Abstract
Mechanical stimulation is an essential factor affecting the metabolism of bone cells and their precursors. We hypothesized that vibration loading would stimulate differentiation of human adipose stem cells (hASCs) towards bone-forming cells and simultaneously inhibit differentiation towards fat tissue. We developed a vibration-loading device that produces 3g peak acceleration at frequencies of 50 and 100 Hz to cells cultured on well plates. hASCs were cultured using either basal medium (BM), osteogenic medium (OM) or adipogenic medium (AM), and subjected to vibration loading for 3 h d–1 for 1, 7 and 14 day. Osteogenesis, i.e. differentiation of hASCs towards bone-forming cells, was analysed using markers such as alkaline phosphatase (ALP) activity, collagen production and mineralization. Both 50 and 100 Hz vibration frequencies induced significantly increased ALP activity and collagen production of hASCs compared with the static control at 14 day in OM. A similar trend was detected for mineralization, but the increase was not statistically significant. Furthermore, vibration loading inhibited adipocyte differentiation of hASCs. Vibration did not affect cell number or viability. These findings suggest that osteogenic culture conditions amplify the stimulatory effect of vibration loading on differentiation of hASCs towards bone-forming cells.
Keywords: adipose stem cells, in vitro culturing, vibration loading, mechanical stimulation
1. Introduction
Bone tissue engineering has emerged as a new interdisciplinary approach to address the increasing demand for bone substitutes and to combat the limitations related to traditional bone grafts. In addition to surgical methods, such as grafting, the development of non-surgical interventions would be beneficial to enhance bone formation. Bone is a dynamic tissue that adapts to its prevailing mechanical environment through the activation of bone remodelling cells. The mechanosensory system of bone modifies skeletal strength by sensing the strains caused by mechanical loading and then inducing changes in skeletal mass through bone formation or resorption [1]. The significance of this mechanosensitivity in the maintenance of bone architecture becomes crucially evident when physical signals are removed because reduced loading results in rapid bone loss, e.g. during prolonged bedrest, paraplegia or spaceflight [2–4]. The strong correlation between bone structure and mechanical stimuli has led to increased medical interest towards this feature of bone tissue. Several studies conducted in vivo indicate that mechanical loading, such as high-frequency vibration, could be a feasible method of enhancing bone tissue formation [5–8]. Vibrational studies have been conducted using various combinations of different magnitudes and frequencies. Besides the high-magnitude low-frequency (HMLF) vibrations apparently involved in natural, high-impact movements [9,10], low-magnitude high-frequency (LMHF) [7,8,11,12,13] and high-magnitude high-frequency (HMHF) [14,15] vibrations generated by dedicated devices all have an anabolic effect on bone tissue.
LMHF vibration stimulates the proliferation and osteogenic differentiation capacity of mesenchymal stem cells (MSCs) in mouse bone marrow [6,16]. Mechanical disuse, in turn, induces fat formation, i.e. adipogenesis in bone marrow MSCs (BMSCs), which may compromise the regenerative potential of bone and predispose to diseases like osteoporosis. Rubin et al. [17] reported that a brief daily exposure to LMHF vibration inhibits fat production in mice by preventing MSCs from committing to an adipogenic lineage. Sen et al. [18] reported that MSCs cultured on well plates respond to both HMLF and LMHF by inhibiting adipogenesis. These remarkable findings suggest that LMHF vibration might be used to simultaneously prevent both obesity and bone loss by controlling BMSCs differentiation.
In contrast to LMHF vibration, HMHF vibration also has osteogenic effects [14,15]. Oxlund et al. showed that HMHF vibration increases the periosteal bone formation rate and inhibits endocortical resorption in ovariectomized rats compared with lower frequencies and accelerations. Although the role of mechanical loading in bone tissue maintenance is well established, the ideal vibration stimulation parameters and the molecular mechanisms behind the adaptive responses remain to be elucidated. The commonalities in the responses to different types of vibration loading suggest a common signalling pathway for cellular excitation [19].
The strain stimuli required to initiate intracellular signalling in vitro are often greater than those required in vivo. Hence, the advantage of vibration loading over other bioreactor conditions is the capacity to provide high g-forces (1g is equal to the Earth's gravitational field) compared with, for example, bioreactor generated shear or bending forces. For example, everyday tissue-level strains range from 400 to 1500 µε; and rarely exceed 2000 µε, which would have little or no effect on cells in vitro, whereas in vitro strains (greater than 5000 µε) could cause bone tissue damage in vivo [20]. Therefore, some kind of amplification system is likely present in bone tissue [20,21]. Such a system has been suggested at least for osteocytes, enabling 10- to 100-fold amplification of low tissue-level strains [22,23]. Nevertheless, it is not clear how osteoblasts and their precursors, for example, are able to sense extremely low-intensity mechanical signals [5,18,24].
Given the accumulating encouraging results with osteoblasts and BMSCs, high-frequency vibration loading might also promote osteogenic differentiation of adipose stem cells (ASCs). ASCs are stem cells of mesenchymal origin and are capable of differentiating towards osteogenic, adipogenic, myogenic and chondrogenic lineages in vitro when cultured under appropriate conditions [25]. The osteogenic capacity of ASCs has been demonstrated in several in vitro [26–28] and in vivo studies [29–32]. Adipose tissue provides an abundant and accessible source of MSCs particularly when compared with bone marrow. ASC-based applications are also successfully used in clinical bone tissue engineering [33,34].
Vibration stimuli could be used to inhibit or even reverse bone loss caused by diseases such as osteoporosis [17]. Specific vibration loading could also be used to improve the osseointegration of bone-anchored implants [21,35]. In addition, vibration loading could be used ex vivo for the production of ASC-based bone tissue engineering constructs. As recent findings regarding the mechanobiology of MSCs demonstrate, a better understanding of the behaviour of ASCs under mechanical stimulation is of fundamental importance. The aim of this study was to test whether HMHF vibration affects the cell number, viability or attachment of hASCs. Based on previous studies, we also hypothesized that HMHF vibration could enhance osteogenesis and simultaneously inhibit the adipogenic differentiation of hASCs.
2. Material and methods
2.1. Vibration-loading device and principle of operation
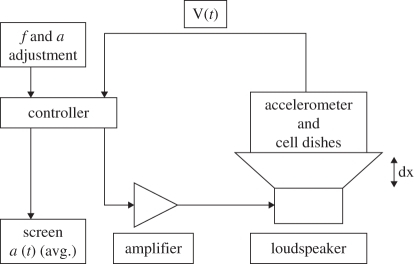
A controllable vibration-loading device, designed and built at the Biomedical Engineering Department, Tampere University of Technology, Finland, was used in the experiment. A block diagram of the complete system is shown in figure 1. An electromagnetic linear motion engine composed of a sound-producing Lab 12 subwoofer (Eminence Speaker LLC, KY, USA) was employed to produce the vibration loading. The unit was driven by a microcontroller-based signal source that was provided with a direct accelerometer feedback. The ADXL321 accelerometer (Analogue Devices Inc., MA, USA) was mounted on the same attachment system as the cell culture plates (figure 2). The modulated square wave signal was amplified by a T.AMP E800 audio amplifier (Musikhaus Thomann e.K., Germany) to produce the desired g-force. Peak acceleration of 3g was achieved by adjusting the amplitude of the output signal, corresponding to the vertical displacement magnitude of the cone. The experiment was repeated three times, and in two of the stimulation experiments the controller operated in a simple open-loop mode, while in the third experiment it was reprogrammed to perform a closed-loop regulation. During the open-loop mode the amplitude was adjusted manually, but in the closed-loop mode the controller adjusted the amplitude automatically by comparing the measured acceleration feedback to a target value of 3g. Upon start-up, the controller increased acceleration quickly to 2.5 G and then approached the target value by small adjustments within 30 s time. The dynamic response of the controller was deliberately made slow to ensure both short- and long-term stability and to prevent overshooting and generation of excessive forces. Frequency was set manually and was accurate within 1 Hz.
