined by gel permeation chromatography (GPC) (Waters, Milford, MA, USA) using tetrahydrofuran (THF) as an eluent and polystyrene as calibration standard. Lyophilized PNiPAAm was dissolved in the THF solvent and filtered through the THF-based 0.2 µm filter (Millipore, Billerica, MA, USA) and passed through the Stryagel HR 3&4 THF 7.8 × 300 mm column. A differential refractive index detector (Waters) was used for detection. PNiPAAm was injected at a flow rate of 1.0 ml min−1 (Waters 515 HPLC pump) and the molecular weight was calculated using Empower 2 build 2154 Waters software (Waters) using the polystyrene standard plot.
2.6. Covalent coupling of collagen type II with poly(N-isopropylacrylamide)
The covalent coupling of CII with PNiPAAm was performed by a copolymerization approach [15]. At first, chemical modification of CII was carried out by itaconic anhydride. Briefly, the CII protein (1 mg ml–1) was dissolved in 50 mM potassium phosphate buffer, pH 6.0. Itaconic anhydride (2.75 mg ml–1) and 1.5 M glucose were added simultaneously at 4°C for 2 h in the reaction mixture. After completion of reaction, unreacted itaconic anhydride was removed through dialysis in potassium phosphate buffer, pH 7.0, at 4°C. Chemically modified CII thus obtained was copolymerized with NiPAAm monomers at 4°C for 12 h. PNiPAAm copolymerized with CII was precipitated by adding 0.05 M NaCl at 40°C and dissolved in cold phosphate buffer saline for further use.
2.7. Biotinylation and modification of poly(N-isopropylacrylamide)
For biotinylation of the polymer, NiPAAm monomers were copolymerized with allylamine monomers through free radical polymerization. The resulting block polymer PNiPAAm-co-allylamine has the primary amino groups (-NH2), which is the preferential site for biotinylation reagents containing N-hydroxysulphosuccinimide (sulpho-NHS) esters. Briefly, the PNiPAAm-co-allylamine block polymer was dissolved in 50 mM phosphate buffer, pH 6.5, and incubated with 10 mM sulpho-NHS-biotin reagent (Pierce, Rockford, IL, USA) for 24 h at 4°C. The unreacted impurities were removed by dialysis. Similarly, increasing the amount of primary amino groups by incorporating allylamine modified the hydrophilicity of PNiPAAm. NiPAAm and allylamine monomers have been copolymerized through the free radical process in the ratio of 5 : 1 and 5 : 2 for this purpose and used in further experiments.
2.8. In vitro release of collagen II from poly(N-isopropylacrylamide)
One hundred microlitres of CII (1 mg ml–1) was mixed with 100 µg of PNiPAAm and incubated at 37°C. At different time intervals, supernatants were collected. The released protein content was estimated by the bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA). To eliminate background signal, a control consisting of only polymers without protein was used and experiments were performed in triplicates.
2.9. In vitro biocompatibility of poly(N-isopropylacrylamide)
For determination of cell viability and proliferation of cells on the PNiPAAm surface, a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was carried out [16]. It is one of the colorimetric methods for estimation of cell growth and proliferation, based on the principle of live cell mitochondria oxidizing the MTT reagent and giving a blue–violet colour, which was quantified by spectrophotometric analysis. Briefly, human fibrosarcoma HT-1080 cells were incubated on the polymer surface for the purpose of determining cell proliferation and cytotoxicity effects of the material on the cells. The culture medium was removed after allowing the cells to attain 70 per cent confluence, followed by monitoring for cell viability. The serum-free DMEM culture medium containing MTT (0.5 mg ml–1) was added to each sample and incubated for 4 h. The medium containing MTT was removed, followed by the addition of dimethylsulphoxide (1.5 ml) into each sample to dissolve the formazan crystals formed by the living cells, and the sample was incubated for 15 min at 37°C. Supernatants were removed from each sample and the absorbance was read at 540 nm. All the above experiments were performed in triplicates.
2.10. Arthritis induction
Adjuvancity of PNiPAAm was evaluated in 7–10-week-old B10.RIII mice. Mice were distributed equally in all three different groups, viz. CFA–CII, PNiPAAm–CII and PNiPAAm–Ova. CII or ovalbumin emulsified in complete Freund adjuvant (CFA; Difco, Detroit, MI, USA) constituted positive and negative controls, respectively. Both CII and ovalbumin proteins (1 mg ml–1) were mixed with PNiPAAm separately and 100 µg of antigen–polymer mixture (1 : 1) was injected subcutaneously on day 0 and boosted with 50 µg of the respective antigen–polymer mixture (1 : 1) on day 21 or 35. Serum samples were collected on different days for analysis of antibody levels and cytokines.
2.11. Detection of collagen type II conformation with poly(N-isopropylacrylamide)
Immunostaining was performed to detect changes in the conformation of CII after mixing it with PNiPAAm. PNiPAAm–CII was injected subcutaneously and after 3 h, tissues surrounding the injection site were collected for immunohistochemistry. Sections of 5–10 µm were fixed in acetone, dried and incubated with a blocking solution containing 10 per cent bovine serum albumin, 2 per cent rat serum and avidin for 30 min. After washing with trisbuffered saline, inherent avidin present in the tissues was blocked using biotin solution for 30 min, followed by washing and incubation with biotinylated CB20 or GB8 monoclonal antibodies. Sections were blocked for endogenous peroxidase using H2O2 (3%, v/v) in methanol before adding DAB peroxidase substrate solution (Vector Laboratories Inc., Burlingame, CA, USA). Counterstaining was performed using haematoxylin.
2.12. Anti-collagen type II antibody response
The amounts of total anti-CII IgG were determined through quantitative ELISA as described earlier [17]. Affinity-purified anti-CII antibody from pooled sera of CII-immunized mice or pooled sera were used as the standard. Biotinylated goat anti-mouse IgG (Southern Biotech, Birmingham, AL, USA) or mouse anti-mouse IgG2c (BD), or peroxidase-conjugated goat anti-mouse antibodies specific for IgG1, IgG2b or IgG3 (Southern Biotech) were used as detecting antibodies. Binding of biotinylated antibodies was revealed by extravidin peroxidase (Sigma-Aldrich). It is of interest to note that B10 mice express IgG2a-related allele, viz. IgG2c [18]. Plates were developed using ABTS (Roche Diagnostic Systems) as the substrate, and measured at 405 nm (Synergy-2, BioTek Instruments Inc., Winooski, VT, USA). Isotype levels were measured as arbitrary units using pooled sera from arthritic mice as the standard.