Figure 1.
The voice coil movement amplitude (dx) was adjusted according to the accelerometer-provided direct feedback (V(t)). The adjustment was done either by the user (open-loop mode) or the controller (closed-loop mode). The frequency was user-selected in both modes. Signal amplification was done by standard PA-audio amplifier.
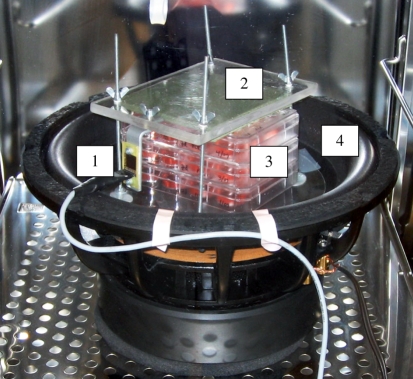
Figure 2.
The vibration-loading device assembled inside a cell culture incubator. The ADXL321 accelerometer (1) was attached to the top plastic plate (2) that was holding the cell-culturing plates (3) in place when the loudspeaker cone (4) was vibrating vertically.
2.2. Adipose stem cell isolation and culture
The human ASCs (hASCs) were isolated from adipose tissue samples collected from four female donors (mean age 56 ± 12.6 years) during surgical procedures in the Department of Plastic Surgery, Tampere University Hospital. In brief, the adipose tissue samples were digested with collagenase type I (1.5 mg ml–1; Invitrogen, CA, USA). After centrifugation and filtration, the isolated ASCs were maintained and expanded in T-75 cm2 polystyrene flasks (Nunc, Roskilde, Denmark) in basal medium (BM) consisting of Dulbecco's modified Eagle medium/Ham's nutrient mixture F-12 (DMEM/F-12 1:1; Invitrogen), 10 per cent human serum (PAA Laboratories GmbH, Pasching, Austria), 1 per cent l-glutamine (GlutaMAX; Invitrogen) and 1 per cent antibiotics (100 U ml–1 penicillin, 0.1 mg ml–1 streptomycin; Invitrogen). The ASCs cultured in BM were detached with TrypLe Select (Invitrogen). The expanded ASCs were cryo-preserved in liquid nitrogen in freezing solution containing 10 per cent dimethyl sulphoxide (DMSO; Hybri-Max, Sigma-Aldrich, MO, USA) and human serum. The ASCs needed for the experiments were thawed and expanded in BM.
2.3. Vibration-loading stimulation
The vibration-loading device was placed inside a cell culture incubator and stimulation was conducted under standard cell culture conditions (+37°C, 5% CO2) at all times. Vibration loading was started 48 h after plating the hASCs on well plates. The cells were vibrated with a peak acceleration of 3g, using square wave at either 50 or 100 Hz with an effective vibration period of 1.5 h during a 3 h period. Specifically, the stimulation pattern for 3 h consisted of 1 s of vibration followed by a 1 s rest period. Thereafter, a 21 h rest period ensued. The 3 h stimulation period was repeated every day for 1, 7 or 14 days. The control cells were cultured similarly under standard cell-culturing conditions without vibration.
2.4. Flow cytometric surface marker expression analysis
After primary culture in T-75 flasks (at passage 1–2), the hASCs were harvested and characterized by a fluorescence-activated cell sorter (FACSAria; BD Biosciences, Erembodegem, Belgium) as described earlier by Lindroos et al. [36]. Monoclonal antibodies against CD14, CD19, CD49d-PE, CD90-APC, CD106-PE-Cy5 (BD Biosciences); CD45-FITC (Miltenyi Biotech, Bergisch Gladbach, Germany); CD34-APC, HLA-ABC-PE, HLA-DR-PE (Immunotools GmbH, Friesoythe, Germany) and CD105-PE (R&D Systems Inc, MN, USA) were used. A total of 10 000 cells per sample were used, and positive expression was defined as a level of fluorescence greater than 99 per cent of the corresponding unstained cell sample.
2.5. Cell number and viability
Cell attachment and viability were evaluated qualitatively using live–dead-staining probes (Molecular Probes, Eugene, OR, USA) at 1, 7 and 14 day. The hASCs were incubated for 45 min at room temperature with a mixture of 0.5 µM calcein acetoxymethyl ester (Molecular Probes) and 0.25 µM ethidium homodimer-1 (Molecular Probes). Images of the viable cells (green fluorescence) and necrotic cells (red fluorescence) were taken using a fluorescence microscope.
The cell number of hASCs cultured in BM and OM was studied at the 1, 7 and 14 day time points by CyQUANT Cell Proliferation Assay Kit (CyQUANT; Molecular Probes, Invitrogen), which is based on the amount of total DNA in the sample. The method was performed as described previously [37]. Briefly, the cells were lysed with 0.1 per cent Triton-X 100 buffer (Sigma-Aldrich) and stored at −70°C until analysis. After a freeze–thaw cycle 20 µl of each sample was mixed with CyQUANT GR dye and lysis buffer. The fluorescence was measured with a multiplate reader (Victor 1420 Multilabel Counter; Wallac; Turku, Finland) at 480/520 nm.
2.6. Osteogenic differentiation of adipose stem cells
The pooled hASCs at passage three to four were seeded onto a 12-well plate at a density of 7 × 103 cell cm–2 in 1 ml of BM. Osteogenic differentiation was initiated 24 h after plating by adding osteogenic medium (OM), consisting of BM with osteogenic supplements: 250 µM ascorbic acid 2-phosphate (Sigma-Aldrich), 5 nM dexamethasone (Sigma-Aldrich) and 10 mM β-glycerophosphate (Sigma-Aldrich). The control cell cultures were maintained in BM. The medium was changed every other day.
The alkaline phosphatase (ALP) activity, mineralization and collagen production were analysed to detect the osteogenic differentiation of hASCs. The ALP activity was studied at day 1, 7 and 14 of culturing. The quantitative ALP analysis was done according to the Sigma ALP (Sigma-Aldrich) procedure as described previously [38]. The ALP activity was determined from the same Triton-X 100 lysates as the cell number. The absorbance was measured with a multiplate reader (Victor 1420) at 405 nm.
A quantitative Alizarin Red S method was used at 7 and 14 day to detect calcium compounds deposited in the extracellular matrix (ECM) as a result of mineralization. Briefly, the cells were rinsed with Dulbecco's phosphate-buffered saline (DPBS; Lonza Biowhittaker, Switzerland) followed by fixation in 4 per cent paraformaldehyde for 20 min. The fixed cultures were stained with 2 per cent Alizarin Red S (Sigma-Aldrich), pH 4.2 for 5 min and the stained cells were photographed after several steps of washing. The dye from the stained cells was extracted with 100 mM cetylpyridinium chloride (Sigma-Aldrich) at gentle shaking for 3 h. The dye intensity was determined at 540 nm with a multiplate reader (Victor 1420).