2.13. Clinical evaluation of arthritis and histology
Mice were examined for arthritis development twice per week. Scoring of animals was done blindly using a scoring system based on the number of inflamed joints in each paw, inflammation being defined by swelling and redness [19]. In this scoring system, each inflamed toe or knuckle gives one point, whereas an inflamed wrist or ankle gives five points, resulting in a score of 0–15 (five toes + five knuckles + one wrist/ankle) for each paw and 0–60 points for each mouse. After fixation with phosphate-buffered paraformaldehyde solution for 24 h and decalcification for three to four weeks in an ethylenediaminetetraacetic acid solution containing polyvinylpyrrolidone and tris (pH 6.9), paws were dehydrated and embedded in paraffin blocks. Joint sections (6 µm) were stained with haematoxylin–eosin to visualize morphology and infiltration of immune cells.
2.14. Proliferation and cytokine assays
Proliferation studies of cells from splenocytes (10 days after immunization with 100 µg of CII and PNiPAAm at the base of the tail of B10. RIII mice) were performed as described earlier [20]. Briefly, lymphocytes were cultured at a concentration of 1 × 106 cells per well for 72 h with medium alone, 50 µg ml–1 bovine CII, 5 µg ml–1 of concanavalin A (ConA; Sigma) or 0.5 µg ml–1 of anti-CD3 (clone 17A2, eBioscience, Inc., San Diego, CA, USA) in DMEM + Glutamax-I (Gibco) supplemented with 5 per cent heat-inactivated foetal calf serum and penicillin/streptomycin and then pulsed with [3H] thymidine (Amersham Biosciences) for a further period of 15–18 h, as previously described. Supernatant of cultured cells was collected after 72 h for IFN-γ measurement. IFN-γ levels were determined by coating microtitre plates with anti-IFN-γ (AN18) in PBS overnight. After blocking with BSA (1%, w/v) in PBS, supernatant was added to these plates. Biotinylated anti-IFN-γ-bio (R46-A2) was used as the secondary antibody. Mice immunized with PNiPAAm–ovalbumin were used to measure serum IL-1, IL-4, IFN-γ and IL-17 levels. IL-1 levels were measured using the assay kit supplied by eBioscience Inc. Serum IL-4 (using 11B11 and BVD6-24G2-bio, BD biosciences) and IL-17 (using TC11-18H10 and TCH11-8H4.1-bio, BD biosciences) levels were measured by ELISA similar to IFN-γ. Recombinant cytokines were used as standards.
2.15. Statistical analyses
A Mann–Whitney U-ranking test in Statview 5.0.1 software version (SAS Institute, NC, USA) was used for statistical calculations. Significance was considered when p < 0.05 for a 95% confidence interval.
3. Results
3.1. In vitro and in vivo characterization of poly(N-isopropylacrylamide)
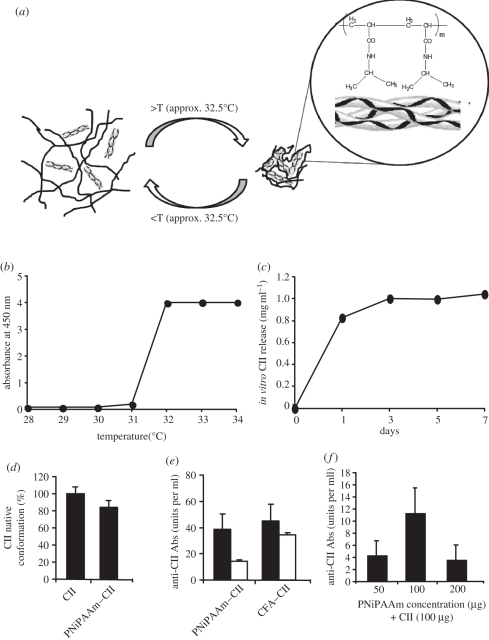
PNiPAAm was synthesized by the free radical polymerization procedure as described in detail earlier [21]. The number-average (Mn) and weight-average molecular weight (Mw) of the polymer as determined by the GPC was used to calculate the polydispersity index (PDI). The Mw and the Mn of PNiPAAm were found to be 120 and 110 kDa, respectively, with a PDI of 1.1, which ensures homogeneity of the polymer. We used PNiPAAm with CII to understand whether PNiPAAm can act as an adjuvant in arthritis induction in mice. To do this, at first, we characterized the polymer both in vitro and in vivo. PNiPAAm entraps an antigen by its precipitation property [22], and the possible interactions of PNiPAAm with CII are schematically represented in figure 1a. LCST of the newly synthesized PNiPAAm was found to be 32°C (figure 1b). We studied the pattern of CII released from PNiPAAm at 37°C (figure 1c). After 24 h, the release of CII from PNiPAAm was high and then it increased slowly to reach the plateau level. Interestingly, CII thus released was found to mostly retain the native triple helical conformation (figure 1d), and the majority of the antibodies generated in mice immunized with PNiPAAm–CII, similar to the CFA–CII group, recognized the native CII conformation (figure 1e). We found 100 µg each of PNiPAAm and CII (1 : 1) as the optimal dose for immunization that induced a high level of anti-CII antibody response (figure 1f).
Figure 1.
In vitro and in vivo characterization of PNiPAAm. Schematic of thermo-responsive behaviour of PNiPAAm with CII (a). Below LCST (approx. 32°C) (b), 100 µg of PNiPAAm mixed with 100 µg of CII (1 mg ml−1) remains in solution and above this temperature forms a lattice incorporating CII; in vitro release profile of CII from PNiPAAm at 37°C (c); percentage of released CII retaining native CII conformation from PNiPAAm–CII mixture (100 µg each at 1 : 1 ratio) in vitro (d); serum antibody response recognizing native and/or denatured conformation of CII (units per ml) (e). Optimization of PNiPAAm concentration (50–200 µg) for immunization mixed with 100 µg of CII (f) after 21 days of injection. Error bars denote ±s.e.m. (e) Black bars, native CII; white bars, denatured CII.
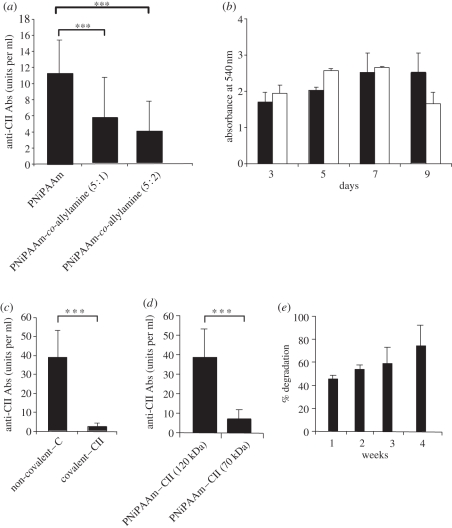
Furthermore, hydrophilicity effect on the adjuvant potential of PNiPAAm has been analysed by increasing the amount of primary amino groups with PNiPAAm by allylamine incorporation. NiPAAm and allylamine monomers were copolymerized through the free radical process in ratios of 5 : 1 and 5 : 2. The synthesized PNiPAAm and its copolymers were injected with CII, and serum anti-CII antibody levels were measured after 21 days of immunization. Interestingly, increasing the hydrophilicity of PNiPAAm significantly reduced the adjuvant properties of PNiPAAm (figure 2a). Using the MTT assay with the human fibroblast cell line, we could ascertain the biocompatibility function (support for cell growth) of PNiPAAm in vitro (figure 2b). Similarly, we found that CII interacting with PNiPAAm non-covalently induced a highly significant level of anti-CII antibody response compared with CII that was covalently linked to PNiPAAm (figure 2c), suggesting a depot effect (sustained release of the antigen from a depot) might be one of the major modes of actions of this polymer. We also found that high- but not low-molecular-weight (120 versus 70 kDa) PNiPAAm mixed with CII induced a significant level of antibody response (figure 2d). To understand the in vivo gravimetric degradation kinetics of PNiPAAm, we implanted PNiPAAm in the form of a hydrogel at the back of the B10.RIII mice. As shown in figure 2e, nearly 75 per cent of the implanted polymer by weight was degraded within four weeks.
Figure 2.
Physical and biochemical characterization of PNiPAAm. Hydrophilicity effect on the adjuvant potential of PNiPAAm (a); MTT cell proliferation assay of HT1080 cell line grown over the PNiPAAm surface (b); antibody response to CII (units per ml) in mice immunized with PNiPAAm incorporating CII in a non-covalent and covalent method (c); antibody response to CII (units per ml) in mice immunized with CII mixed with two different molecular weight forms of PNiPAAm (120 and 70 kDa) (d); gravimetric degradation kinetics of PNiPAAm at different time points in vivo (e). ***p < 0.001. Error bars denote ±s.e.m. (b) Black bars, PNiPAAm; white bars, control.
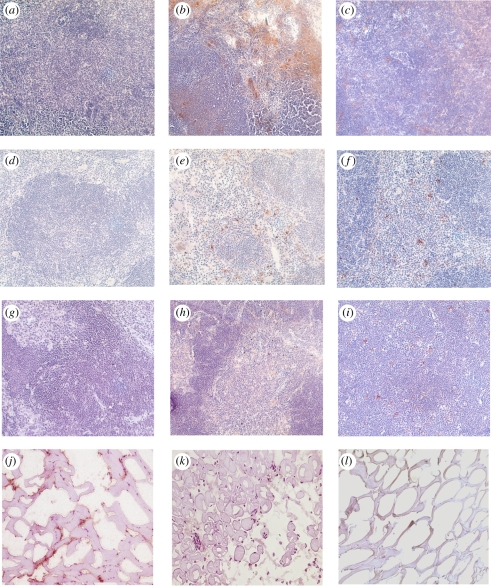
To assess the bio-distribution of PNiPAAm in vivo, we synthesized the block polymer PNiPAAm-co-allylamine, conjugated with biotin and injected into the mice at the base of the tail. The lymphoid organs were harvested at different time points for analysis. As shown in figure 3a–i, PNiPAAm can be detected strongly in the peripheral lymphoid organs but weakly in the thymus after 24 h. After four weeks, PNiPAAm staining was weak in all the organs tested. Furthermore, a monoclonal antibody specific for the native (CB20) but not the denatured form (GB8) of CII detected the collagen present in the surrounding tissue at the site of PNiPAAm–CII immunization after 3 h (figure 3j–k). Further characterization of PNiPAAm was done for in vivo gelation and systemic toxicity (data not shown). Briefly, after injection into the base of the tail, we found relatively stable gelation of PNiPAAm after 20 min because of its LCST, which is below the body temperature. Systemic toxicity occurs owing to leaching of the injected polymer and its distribution in the blood and lymphatic system. However, we did not find any detectable serum TNF-α response, which was used as a measurement of systemic toxic response to the injected polymer.
Figure 3.
Bio-distribution of the biotinylated polymer in different lymphoid organs. Tissue sections from control mice (a,d,g) and mice sacrificed on day 1 (b,e,h) and day 30 (c,f,i) after biotinylated PNiPAAm-co-allylamine injection are shown. Lymph nodes (a–c), spleen (d–f) and thymus (g–i) samples were used. Liver sections did not show any staining for the polymer (data not shown). Immunostaining of CII with monoclonal antibodies recognizing native (j) CB20 or denatured (k) GB8 conformations. Control staining (l). All the images were taken at 20× magnification. (Online version in colour.)