Collagen content of the cells was quantified at 7 and 14 day by using a quantitative Sircol Soluble Collagen Assay (Biocolor Ltd, Carrickfergus, Northern Ireland), which is based on the selective binding of the Sirius red dye to the [Gly–X–Y] tripeptide sequence of mammalian collagen. In brief, the acid-soluble collagen was extracted from the cell cultures over night at +4°C in 0.5 M acetic acid (Merck KGaA, Darmstadt, Germany) containing 0.1 mg ml–1 pepsin (Sigma-Aldrich). The collagen extraction was followed by a concentration step according to the manufacturer's protocol. To determine the collagen content, Sircol Dye reagent (Biocolor Ltd) comprised Sirius Red and picric acid, was added to the concentrated collagen samples and incubated for 30 min. The samples were centrifuged 12 000 r.p.m. for 10 min and the collagen-dye pellet was washed once with ice-cold Acid-Salt buffer (Biocolor Ltd) to remove the unbound dye. Finally, an alkali reagent (0.5 M sodium hydroxide solution, Biocolor Ltd) was added to resolubilize the collagen, and the dye intensity was determined with a multiplate reader at 540 nm (Victor 1420).
2.7. Adipogenic differentiation of adipose stem cells
The pooled hASCs at passage four to five were seeded onto a 12-well plate at a density of 1.4 × 104 cells cm–2 in 1 ml of BM. Adipogenic induction was started 24 h after plating by adding adipogenic medium (AM) consisting of BM supplemented with 33 µM biotin (Sigma-Aldrich), 1 µM dexamethasone (Sigma-Aldrich), 100 nM insulin (Invitrogen) and 17 µM pantothenate (Fluka, Buchs, Switzerland). Upon seeding the cells, 250 µM isobutylmethylxanthine (Sigma-Aldrich) was added to the differentiation medium and was removed from the culture after 24 h. The control cell cultures were maintained in BM. The medium was changed every other day.
The adipogenic differentiation was analysed at day 7 and 14 using Oil red O (Sigma-Aldrich) staining as an indicator of intracellular lipid accumulation, as described previously [36]. Stained cells were visualized by both light microscope and epifluorescence using a 560 nm excitation/emission filter [39]. For quantification, the stained oil droplets were dissolved in 100 per cent 2-propanol (Merck) and the absorbance was measured at 450 nm with a multiplate reader (Victor 1420).
2.8. Statistical analysis
A one-way ANOVA with Tukey post hoc test was used to analyse the effect of different stimulation conditions on cell number, ALP activity, mineralization, collagen production and adipogenesis. The effect of culture duration was analysed using a Student's t-test for independent samples for mineralization, collagen production and adipogenesis (7 d and 14 day), and a one-way ANOVA with Tukey post hoc test for cell number and ALP activity (1, 7 and 14 day). The experiment was repeated three times (n = 9), except the analyses for collagen production and adipogenesis were conducted only once with four parallel samples (n = 4). For mineralization, number of samples (n) was only 7, since cells in some wells were detaching during the third experiment replication and could not be measured reliably. The results were considered statistically significant when p < 0.05. Data were described by mean and standard deviation (s.d.) for cell number, normalized ALP activity (ALP/DNA ratio), collagen production and adipogenesis. Mineralization was described using a scatter chart showing the individual data points for each experiment replication. Statistical analyses were performed using PASW (former SPSS) software v. 18.
3. Results
3.1. Flow cytometric surface marker expression analysis
Flow cytometric analysis was used for the characterization of hASCs. The hASCs used in the study expressed the surface markers CD49d, CD73, CD90, CD105 and HLA-ABC as shown in table 1. The CD14, CD19, CD34, HLA-DR, the vascular cell adhesion molecule CD106, and the haematopoietic marker CD45 were not expressed in the hASCs.
Table 1.
Surface marker expression of undifferentiated hASCs cultured in BM (passage 1–2). The results are displayed as mean percentage of the surface marker expression of the four cell lineages used in the study.
| surface protein | antigen | mean | s.d. | expression |
|---|---|---|---|---|
| CD14 | serum lipopolysaccharide binding protein | 2.7 | 1.2 | negative |
| CD19 | B lymphocyte-lineage differentiation antigen | 1.1 | 0.3 | negative |
| CD34 | sialomucin-like adhesion molecule | 19.7 | 2.0 | moderate expression |
| CD45 | leukocyte common antigen | 2.9 | 0.9 | negative |
| CD49d | integrin α2, VLA-4 | 62.3 | 18.2 | positive |
| CD73 | ecto-5′-nucleotidase | 98.1 | 1.7 | positive |
| CD90 | Thy-1 (T cell surface glycoprotein) | 97.6 | 2.5 | positive |
| CD105 | SH-2, endoglin | 98.9 | 0.9 | positive |
| CD106 | VCAM-1 (vascular cell adhesion molecule) | 1.2 | 0.7 | negative |
| HLA-ABC | major histocompatibility class I antigens | 73.9 | 18.4 | positive |
| HLA-DR | major histocompatibility class II antigens | 1 | 0.3 | negative |
3.2. Adipose stem cell viability and cell number
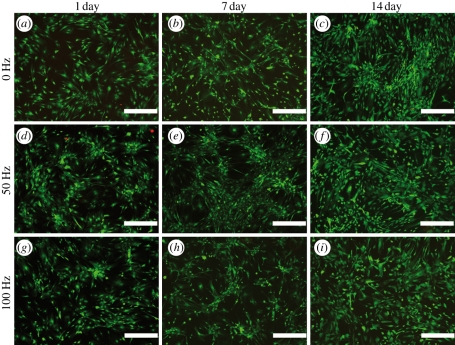
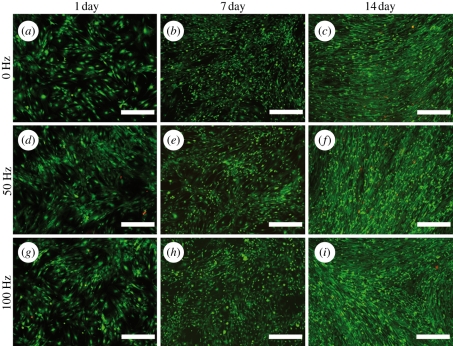
The attachment and viability of hASCs were studied using live–dead staining. As shown in figures 3 and 4, the vibrated cells were slightly less homogeneously attached at 1 day than the control cells (figures 3a,d,g and 4a,d,g). The vibrated cells also tended to grow in clusters, especially at 7 day when cultured in BM (figure 3e,h). Vibration loading did not greatly affect the viability of hASCs, since only a few dead cells were detected at any time point. The number of viable cells also increased over time in all conditions. However, it was observed that cells cultured in BM detached more easily under vibration than cells cultured in OM (figure 5).
Figure 3.
Representative fluorescence microscope images of the static (0 Hz: a,b,c) and the vibration-stimulated (50 Hz: d,e,f and 100 Hz: g,h,i) hASCs in BM at 1, 7 and 14 day time points as visualized by live–dead staining of live cells (green) and dead cells (red). Scale bar 500 µm.
Figure 4.
Representative fluorescence microscope images of the static (0 Hz: a,b,c) and the vibration-stimulated (50 Hz: d,e,f and 100 Hz: g,h,i) hASCs in OM at 1, 7 and 14 day time points as visualized by live–dead staining of live cells (green) and dead cells (red). Scale bar 500 µm.
Figure 5.
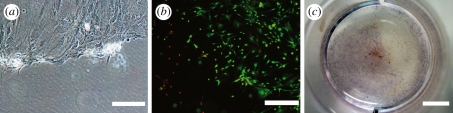
Images showing areas of dead or detached cells in 50 Hz vibration-stimulated hASCs in BM. Similar effects were detected in cells stimulated with 100 Hz vibration in BM, but not in any of the stimulated cells cultured in OM. (a) Light microscope image at 7 day, (b) live–dead staining at 7 day, and (c) Alizarin Red staining at 14 day. Scale bar (a) 100 µm, (b) 500 µm and (c) 5 mm.