3.2. Arthritis induction with collagen type II and the polymeric adjuvant
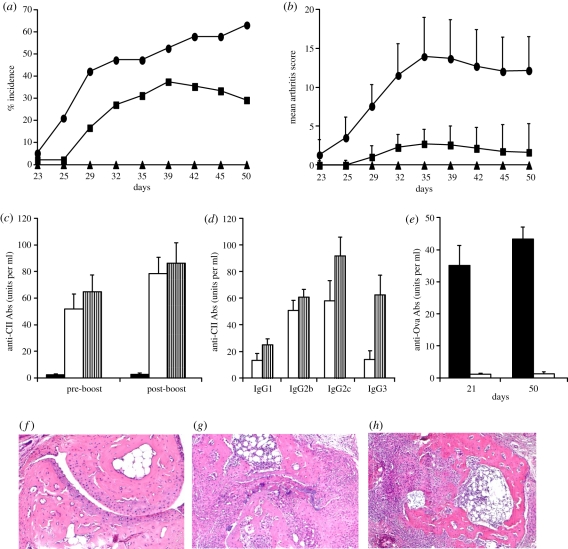
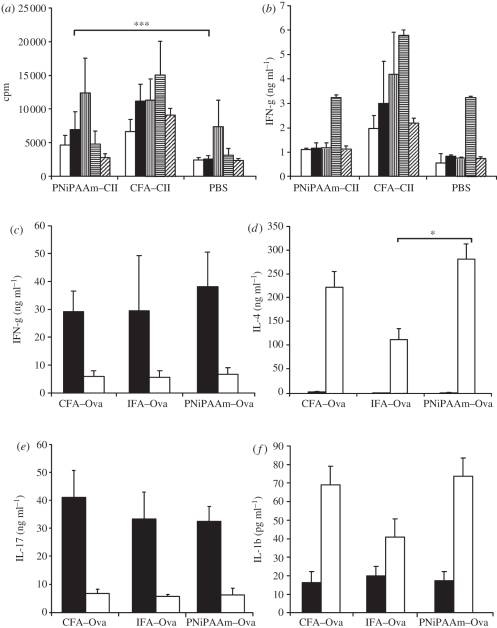
In order to use the synthetic polymer as an adjuvant, we tested PNiPAAm in arthritis experiments. Thirty-eight per cent of the B10.RIII mice developed arthritis after PNiPAAm–CII immunization with a mean maximum score of 22 ± 3 (figure 4a,b). Antibody response in the PNiPAAm–CII group (figure 4c) was found to be similar to the CFA–CII group, and there was no difference in the distribution of major IgG subclasses between the groups except in the IgG3 subclass (figure 4d). Antibodies thus produced were specific to CII, which showed negligible binding to ovalbumin (figure 4e), suggesting the absence of any B-cell mitogenic response owing to the injected polymer. Massive infiltration of immune cells was observed in the joints of mice that developed arthritis in the PNiPAAm–CII group with extensive damage to cartilage and bone structures (figure 4f–h). Furthermore, similar to the PNiPAAm–CII group, a high level of antibody response to the globular protein, ovalbumin, was observed in PNiPAAm–Ova-immunized mice (figure 4e) with negligible cross-reactivity to CII. We also found a significant antigen-specific T cell recall response, when splenocytes from PNiPAAm–CII mice were tested in the lymphocyte proliferation assay (figure 5a), but IFN-γ levels were found to be similar to that of the control group (figure 5b).
Figure 4.
PNiPAAm as an adjuvant in the induction of autoimmunity and arthritis. (a) Arthritis incidence and (b) mean arthritis score on different days are shown. Groups of 8-week-old B10.RIII mice were immunized subcutaneously at the base of the tail with 100 µg of CII emulsified in complete Freund's adjuvant (CFA–CII; n = 19), CII mixed with 100 µg of PNiPAAm (PNiPAAm–CII; n = 48) or 100 µg of ovalbumin mixed with 100 µg of PNiPAAm (PNiPAAm–Ova; n = 10) on day 0 in 200 µl volume. Mice were boosted with 50 µg of CII emulsified in incomplete Freund's adjuvant, CII mixed with PNiPAAm or ovalbumin mixed with PNiPAAm, respectively, on day 21 or 35. Results are from three experiments and all the animals were used for calculations. Sera collected on either days 21/35 (pre-boost) or days 50/60 (post-boost) were used for antibody analysis (c–e) as described in §2. CII without any adjuvant induced neither arthritis nor an anti-CII antibody response in a group of mice (n = 8). IgG subclass analysis was done in sera collected after 50 days of injection. Representative histology joints of mice (n = 3–4 in each group) from PBS (f), PNiPAAm–CII (g) and CFA–CII (h) groups. n indicates the number of mice in each group. Error bars denote ±s.e.m. All the images were taken at 20× magnification. (a,b) Solid line with oval-shaped symbols, CFA–CII; solid line with squares, PNiPAAm–CII; solid line with triangles, PNiPAAm–Ova. (c) Black bars, PNiPAAm–Ova; white bars, PNiPAAm–CII; striped bars, CFA–CII. (d) White bars, PNiPAAm–CII; striped bars, CFA–CII. (e) Black bars, PNiPAAm–Ova; white bars, PNiPAAm–CII. (Online version in colour.)
Figure 5.
PNiPAAm enhances cellular activation and serum cytokine production. Groups of B10.RIII mice (n = 15) were immunized with bovine PNiPAAm–CII, CII emulsified in CFA (CFA–CII) or PBS and 10 days later splenocytes were cultured in triplicates at a concentration of 1 × 106 cells per well for 72 h with medium alone, 50 µg ml−1 bovine CII, 5 µg ml−1 of concanavalin A or 0.5 µg ml−1 of anti-CD3 (clone 17A2) in DMEM + Glutamax-I supplemented with 5% heat-inactivated foetal calf serum and penicillin/streptomycin and then pulsed with [3H] thymidine for a further 15–18 h before harvesting the cells. (a) cpm denotes counts per minute. (b) Supernatant of cultured cells were collected after 72 h for IFN-γ estimation. For serum cytokine measurements, C57BL/6NJ mice (n = 15) were immunized at the base of the tail with 100 µg of ovalbumin emulsified with complete Freund's adjuvant (CFA–Ova), incomplete Freund's adjuvant (IFA–Ova) or mixed with PNiPAAm. Sera collected on days 10 and 19 were analysed for various cytokines (c–f) as described in §2. *p < 0.05; ***p < 0.001. Error bars denote ±s.e.m. n indicates the number of mice in each group. (a,b) White bars, medium; black bars, CII; bars with vertical stripes, ConA; bars with horizontal stripes, anti-CD3; bars with oblique stripes, Ova. (c–f) Black bars, 10 days; white bars, 19 days.
3.3. Serum cytokine levels
Th1 cells produce IFN-γ and mediate protection against intracellular pathogens, Th2 cells produce IL-4, IL-13 and IL-25 and are involved in the clearance of extracellular pathogens, whereas Th17 cells produce IL-17 and induce the production of proinflammatory cytokines, chemokines and metalloproteinases [23]. To understand whether the PNiPAAm adjuvant is capable of inducing all the three T-helper cell populations, we measured serum IFN-γ, IL-4 and IL-17 levels as an indicator of activation of these cells. Interestingly, all these three cytokines were found to be elevated in the PNiPAAm adjuvant group (figure 5c–e). Similarly, the serum IL-1β level was enhanced when PNiPAAm was used as an adjuvant (figure 5f), suggesting the possible involvement of the inflammasome pathway.
4. Discussion
Animal models are important tools for understanding the disease mechanisms operating during the development of autoimmune diseases, and in most of the induced arthritis models, adjuvants are needed to induce or enhance disease development. However, currently used adjuvants often tend to strongly deviate the ensuing immune response [3,4]. Hence, there is a tremendous need for mild adjuvants that are well defined, biocompatible and amenable to modification but which can also be used as a carrier for testing other immune-responsive molecules. Biodegradable, biocompatible and stimulus-responsive polymers offer such an opportunity because of the following advantages: polymers have the ability to sustain the release of antigens over an extended period of time, the immunomodulatory property can also be modified by polymer chemistry, other immunomodulatory molecules or motifs can be incorporated to create a pathogen-mimicking solid particle and most of the polymers are manufactured from synthetic parent compounds, eliminating many potentially reactive antigenic or allergenic epitopes that can accompany the use of prokaryotic, animal- or plant-derived materials.
In this study, for the first time, we demonstrate the adjuvant capacity of a thermo-responsive carrier, PNiPAAm. Mice immunized with PNiPAAm–CII developed CII-specific antibody responses leading to arthritis induction. Most of the CII protein retained its native configuration after mixing it with PNiPAAm, and sera from PNiPAAm–CII-immunized mice recognized native CII triple helical structure comparatively higher than the denatured form. Generally, adjuvants enhance the immunogenicity of an antigen either by creating a reservoir for the slow release of protein by entrapping or sequestering (depot effect), by endocytosis/phagocytosis of polymer-protein by facilitating targeting of the antigen to immune cells and/or by modulating and enhancing the type of immune response induced by the antigen alone. Understanding the mechanisms underlying the mode of action of adjuvants is a pre-requisite for their use in humans [24,25]. Physical adsorption and release but not covalent coupling of CII with PNiPAAm induced strong arthritis, which suggests that the depot effect might be one of the major mechanisms of the adjuvant action of PNiPAAm. Moreover, a higher anti-CII response was observed in mice immunized with CII and the high Mw form of PNiPAAm (120 kDa) than the low Mw form (70 kDa); this might be due to the better precipitating property with the high Mw polymer, which could retain the antigen for a longer period of time. From bio-distribution study, we found that the biotinylated polymer injected subcutaneously followed a similar lymphoid pathway to that of a protein antigen. It was drained by the lymphatic system within 24 h and retained for more than 30 days, which could have helped for antigen presentation for a longer duration. With the progression of time, PNiPAAm was degraded systematically as shown by the gravimetric analysis of excised PNiPAAm after implantation, at different time intervals. Furthermore, epitope modifications may occur during formulation or conjugation with an adjuvant. However, the monoclonal antibody (CB20) recognizing the triple helical C1III epitope still recognized the CII present in the tissues surrounding the PNiPAAm–CII injection site after 3 h in vivo, which clearly demonstrated the absence of any modifications to the epitope structure of CII.
However, arthritis incidence in the PNiPAAm–CII group was low (38%) compared with the CFA–CII (68%) group. CFA is a mineral oil suspended with mycobacterium, which contains several pathogen-associated molecular patterns and danger-associated molecular patterns that are recognized by the receptors present on the antigen-presenting cells (APCs), for example, Toll-like receptors (TLRs). Since the costimulatory function of cells during the immune response to collagen is very essential to break the tolerance to this self-antigen for eventual induction of arthritis, as evidenced by the lack of arthritis induction by collagen itself [26], we propose that PNiPAAm may induce the immune response that is independent of TLRs. Another possibility is the genetic background of the mice used. Although induced cytokine difference with the adjuvants may have contributed in causing the difference in the incidence of arthritis between the groups, it is, however, most unlikely because all these cytokines have pathogenic roles depending on the location of their response (systemic versus local). In CIA, Th1 cells producing IFN-γ and IL-2 are associated with the acute phase [27,28], whereas the Th2 cytokine IL-4 is associated with the remission phase [27,29] of the disease. However, IL-4 can also play a pathogenic role at the effector phase of arthritis [30,31]. Furthermore, Th17 cells producing IL-17 (or IL-17A), IL-6 and TNF-α have an important role in CIA pathogenesis [32,33]. On the other hand, the low incidence of arthritis in the PNiPPAm group will definitely not be due to the increased presence of native collagen in the polymer group compared to CFA group because only native but not denatured collagen can induce arthritis [26]. However, how the mixture of native and denatured collagen in the CFA group contributed to increased arthritis is currently not known because only antibodies that recognize native structures are pathogenic, while T cells can be presented with linear peptides derived from denatured collagen by APCs. Experiments are underway to answer such questions apart from understanding whether PNiPPAm can alter genetic susceptibility to collagen in mice.
Earlier, several polymeric systems have been tested as adjuvants, such as poly(glycolide) [34], poly(lactide-co-glycolide) (PLGA) [35,36], poloxamers [37], polyphosphazenes [38], polyoxyethylene [39], polyoxypropylene and chitosan polymers [40]. They are biocompatible, biodegradable and able to incorporate different kinds of antigens with higher loading efficiency. They have advantages over conventional adjuvants, mainly in the manipulation of degradation kinetics by varying the concentration of monomers and cross-linkers [41]. In this way, the release profile of an antigen can be modulated. Moreover, antigen uptake by APCs and induction of cytotoxic T lymphocytes were also enhanced with the use of the polymeric system [42]. Recent studies showed the use of the particulate form of PLGA in adjuvant-induced arthritis in mice [43]. In this study, we used the thermo-responsive PNiPAAm that was synthesized by free radical polymerization and had a high molecular weight (120 kDa) and PDI close to 1, with the cloud point (LCST) at 32°C. PNiPAAm binds to protein mostly non-covalently and releases the protein in the medium significantly at higher levels owing to weak hydrophobic interactions. From immunostaining results, it is clear that the polymer interactions with the collagen protein did not change its native conformation significantly. PNiPAAm is temperature responsive and thus showed the temperature-dependent conformational change (soluble at temperature below the LCST and form aggregation above the LCST). Interestingly, PNiPAAm formed a clear and visible white precipitate at the site of injection, which demonstrated its in vivo gelation property. Furthermore, in order to find out whether PNiPAAm can be used in immunological studies other than autoimmunity, we tested the polymer with ovalbumin as an antigen. As we observed for CII, immune responses to ovalbumin were robust with PNiPAAm–Ova immunization, suggesting PNiPAAm can be used as a general adjuvant for several immunological applications including vaccine formulations.