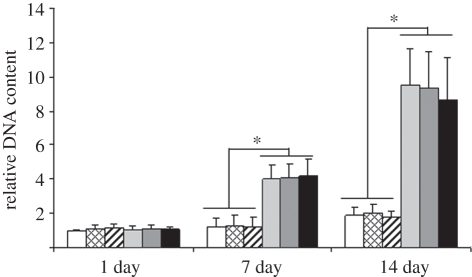
The cell number of hASCs cultured in BM and OM was evaluated quantitatively by measuring the total DNA content. The number of cells increased significantly when cultured in OM (p < 0.05) in contrast to cells cultured in BM at both 7 and 14 day time points (figure 6). The number of cells also increased significantly (p < 0.05) with time in all conditions, except for BM-groups when comparing the 1 and 7 day time points.
Figure 6.
The relative DNA content of hASCs cultured in BM or OM, non-stimulated or stimulated with 50 or 100 Hz vibration loading at the 1, 7 and 14 day time points. Results are expressed as mean + s.d. and normalized relative to control culture (BM). *p < 0.05 for the effect of OM versus BM. White bars, BM; bars with squares, BM 50 Hz; bars with cross lines, BM 100 Hz; light grey bars, OM; dark grey bars, OM 50 Hz; black bars, OM 100 Hz.
No statistically significant differences were observed in cell number between vibration-stimulated and the control hASCs in either cell-culturing condition (BM or OM) in any of the time points.
3.3. Alkaline phosphatase activity of adipose stem cells
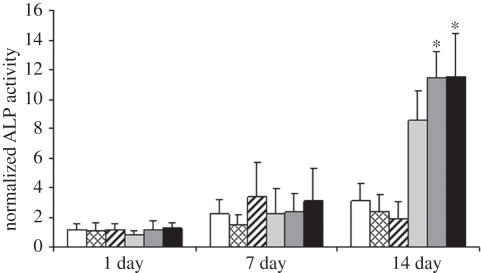
ALP activity was used as an early marker for osteoblast differentiation of hASCs. The quantification of ALP activity was performed on the same samples as the DNA measurement, enabling normalization of the ALP activity relative to the DNA content of the sample. At the 14 day time point, ALP activity of the vibration stimulated hASCs in BM seemed to be lower than in the control, but the difference was not statistically significant (figure 7). Conversely, both 50 Hz (p = 0.018) and 100 Hz (p = 0.013) frequencies increased ALP activity of the hASCs cultured in OM significantly at 14 days in contrast to the static control (OM). Both frequencies were equally effective in stimulating ALP activity in OM-cultured hASCs, because there was no significant effect between these frequencies.
Figure 7.
The normalized ALP activity of hASCs cultured in BM or OM, non-stimulated or stimulated with 50 or 100 Hz vibration loading at the 1, 7, and 14 day time points. Results are expressed as mean + s.d. and normalized relative to amount of DNA. *p < 0.05 with respect to OM, BM, BM 50 Hz and BM 100 Hz. White bars, BM; bars with squares, BM 50 Hz; bars with cross lines, BM 100 Hz; light grey bars, OM; dark grey bars, OM 50 Hz; black bars, OM 100 Hz.
3.4. Mineralization of adipose stem cells
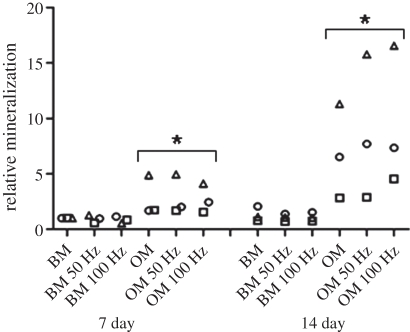
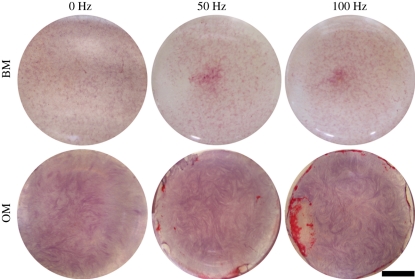
In addition to using ALP activity as an early marker for osteogenic differentiation, mineralization of the ECM was used as a late marker of in vitro bone formation. Mineralization was measured quantitatively from the Alizarin Red-stained hASCs at the 7 and 14 day time points. Mineralization of hASCs increased significantly with time when hASCs were cultured in OM (figure 8). Although mineralization was higher in the vibration-stimulated hASCs cultured in OM in some of the experiment replications, significant differences were not detected in the combined results between the vibration-stimulated cells and static control in any of the culturing conditions or time points. However, a qualitative Alizarin Red staining at day 14 showed mineralized areas in the OM-cultured hASCs stimulated with 50 and 100 Hz frequencies whereas the static control showed no mineralization (figure 9). Mineralized areas were detected in the stimulated cells cultured in OM in all the experiment replications and mineralization was consistently more intense in 100 Hz than in 50 Hz stimulation.
Figure 8.
Scatter chart of the relative mineralization of hASCs showing the individual data points for each experiment replication at the 7 and 14 day time points. *p < 0.05 for the effect of OM versus BM. Squares, experiment 1; circles, experiment 2; triangles, experiment 3.
Figure 9.
Representative images of Alizarin Red-stained hASCs cultured either in BM or OM at the 14 day time point. Mineralization was observed in the OM-cultured hASCs stimulated with 50 and 100 Hz vibration. Scale bar 5 mm.
3.5. Collagen production
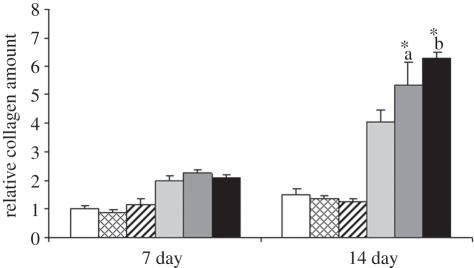
Collagen production of hASCs was analysed at the 7 and 14 day time points to examine the in vitro formation of organic bone matrix. Vibration loading had no effect on the collagen production of hASCs cultured in BM, whereas the vibration-stimulated hASCs cultured in OM produced significantly more (p < 0.01) collagen at the 14 day time point when compared with the static control (figure 10). The collagen production was enhanced the most by vibration loading at 100 Hz frequency (p = 0.034 when compared with 50 Hz vibration).
Figure 10.
The relative collagen production of hASCs cultured in BM or OM, non-stimulated or stimulated with 50 or 100 Hz vibration loading at the 7 and 14 day time points. Results are expressed as mean + s.d. *a p < 0.05 with respect to OM, OM 100 Hz, BM, BM 50 Hz and BM 100 Hz. *b p < 0.05 with respect to OM, OM 50 Hz, BM, BM 50 Hz and BM 100 Hz. White bars, BM; bars with squares, BM 50 Hz; bars with cross lines, BM 100 Hz; light grey bars, OM; dark grey bars, OM 50 Hz; black bars, OM 100 Hz.