Since we observed induction of all the major IgG subclasses when PNiPAAm was used as an adjuvant, we measured the serum IFN-γ, IL-4 and IL-17 levels as an indicator for the activation of all the three major T-helper cell populations [23]. Interestingly, we found that all these three cytokine levels in PNiPAAm–Ova immunized mice were enhanced, suggesting no major deviation towards any one type of an immune response. Recent studies have also shown that the commonly used adjuvant, alum, induced the release of IL-1β, IL-18 and IL-33 [44,45], which is mediated by the protein NALP3 [46]. Hence, to check whether the adjuvancity of PNiPAAm also involves the inflammasome pathway, we analysed IL-1β levels in the sera from PNiPAAm–Ova-immunized mice and compared it with ovalbumin emulsified with Freund's adjuvant(s). PNiPAAm–Ova immunization induced a comparable level of IL-1 production to Freund's adjuvant groups, suggesting the possible involvement of this inflammasome pathway when PNiPAAm was used as an adjuvant. It is of interest to note that IL-1 is synthesized by various cells including monocytes, macrophages, neutrophils, hepatocytes and tissue macrophages [47] and IL-1 is an important mediator of inflammation induced by immune complexes [48]. For example, after collagen immunization, around day 14, antibodies to collagen are significantly developed and, IL-1 plays a critical role in the antibody-mediated cartilage damage [49]. Hence, the increased level of IL-1 during day 19 compared with day 10 might be due to the presence of the enhanced level of anti-Ova antibodies around this time point, as shown in figure 4c. However, further experiments are needed to specifically address different pathways involved in the adjuvant action of PNiPAAm.
5. Conclusions
We found, for the first time, that the biocompatible and biodegradable thermo-responsive PNiPAAm can be used as an adjuvant to study several immunological parameters, including development of autoimmunity and arthritis. Antigen-specific immune responses were observed when PNiPAAm was used as an adjuvant without any major deviation towards any one type of an immune response. More studies are needed to explore the mechanisms of the adjuvant action of PNiPAAm in detail.
Acknowledgements
We thank Prof. Rikard Holmdahl for support and encouragement. We are grateful to Carlos and Kristina Palestro for taking care of the animals. The following foundations provided financial support: Alex and Eva Wallström, Professor Nanna Svartz, Åke Wieberg, Anne Greta Holger Crafoord, KI (Fobi), Swedish Rheumatism Association, King Gustaf V:s 80-years Foundation and Swedish Research Council, VR-Link (2008-6007) and VR-project grant (2009-2338). A.K.S. acknowledges the senior research fellowship (SRF) from Council of Scientific and Industrial Research, India.
References
- 1.Monach P. A., Benoist C., Mathis D. 2004. The role of antibodies in mouse models of rheumatoid arthritis, and relevance to human disease. Adv. Immunol. 82, 217–248 10.1016/S0065-2776(04)82005-4 (doi:10.1016/S0065-2776(04)82005-4) [DOI] [PubMed] [Google Scholar]
- 2.Kannan K., Ortmann R. A., Kimpel D. 2005. Animal models of rheumatoid arthritis and their relevance to human disease. Pathophysiology 12, 167–181 10.1016/j.pathophys.2005.07.011 (doi:10.1016/j.pathophys.2005.07.011) [DOI] [PubMed] [Google Scholar]
- 3.Reed S. G., Bertholet S., Coler R. N., Friede M. 2009. New horizons in adjuvants for vaccine development. Trends Immunol. 30, 23–32 10.1016/j.it.2008.09.006 (doi:10.1016/j.it.2008.09.006) [DOI] [PubMed] [Google Scholar]
- 4.Wilson-Welder J. H., Torres M. P., Kipper M. J., Mallapragada S. K., Wannemuehler M. J., Narasimhan B. 2009. Vaccine adjuvants: current challenges and future approaches. J. Pharm. Sci. 98, 1278–1316 10.1002/jps.21523 (doi:10.1002/jps.21523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edelman R. 2002. The development and use of vaccine adjuvants. Mol. Biotechnol. 21, 129–148 10.1385/MB:21:2:129 (doi:10.1385/MB:21:2:129) [DOI] [PubMed] [Google Scholar]
- 6.Galaev I. Y., Mattiasson B. 1999. ‘Smart’ polymers and what they could do in biotechnology and medicine. Trends Biotechnol. 17, 335–340 10.1016/S0167-7799(99)01345-1 (doi:10.1016/S0167-7799(99)01345-1) [DOI] [PubMed] [Google Scholar]
- 7.Jeong B., Gutowska A. 2002. Lessons from nature: stimuli-responsive polymers and their biomedical applications. Trends Biotechnol. 20, 305–311 10.1016/S0167-7799(02)01962-5 (doi:10.1016/S0167-7799(02)01962-5) [DOI] [PubMed] [Google Scholar]
- 8.Cooperstein M. A., Canavan H. E. 2010. Biological cell detachment from poly(N-isopropyl acrylamide) and its applications. Langmuir 26, 7695–7707 10.1021/la902587p (doi:10.1021/la902587p) [DOI] [PubMed] [Google Scholar]
- 9.Kumar A., Srivastava A., Galaev I. Y., Mattiasson B. 2007. Smart polymers: physical forms and bioengineering applications. Prog. Polym. Sci. 32, 1205–1237 10.1016/j.progpolymsci.2007.05.003 (doi:10.1016/j.progpolymsci.2007.05.003) [DOI] [Google Scholar]
- 10.Shin B. C., Jhon M. S., Lee H. B., Yuk S. H. 1998. Temperature-induced phase transition of semi-interpenetrating polymer networks composed of poly(N-isopropyl acrylamide) and hydrophilic polymers. Eur. Polym. J. 34, 171–174 10.1016/S0014-3057(98)00025-1 (doi:10.1016/S0014-3057(98)00025-1) [DOI] [Google Scholar]