3.6. Adipogenic differentiation of adipose stem cells
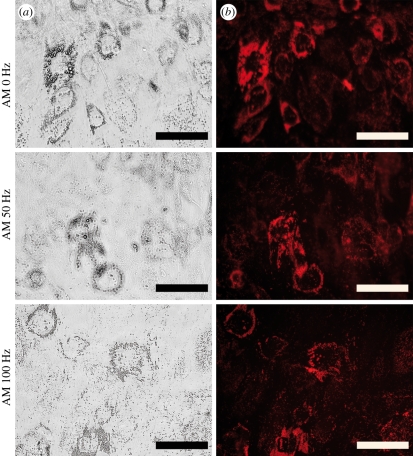
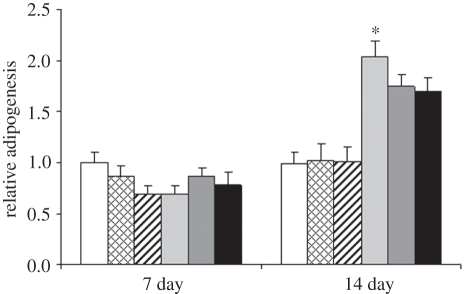
The effect of vibration loading on adipogenic differentiation of hASCs was studied by Oil red O staining of the lipid droplets. The stained cells were visualized both by light microscopy and epifluorescence using a 560 nm excitation/emission filter. Representative images of the vibration-stimulated and static hASCs under adipogenic differentiation at the 14 day time point are shown in figure 11. The lipid droplet formation under adipogenic differentiation conditions was more extensive in the static control cells than in the vibration-stimulated cells (figure 11). The quantified staining result demonstrated reduced adipogenesis of the vibration-stimulated hASCs under adipogenic induction at the 14 day time point (figure 12). However, the difference was statistically significant (p = 0.048) only between the static control and hASCs stimulated with 100 Hz frequency vibration in AM.
Figure 11.
Representative images of the vibration-stimulated (50 and 100 Hz) and static (0 Hz) hASCs under adipogenic differentiation (AM) at the 14 day time point as visualized by (a) light microscope and (b) epifluorescence at 560 nm after Oil red O staining. Scale bar 500 µm.
Figure 12.
Relative adipogenesis of hASCs cultured in BM or AM, non-stimulated or stimulated with 50 or 100 Hz vibration loading at 7 and 14 day time points. Results are expressed as mean + s.d. *p < 0.05 with respect to AM 100 Hz, BM, BM 50 Hz and BM 100 Hz. White bars, BM; bars with squares, BM 50 Hz; bars with cross lines, BM 100 Hz; light grey bars, AM; dark grey bars, AM 50 Hz; black bars, AM 100 Hz.
4. Discussion
To our knowledge, this is the first study to report that hASCs respond to HMHF vibration loading. The effects of mechanical [40,41] and electrical [42] stimulation on ASCs using low frequencies (0.5–1 Hz) were previously reported. We chose to use high-frequency vibration in the present study because of its osteogenic effect on osteoblasts or osteoblast-like cells [43–47] as well as on MSCs [6,16]. HMHF vibration has also been tested in vitro with fibroblasts [48] and in vivo with ovariectomized rats [14,15]. The accelerations used in these HMHF studies vary from 2 to 3.4 G and the frequencies range from 45 to 100 Hz [14,15,48]. In addition, high-impact exercising involving high tissue-level strains is essential for bone remodelling in athletes [10]. Based on these studies, a square wave was used as the vibration stimulation, providing high strain rates for hASCs. Because it is not yet clear whether frequency has a greater influence on vibration loaded cells, we used two different high frequencies (50 and 100 Hz) in combination with a high-magnitude (3g) vibration in this study. Furthermore, parameters other than magnitude and frequency affect the response of cells to mechanical loading. Sen et al. [18] suggest that the schedule of mechanical signals could be at least as important as the signals themselves. Bone adaptation is driven mostly by dynamic rather than static mechanical stimulation [49]. For example, insertion of short rest periods between stimulations can prevent saturation of an adaptive response [50,51]. In addition, a recovery period of at least 8 h is reported to restore mechanosensitivity of saturated bone cells [52]. Therefore, we used resting periods both during the vibration stimulation (stimulation for 1 s was followed by a 1 s rest period) and after the stimulation bouts (a 3 h stimulation bout was followed by 21 h of recovery).
Human ASCs pooled from four donors were used in this study to reduce the effects of donor variability. It is important to note that donor age, sex and individual differences can impact cell behaviour, especially in clinical treatments. Although the relatively high mean donor age (56 ± 12.6 years) in this study might have affected the proliferation capacity of the cells, the osteogenic potential of ASCs is not reduced by ageing [40,53,54]. The hASC populations used in this study were characterized by flow cytometry. The surface marker profiles were consistent with the previous results for hASCs [36,55], with the expression of markers confirming the mesenchymal origin of the cells and the lack of haematopoietic and angiogenic markers.
The most significant finding of the present study was that hASCs cultured under osteogenic conditions were sensitive to vibration loading and that their osteogenic differentiation could be intensified with high-frequency vibration, especially by 100 Hz vibration. This is consistent with the findings by Judex et al. [56] showing increased bone formation at 90 Hz compared with 45 Hz vibration. Because vibration loading had no significant effect on hASCs cultured in BM, it is likely that hASCs become sensitive to mechanical stimulation as they begin to differentiate towards osteogenic lineages when cultured in OM. Our results suggest that osteogenic culturing conditions were more optimal for vibration stimulation of hASCs owing to the enhanced cell attachment. Live–dead staining showed that the vibrated cells attached slightly less homogeneously than the static control cells and also formed clusters during the first week of culturing. This effect was more pronounced in hASCs cultured in BM. The viability of the cells, however, was not affected by the vibration loading, because the number of viable cells increased over time in all conditions. Some dead or detached cells were occasionally detected among stimulated cells cultured in BM, but to a lesser extent than in cells cultured in OM. It seems that osteogenic culture conditions enhanced the attachment of the hASCs, possibly owing to the increased secretion of ECM proteins. The OM used in this study was specifically optimized for hASCs cultured in human serum (L. Tirkkonen et al. 2011, unpublished data) containing a higher concentration of Asc 2-P (250 µM) than other types of OM described in the literature [25–27,57]. Asc 2-P stimulates the secretion of ECM proteins such as collagen and glycosaminoglycan in MSCs [58]. Overall, the cell number, ALP activity, collagen content and mineralization were significantly higher in OM-cultured hASCs than in BM-cultured cells at the 14 day time point. Despite the occasional detachment, the results indicated that vibration loading had no significant effect on hASC cell number in OM or BM. Previous reports of the effects of vibration on proliferation of bone cells or their precursors are somewhat divergent. Patel et al. [44] reported that proliferation of pre-osteoblasts in BM was not affected by 30 Hz stimulation at the 3 day time point. Dumas et al. [59] reported that osteoblast number and viability were not affected after 3 or 7 days of culturing in BM under LMHF vibration. On the other hand, Rosenberg et al. [46] showed that osteoblast proliferation decreases as frequency increases, with the highest proliferation at 20 Hz and lowest at 60 Hz at the 5 day time point using osteogenic culturing conditions. HMLF stretching (10% cyclic strain, 0.5 Hz), in turn, enhances proliferation of mouse ASCs and even counteracts the reduced proliferation rate related to ageing [40].
In contrast to cell number, ALP activity was significantly higher in vibration-stimulated hASCs cultured in OM when compared with static control at the 14 day time point. Similarly, vibration-stimulated hASCs cultured in OM produced significantly more collagen than the static control at 14 day. The two vibration frequencies did not differ in their effect on ALP activity, but 100 Hz vibration stimulated collagen production significantly more than 50 Hz vibration did. Previous studies reported that high-frequency vibration stimulation enhanced ECM protein accumulation in pre-osteoblasts [44], fibroblasts [48] and chondrocytes [60,61].
In the present study, vibration loading enhanced the mineralization of OM-cultured hASCs compared with static control at the 14 day time point, although the difference was not statistically significant. The difference between the stimulated and static groups in OM was greatest in the third experiment replicate, but a similar trend was seen in all the three experiment replications. This may have resulted from a variation in the cell pool used in each of the replicated experiments, because the passage and ratio of cells can vary slightly owing to the differing growth rates of the cells. Also, during the third experiment replication, the microcontroller of the vibration device was reprogrammed to adjust the acceleration automatically to improve the accuracy of the acceleration adjustment. The effect of vibration on mineralization was further supported by the qualitative Alizarin Red staining, showing mineralization on the vibration-stimulated OM-cultured hASCs at the 14 day time point, detected in all replicates of the experiment. Mineralization was more intense in hASCs stimulated with 100 Hz than 50 Hz vibration. In summary, the findings regarding ALP activity, collagen production and mineralization are consistent with those of other vibration-loading studies. High-frequency vibration stimulates ALP activity of osteoblasts [44,46], mineralization of pre-osteoblasts [44] and collagen accumulation of fibroblasts [48] in vitro.
Finally, the effect of vibration loading on adipogenic differentiation of hASCs was studied. MSCs are the progenitors of both osteoblasts and adipocytes, and as previously reported, mechanical signals can direct MSC differentiation towards osteoblastogenesis and inhibit adipogenesis of MSCs [6,17,18,40]. Similar to the studies conducted with bone marrow MSCs, our results showed that the adipogenic differentiation of hASCs is inhibited by high-frequency vibration, especially by 100 Hz vibration.
In conclusion, the vibration-loading device described in this study successfully generated controlled vibrational forces to cells cultured on well plates. Osteogenic culturing conditions were more optimal for vibration-loading studies performed with hASCs than basal conditions. The findings of this study suggest that osteogenic culture conditions combined with HMHF vibration enhance hASC differentiation towards bone-forming cells. Consistent with the reciprocal relationship between osteogenesis and adipogenesis in MSCs [62,63], HMHF vibration inhibited adipogenic differentiation of induced hASCs. Finally, these findings combined with previously published data indicate the significance of mechanical signals in controlling adult stem cell fate, a potential that may be harnessed to combat bone loss or even obesity in the future.
Acknowledgements
The study was conducted in accordance with the Ethics Committee of the Pirkanmaa Hospital District, Tampere, Finland.
The authors thank Ms Anna-Maija Honkala, Ms Miia Juntunen, Ms Minna Salomäki and Ms Sari Kalliokoski for their excellent technical assistance. Special thanks to Mr Nikolai Beev from the Technical University of Tampere for technical assistance in constructing the vibration-loading device. This study was financially supported by the Competitive Research Funding of Tampere University Hospital (grants 9L057, 9K117, 9L100, 9M058 and 9J014), the Finnish Funding Agency for Technology and Innovation (TEKES) and Stryker Leibinger GmbH & Co KG.
References
- 1.Frost H. M. 2003. Bone's mechanostat: a 2003 update. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 275, 1081–1101 10.1002/ar.a.10119 (doi:10.1002/ar.a.10119) [DOI] [PubMed] [Google Scholar]
- 2.Leblanc A. D., Schneider V. S., Evans H. J., Engelbretson D. A., Krebs J. M. 1990. Bone mineral loss and recovery after 17 weeks of bed rest. J. Bone Miner. Res. 5, 843–850 10.1002/jbmr.5650050807 (doi:10.1002/jbmr.5650050807) [DOI] [PubMed] [Google Scholar]
- 3.Sievanen H. 2010. Immobilization and bone structure in humans. Arch Biochem. Biophys. 503, 146–152 10.1016/j.abb.2010.07.008 (doi:10.1016/j.abb.2010.07.008) [DOI] [PubMed] [Google Scholar]
- 4.Vico L., Collet P., Guignandon A., Lafage-Proust M. H., Thomas T., Rehaillia M., Alexandre C. 2000. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 355, 1607–1611 10.1016/S0140-6736(00)02217-0 (doi:10.1016/S0140-6736(00)02217-0) [DOI] [PubMed] [Google Scholar]
- 5.Ozcivici E., Luu Y. K., Adler B., Qin Y. X., Rubin J., Judex S., Rubin C. T. 2010. Mechanical signals as anabolic agents in bone. Nat. Rev. Rheumatol. 6, 50–59 10.1038/nrrheum.2009.239 (doi:10.1038/nrrheum.2009.239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozcivici E., Luu Y. K., Rubin C. T., Judex S. 2010. Low-level vibrations retain bone marrow's osteogenic potential and augment recovery of trabecular bone during reambulation. PLoS ONE 5, e11178. 10.1371/journal.pone.0011178 (doi:10.1371/journal.pone.0011178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin C., Turner A. S., Bain S., Mallinckrodt C., McLeod K. 2001. Anabolism low mechanical signals strengthen long bones. Nature 412, 603–604 10.1038/35088122 (doi:10.1038/35088122) [DOI] [PubMed] [Google Scholar]
- 8.Rubin C., Xu G., Judex S. 2001. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J. 15, 2225–2229 10.1096/fj.01-0166com (doi:10.1096/fj.01-0166com) [DOI] [PubMed] [Google Scholar]
- 9.Heinonen A., Sievanen H., Kyrolainen H., Perttunen J., Kannus P. 2001. Mineral mass, size, and estimated mechanical strength of triple jumpers' lower limb. Bone 29, 279–285 10.1016/S8756-3282(01)00574-9 (doi:10.1016/S8756-3282(01)00574-9) [DOI] [PubMed] [Google Scholar]
- 10.Nikander R., et al. 2009. Targeted exercises against hip fragility. Osteoporos. Int. 20, 1321–1328 10.1007/s00198-008-0785-x (doi:10.1007/s00198-008-0785-x) [DOI] [PubMed] [Google Scholar]
- 11.Ward K., Alsop C., Caulton J., Rubin C., Adams J., Mughal Z. 2004. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J. Bone Miner. Res. 19, 360–369 10.1359/JBMR.040129 (doi:10.1359/JBMR.040129) [DOI] [PubMed] [Google Scholar]
- 12.Gilsanz V., Wren T. A., Sanchez M., Dorey F., Judex S., Rubin C. 2006. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J. Bone Miner. Res. 21, 1464–1474 10.1359/jbmr.060612 (doi:10.1359/jbmr.060612) [DOI] [PubMed] [Google Scholar]
- 13.Rubin C., Recker R., Cullen D., Ryaby J., McCabe J., McLeod K. 2004. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J. Bone Miner. Res. 19, 343–351 10.1359/JBMR.0301251 (doi:10.1359/JBMR.0301251) [DOI] [PubMed] [Google Scholar]
- 14.Flieger J., Karachalios T., Khaldi L., Raptou P., Lyritis G. 1998. Mechanical stimulation in the form of vibration prevents postmenopausal bone loss in ovariectomized rats. Calcif. Tissue Int. 63, 510–514 10.1007/s002239900566 (doi:10.1007/s002239900566) [DOI] [PubMed] [Google Scholar]