- 11.Grab B., Miles A. J., Furcht L. T., Fields G. B. 1996. Promotion of fibroblast adhesion by triple-helical peptide models of type I collagen-derived sequences. J. Biol. Chem. 271, 12 234–12 240 10.1074/jbc.271.21.12234 (doi:10.1074/jbc.271.21.12234) [DOI] [PubMed] [Google Scholar]
- 12.Mo J. A., Bona C. A., Holmdahl R. 1993. Variable region gene selection of immunoglobulin G-expressing B cells with specificity for a defined epitope on type II collagen. Eur. J. Immunol. 23, 2503–2510 10.1002/eji.1830231019 (doi:10.1002/eji.1830231019) [DOI] [PubMed] [Google Scholar]
- 13.Uysal H., et al. 2009. Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J. Exp. Med. 206, 449–462 10.1084/jem.20081862 (doi:10.1084/jem.20081862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar A., Kamihira M., Galaev I. Y., Mattiasson B., Iijima S. 2001. Type-specific separation of animal cells in aqueous two-phase systems using antibody conjugates with temperature-sensitive polymers. Biotechnol. Bioeng. 75, 570–580 10.1002/bit.10080 (doi:10.1002/bit.10080) [DOI] [PubMed] [Google Scholar]
- 15.Shakya A. K., Sharma P., Kumar A. 2010. Synthesis and characterization of thermo-responsive poly(N-isopropylacrylamide)-bovine liver catalase bioconjugate. Enzyme Microb. Tech. 47, 277–282 10.1016/j.enzmictec.2010.07.018 (doi:10.1016/j.enzmictec.2010.07.018) [DOI] [Google Scholar]
- 16.Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63 10.1016/0022-1759(83)90303-4 (doi:10.1016/0022-1759(83)90303-4) [DOI] [PubMed] [Google Scholar]
- 17.Holmdahl R., Rubin K., Klareskog L., Larsson E., Wigzell H. 1986. Characterization of the antibody response in mice with type II collagen-induced arthritis, using monoclonal anti-type II collagen antibodies. Arthritis Rheum. 29, 400–410 10.1002/art.1780290314 (doi:10.1002/art.1780290314) [DOI] [PubMed] [Google Scholar]
- 18.Morgado M. G., Cam P., Gris-Liebe C., Cazenave P. A., Jouvin-Marche E. 1989. Further evidence that BALB/c and C57BL/6 gamma 2a genes originate from two distinct isotypes. EMBO J. 8, 3245–3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmdahl R., Carlsen S., Mikulowska A., Vestberg M., Brunsberg U., Hansson A.-S., Sundvall M., Jansson L., Pettersson U. 1998. Genetic analysis of murine models for rheumatoid arthritis. In Human genome methods (ed. Adolpho K. W.), pp. 215–238 New York, NY: CRC Press [Google Scholar]
- 20.Svensson L., Jirholt J., Holmdahl R., Jansson L. 1998. B cell-deficient mice do not develop type II collagen-induced arthritis (CIA). Clin. Exp. Immunol. 111, 521–526 10.1046/j.1365-2249.1998.00529.x (doi:10.1046/j.1365-2249.1998.00529.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schild H. G. 1992. Poly(N-isopropylacrylamide): experiment, theory and application. Prog. Polym. Sci. 17, 163–249 10.1016/0079-6700(92)90023-R (doi:10.1016/0079-6700(92)90023-R) [DOI] [Google Scholar]
- 22.Shakya A. K., Sami H., Srivastava A., Kumar A. 2010. Stability of responsive polymer–protein bioconjugates. Prog. Polym. Sci. 35, 459–486 10.1016/j.progpolymsci.2010.01.003 (doi:10.1016/j.progpolymsci.2010.01.003) [DOI] [Google Scholar]
- 23.Bettelli E., Korn T., Oukka M., Kuchroo V. K. 2008. Induction and effector functions of T(H)17 cells. Nature 453, 1051–1057 10.1038/nature07036 (doi:10.1038/nature07036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.HogenEsch H. 2002. Mechanisms of stimulation of the immune response by aluminum adjuvants. Vaccine 20(Suppl. 3), S34–S39 10.1016/S0264-410X(02)00169-X (doi:10.1016/S0264-410X(02)00169-X) [DOI] [PubMed] [Google Scholar]
- 25.Aimanianda V., Haensler J., Lacroix-Desmazes S., Kaveri S. V., Bayry J. 2009. Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol. Sci. 30, 287–295 10.1016/j.tips.2009.03.005 (doi:10.1016/j.tips.2009.03.005) [DOI] [PubMed] [Google Scholar]
- 26.Trentham D. E., Townes A. S., Kang A. H. 1977. Autoimmunity to type II collagen: an experimental model of arthritis. J. Exp. Med. 146, 857–868 10.1084/jem.146.3.857 (doi:10.1084/jem.146.3.857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauri C., Williams R. O., Walmsley M., Feldmann M. 1996. Relationship between Th1/Th2 cytokine patterns and the arthritogenic response in collagen-induced arthritis. Eur. J. Immunol. 26, 1511–1518 10.1002/eji.1830260716 (doi:10.1002/eji.1830260716) [DOI] [PubMed] [Google Scholar]
- 28.Brand D. D., Kang A. H., Rosloniec E. F. 2003. Immunopathogenesis of collagen arthritis. Springer Semin. Immunopathol. 25, 3–18 10.1007/s00281-003-0127-1 (doi:10.1007/s00281-003-0127-1) [DOI] [PubMed] [Google Scholar]
- 29.Doncarli A., Stasiuk L. M., Fournier C., Abehsira-Amar O. 1997. Conversion in vivo from an early dominant Th0/Th1 response to a Th2 phenotype during the development of collagen-induced arthritis. Eur. J. Immunol. 27, 1451–1458 10.1002/eji.1830270623 (doi:10.1002/eji.1830270623) [DOI] [PubMed] [Google Scholar]
- 30.Svensson L., Nandakumar K. S., Johansson A., Jansson L., Holmdahl R. 2002. IL-4-deficient mice develop less acute but more chronic relapsing collagen-induced arthritis. Eur. J. Immunol. 32, 2944–2953 (doi:10.1002/1521-4141(2002010)32:10<2944::AID-IMMU2944>3.0.CO;2-4) [DOI] [PubMed] [Google Scholar]
- 31.Nandakumar K. S., Holmdahl R. 2006. Arthritis induced with cartilage-specific antibodies is IL-4-dependent. Eur. J. Immunol. 36, 1608–1618 10.1002/eji.200535633 (doi:10.1002/eji.200535633) [DOI] [PubMed] [Google Scholar]
- 32.Weaver C. T., Hatton R. D., Mangan P. R., Harrington L. E. 2007. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25, 821–852 10.1146/annurev.immunol.25.022106.141557 (doi:10.1146/annurev.immunol.25.022106.141557) [DOI] [PubMed] [Google Scholar]
- 33.Ito Y., et al. 2009. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum. 60, 2294–2303 10.1002/art.24687 (doi:10.1002/art.24687) [DOI] [PubMed] [Google Scholar]
- 34.Nellore R. V., Pande P. G., Young D., Bhagat H. R. 1992. Evaluation of biodegradable microspheres as vaccine adjuvant for hepatitis B surface antigen. J. Parenter. Sci. Technol. 46, 176–180 [PubMed] [Google Scholar]
- 35.Eldridge J. H., Staas J. K., Meulbroek J. A., Tice T. R., Gilley R. M. 1991. Biodegradable and biocompatible poly(dl-lactide-co-glycolide) microspheres as an adjuvant for staphylococcal enterotoxin B toxoid which enhances the level of toxin-neutralizing antibodies. Infect. Immun. 59, 2978–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mutsumi Y., Jessica M., Julia E. B. 2007. Effect of poly(lactic-co-glycolic acid) contact on maturation of murine bone marrow-derived dendritic cells. J. Biomed. Mater. Res. A 80, 7–12 [DOI] [PubMed] [Google Scholar]
- 37.Hartikka J., Geall A., Bozoukova V., Kurniadi D., Rusalov D., Enas J., Yi J. H., Nanci A., Rolland A. 2008. Physical characterization and in vivo evaluation of poloxamer-based DNA vaccine formulations. J. Gene Med. 10, 770–782 10.1002/jgm.1199 (doi:10.1002/jgm.1199) [DOI] [PubMed] [Google Scholar]
- 38.Mutwiri G., Benjamin P., Soita H., Townsend H., Yost R., Roberts B., Andrianov A. K., Babiuk L. 2007. Poly[di(sodium carboxylatoethylphenoxy)phosphazene] (PCEP) is a potent enhancer of mixed Th1/Th2 immune responses in mice immunized with influenza virus antigens. Vaccine 25, 1204–1213 10.1016/j.vaccine.2006.10.011 (doi:10.1016/j.vaccine.2006.10.011) [DOI] [PubMed] [Google Scholar]
- 39.Kleine B., Rapp W., Wiesmuller K. H., Edinger M., Beck W., Metzger J., Ataulakhanov R., Jung GR., Bessler W. G. 1994. Lipopeptide-polyoxyethylene conjugates as mitogens and adjuvants. Immunobiology 190, 53–66 [DOI] [PubMed] [Google Scholar]
- 40.Boonyo W., Junginger H. E., Waranuch N., Polnok A., Pitaksuteepong T. 2007. Chitosan and trimethyl chitosan chloride (TMC) as adjuvants for inducing immune responses to ovalbumin in mice following nasal administration. J. Control Release 121, 168–175 10.1016/j.jconrel.2007.05.025 (doi:10.1016/j.jconrel.2007.05.025) [DOI] [PubMed] [Google Scholar]
- 41.Eldridge J. H., Staas J. K., Meulbroek J. A., McGhee J. R., Tice T. R., Gilley R. M. 1991. Biodegradable microspheres as a vaccine delivery system. Mol. Immunol. 28, 287–294 10.1016/0161-5890(91)90076-V (doi:10.1016/0161-5890(91)90076-V) [DOI] [PubMed] [Google Scholar]
- 42.Eyles J. E., Carpenter Z. C., Alpar H. O., Williamson E. D. 2003. Immunological aspects of polymer microsphere vaccine delivery systems. J. Drug Target. 11, 509–514 10.1080/10611860410001670017 (doi:10.1080/10611860410001670017) [DOI] [PubMed] [Google Scholar]
- 43.Khaled K. A., Sarhan H. A., Ibrahim M. A., Ali A. H., Naguib Y. W. 2010. Prednisolone-loaded PLGA microspheres. In vitro characterization and in vivo application in adjuvant-induced arthritis in mice. AAPS PharmSciTech 11, 859–869 10.1208/s12249-010-9445-5 (doi:10.1208/s12249-010-9445-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutterwala F. S., et al. 2006. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24, 317–327 10.1016/j.immuni.2006.02.004 (doi:10.1016/j.immuni.2006.02.004) [DOI] [PubMed] [Google Scholar]
- 45.Li H., Nookala S., Re F. 2007. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J. Immunol. 178, 5271–5276 [DOI] [PubMed] [Google Scholar]
- 46.Eisenbarth S. C., Colegio O. R., O'Connor W., Sutterwala F. S., Flavell R. A. 2008. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453, 1122–1126 10.1038/nature06939 (doi:10.1038/nature06939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arend W. P., Palmer G., Gabay C. 2008. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 223, 20–38 10.1111/j.1600-065X.2008.00624.x (doi:10.1111/j.1600-065X.2008.00624.x) [DOI] [PubMed] [Google Scholar]
- 48.Mullazehi M., Mathsson L., Lampa J., Ronnelid J. 2006. Surface-bound anti-type II collagen-containing immune complexes induce production of tumor necrosis factor alpha, interleukin-1beta, and interleukin-8 from peripheral blood monocytes via Fc gamma receptor IIA: a potential pathophysiologic mechanism for humoral anti-type II collagen immunity in arthritis. Arthritis Rheum. 54, 1759–1771 10.1002/art.21892 (doi:10.1002/art.21892) [DOI] [PubMed] [Google Scholar]
- 49.Kagari T., Doi H., Shimozato T. 2002. The importance of IL-1 beta and TNF-alpha, and the noninvolvement of IL-6, in the development of monoclonal antibody-induced arthritis. J. Immunol. 169, 1459–1466 [DOI] [PubMed] [Google Scholar]