- 15.Oxlund B. S., Ortoft G., Andreassen T. T., Oxlund H. 2003. Low-intensity, high-frequency vibration appears to prevent the decrease in strength of the femur and tibia associated with ovariectomy of adult rats. Bone 32, 69–77 10.1016/S8756-3282(02)00916-X (doi:10.1016/S8756-3282(02)00916-X) [DOI] [PubMed] [Google Scholar]
- 16.Luu Y. K., Capilla E., Rosen C. J., Gilsanz V., Pessin J. E., Judex S., Rubin C. T. 2009. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J. Bone Miner. Res. 24, 50–61 10.1359/jbmr.080817 (doi:10.1359/jbmr.080817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin C. T., et al. 2007. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc. Natl Acad. Sci. USA 104, 17 879–17 884 10.1073/pnas.0708467104 (doi:10.1073/pnas.0708467104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sen B., Xie Z., Case N., Styner M., Rubin C. T., Rubin J. 2011. Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. J. Biomech. 44, 593–599 10.1016/j.jbiomech.2010.11.022 (doi:10.1016/j.jbiomech.2010.11.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott A., Khan K. M., Duronio V., Hart D. A. 2008. Mechanotransduction in human bone: in vitro cellular physiology that underpins bone changes with exercise. Sports Med. 38, 139–160 10.2165/00007256-200838020-00004 (doi:10.2165/00007256-200838020-00004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fritton S. P., Weinbaum S. 2009. Fluid and solute transport in bone: flow-induced mechanotransduction. Annu. Rev. Fluid Mech. 41, 347–374 10.1146/annurev.fluid.010908.165136 (doi:10.1146/annurev.fluid.010908.165136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torcasio A., van Lenthe G. H., Van Oosterwyck H. 2008. The importance of loading frequency, rate and vibration for enhancing bone adaptation and implant osseointegration. Eur. Cell Mater. 16, 56–68 [PubMed] [Google Scholar]
- 22.Burra S., Nicolella D. P., Francis W. L., Freitas C. J., Mueschke N. J., Poole K., Jiang J. X. 2010. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc. Natl Acad. Sci. USA 107, 13 648–13 653 10.1073/pnas.1009382107 (doi:10.1073/pnas.1009382107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.You L., Cowin S. C., Schaffler M. B., Weinbaum S. 2001. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J. Biomech. 34, 1375–1386 10.1016/S0021-9290(01)00107-5 (doi:10.1016/S0021-9290(01)00107-5) [DOI] [PubMed] [Google Scholar]
- 24.Judex S., Rubin C. T. 2010. Is bone formation induced by high-frequency mechanical signals modulated by muscle activity? J. Musculoskelet. Neuronal Interact. 10, 3–11 [PMC free article] [PubMed] [Google Scholar]
- 25.Zuk P. A., Zhu M., Mizuno H., Huang J., Futrell J. W., Katz A. J., Benhaim P., Lorenz H. P., Hedrick M. H. 2001. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 7, 211–228 10.1089/107632701300062859 (doi:10.1089/107632701300062859) [DOI] [PubMed] [Google Scholar]
- 26.de Girolamo L., Sartori M. F., Albisetti W., Brini A. T. 2007. Osteogenic differentiation of human adipose-derived stem cells: comparison of two different inductive media. J. Tissue Eng. Regen. Med. 1, 154–157 10.1002/term.12 (doi:10.1002/term.12) [DOI] [PubMed] [Google Scholar]
- 27.Halvorsen Y. D., Franklin D., Bond A. L., Hitt D. C., Auchter C., Boskey A. L., Paschalis E. P., Wilkison W. O., Gimble J. M. 2001. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 7, 729–741 10.1089/107632701753337681 (doi:10.1089/107632701753337681) [DOI] [PubMed] [Google Scholar]
- 28.Zuk P. A., et al. 2002. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 13, 4279–4295 10.1091/mbc.E02-02-0105 (doi:10.1091/mbc.E02-02-0105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cowan C. M., et al. 2004. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat. Biotechnol. 22, 560–567 10.1038/nbt958 (doi:10.1038/nbt958) [DOI] [PubMed] [Google Scholar]
- 30.Hattori H., Masuoka K., Sato M., Ishihara M., Asazuma T., Takase B., Kikuchi M., Nemoto K. 2006. Bone formation using human adipose tissue-derived stromal cells and a biodegradable scaffold. J. Biomed. Mater. Res. B Appl. Biomater. 76, 230–239 [DOI] [PubMed] [Google Scholar]
- 31.Hicok K. C., Du Laney T. V., Zhou Y. S., Halvorsen Y. D., Hitt D. C., Cooper L. F., Gimble J. M. 2004. Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng. 10, 371–380 10.1089/107632704323061735 (doi:10.1089/107632704323061735) [DOI] [PubMed] [Google Scholar]
- 32.Lee J. A., Parrett B. M., Conejero J. A., Laser J., Chen J., Kogon A. J., Nanda D., Grant R. T., Breitbart A. S. 2003. Biological alchemy: engineering bone and fat from fat-derived stem cells. Ann. Plast. Surg. 50, 610–617 10.1097/01.SAP.0000069069.23266.35 (doi:10.1097/01.SAP.0000069069.23266.35) [DOI] [PubMed] [Google Scholar]
- 33.Lendeckel S., Jodicke A., Christophis P., Heidinger K., Wolff J., Fraser J. K., Hedrick M. H., Berthold L., Howaldt H. P. 2004. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J. Craniomaxillofac. Surg. 32, 370–373 [DOI] [PubMed] [Google Scholar]
- 34.Mesimaki K., Lindroos B., Tornwall J., Mauno J., Lindqvist C., Kontio R., Miettinen S., Suuronen R. 2009. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int. J. Oral Maxillofac. Surg. 38, 201–209 10.1016/j.ijom.2009.01.001 (doi:10.1016/j.ijom.2009.01.001) [DOI] [PubMed] [Google Scholar]
- 35.De Smet E., Jaecques S. V., Jansen J. J., Walboomers F., Vander Sloten J., Naert I. E. 2008. Effect of strain at low-frequency loading on peri-implant bone (re)modelling: a guinea-pig experimental study. Clin. Oral Implants Res. 19, 733–739 [DOI] [PubMed] [Google Scholar]
- 36.Lindroos B., Boucher S., Chase L., Kuokkanen H., Huhtala H., Haataja R., Vemuri M., Suuronen R., Miettinen S. 2009. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy 11, 958–972 10.3109/14653240903233081 (doi:10.3109/14653240903233081) [DOI] [PubMed] [Google Scholar]
- 37.Lindroos B., Maenpaa K., Ylikomi T., Oja H., Suuronen R., Miettinen S. 2008. Characterisation of human dental stem cells and buccal mucosa fibroblasts. Biochem. Biophys. Res. Commun. 368, 329–335 10.1016/j.bbrc.2008.01.081 (doi:10.1016/j.bbrc.2008.01.081) [DOI] [PubMed] [Google Scholar]
- 38.Haimi S., et al. 2009. Calcium phosphate surface treatment of bioactive glass causes a delay in early osteogenic differentiation of adipose stem cells. J. Biomed. Mater. Res. A 91, 540–547 [DOI] [PubMed] [Google Scholar]
- 39.Koopman R., Schaart G., Hesselink M. K. 2001. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem. Cell Biol. 116, 63–68 [DOI] [PubMed] [Google Scholar]
- 40.Huang S. C., Wu T. C., Yu H. C., Chen M. R., Liu C. M., Chiang W. S., Lin K. M. 2010. Mechanical strain modulates age-related changes in the proliferation and differentiation of mouse adipose-derived stromal cells. BMC Cell Biol. 11, 18. 10.1186/1471-2121-11-18 (doi:10.1186/1471-2121-11-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wall M. E., Rachlin A., Otey C. A., Loboa E. G. 2007. Human adipose-derived adult stem cells upregulate palladin during osteogenesis and in response to cyclic tensile strain. Am. J. Physiol. Cell Physiol. 293, C1532–C1538 10.1152/ajpcell.00065.2007 (doi:10.1152/ajpcell.00065.2007) [DOI] [PubMed] [Google Scholar]
- 42.McCullen S. D., McQuilling J. P., Grossfeld R. M., Lubischer J. L., Clarke L. I., Loboa E. G. 2010. Application of low-frequency alternating current electric fields via interdigitated electrodes: effects on cellular viability, cytoplasmic calcium, and osteogenic differentiation of human adipose-derived stem cells. Tissue Eng. Part C Methods 16, 1377–1386 10.1089/ten.tec.2009.0751 (doi:10.1089/ten.tec.2009.0751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dumas V., Perrier A., Malaval L., Laroche N., Guignandon A., Vico L., Rattner A. 2009. The effect of dual frequency cyclic compression on matrix deposition by osteoblast-like cells grown in 3D scaffolds and on modulation of VEGF variant expression. Biomaterials 30, 3279–3288 10.1016/j.biomaterials.2009.02.048 (doi:10.1016/j.biomaterials.2009.02.048) [DOI] [PubMed] [Google Scholar]
- 44.Patel M. J., Chang K. H., Sykes M. C., Talish R., Rubin C., Jo H. 2009. Low magnitude and high frequency mechanical loading prevents decreased bone formation responses of 2T3 preosteoblasts. J. Cell Biochem. 106, 306–316 10.1002/jcb.22007 (doi:10.1002/jcb.22007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pre D., Ceccarelli G., Benedetti L., Magenes G., De Angelis M. G. 2009. Effects of low-amplitude, high-frequency vibrations on proliferation and differentiation of SAOS-2 human osteogenic cell line. Tissue Eng. Part C Methods 15, 669–679 10.1089/ten.tec.2008.0599 (doi:10.1089/ten.tec.2008.0599) [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg N., Levy M., Francis M. 2002. Experimental model for stimulation of cultured human osteoblast-like cells by high frequency vibration. Cytotechnology 39, 125–130 10.1023/A:1023925230651 (doi:10.1023/A:1023925230651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka S. M., Li J., Duncan R. L., Yokota H., Burr D. B., Turner C. H. 2003. Effects of broad frequency vibration on cultured osteoblasts. J. Biomech. 36, 73–80 10.1016/S0021-9290(02)00245-2 (doi:10.1016/S0021-9290(02)00245-2) [DOI] [PubMed] [Google Scholar]
- 48.Wolchok J. C., Brokopp C., Underwood C. J., Tresco P. A. 2009. The effect of bioreactor induced vibrational stimulation on extracellular matrix production from human derived fibroblasts. Biomaterials 30, 327–335 10.1016/j.biomaterials.2008.08.035 (doi:10.1016/j.biomaterials.2008.08.035) [DOI] [PubMed] [Google Scholar]
- 49.Turner C. H. 1998. Three rules for bone adaptation to mechanical stimuli. Bone 23, 399–407 10.1016/S8756-3282(98)00118-5 (doi:10.1016/S8756-3282(98)00118-5) [DOI] [PubMed] [Google Scholar]
- 50.LaMothe J. M., Zernicke R. F. 2004. Rest insertion combined with high-frequency loading enhances osteogenesis. J. Appl. Physiol. 96, 1788–1793 10.1152/japplphysiol.01145.2003 (doi:10.1152/japplphysiol.01145.2003) [DOI] [PubMed] [Google Scholar]
- 51.Srinivasan S., Weimer D. A., Agans S. C., Bain S. D., Gross T. S. 2002. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J. Bone Miner. Res. 17, 1613–1620 10.1359/jbmr.2002.17.9.1613 (doi:10.1359/jbmr.2002.17.9.1613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robling A. G., Burr D. B., Turner C. H. 2001. Recovery periods restore mechanosensitivity to dynamically loaded bone. J. Exp. Biol. 204, 3389–3399 [DOI] [PubMed] [Google Scholar]
- 53.Khan W. S., Adesida A. B., Tew S. R., Andrew J. G., Hardingham T. E. 2009. The epitope characterisation and the osteogenic differentiation potential of human fat pad-derived stem cells is maintained with ageing in later life. Injury 40, 150–157 10.1016/j.injury.2008.05.029 (doi:10.1016/j.injury.2008.05.029) [DOI] [PubMed] [Google Scholar]
- 54.Shi Y. Y., Nacamuli R. P., Salim A., Longaker M. T. 2005. The osteogenic potential of adipose-derived mesenchymal cells is maintained with aging. Plast. Reconstr. Surg. 116, 1686–1696 10.1097/01.prs.0000185606.03222.a9 (doi:10.1097/01.prs.0000185606.03222.a9) [DOI] [PubMed] [Google Scholar]
- 55.Lindroos B., Aho K. L., Kuokkanen H., Raty S., Huhtala H., Lemponen R., Yli-Harja O., Suuronen R., Miettinen S. 2010. Differential gene expression in adipose stem cells cultured in allogeneic human serum versus fetal bovine serum. Tissue Eng. Part A 16, 2281–2294 10.1089/ten.tea.2009.0621 (doi:10.1089/ten.tea.2009.0621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Judex S., Lei X., Han D., Rubin C. 2007. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J. Biomech. 40, 1333–1339 10.1016/j.jbiomech.2006.05.014 (doi:10.1016/j.jbiomech.2006.05.014) [DOI] [PubMed] [Google Scholar]
- 57.Ogawa R., Mizuno H., Watanabe A., Migita M., Shimada T., Hyakusoku H. 2004. Osteogenic and chondrogenic differentiation by adipose-derived stem cells harvested from GFP transgenic mice. Biochem. Biophys. Res. Commun. 313, 871–877 10.1016/j.bbrc.2003.12.017 (doi:10.1016/j.bbrc.2003.12.017) [DOI] [PubMed] [Google Scholar]
- 58.Choi K. M., Seo Y. K., Yoon H. H., Song K. Y., Kwon S. Y., Lee H. S., Park J. K. 2008. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J. Biosci. Bioeng. 105, 586–594 10.1263/jbb.105.586 (doi:10.1263/jbb.105.586) [DOI] [PubMed] [Google Scholar]
- 59.Dumas V., et al. 2010. Extracellular matrix produced by osteoblasts cultured under low-magnitude, high-frequency stimulation is favourable to osteogenic differentiation of mesenchymal stem cells. Calcif. Tissue Int. 87, 351–364 10.1007/s00223-010-9394-8 (doi:10.1007/s00223-010-9394-8) [DOI] [PubMed] [Google Scholar]
- 60.Liu J., Sekiya I., Asai K., Tada T., Kato T., Matsui N. 2001. Biosynthetic response of cultured articular chondrocytes to mechanical vibration. Res. Exp. Med. (Berl) 200, 183–193 [PubMed] [Google Scholar]
- 61.Takeuchi R., et al. 2006. Effects of vibration and hyaluronic acid on activation of three-dimensional cultured chondrocytes. Arthritis Rheum. 54, 1897–1905 10.1002/art.21895 (doi:10.1002/art.21895) [DOI] [PubMed] [Google Scholar]
- 62.Scheideler M., et al. 2008. Comparative transcriptomics of human multipotent stem cells during adipogenesis and osteoblastogenesis. BMC Genomics 9, 340. 10.1186/1471-2164-9-340 (doi:10.1186/1471-2164-9-340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang D. C., Tsay H. J., Lin S. Y., Chiou S. H., Li M. J., Chang T. J., Hung S. C. 2008. cAMP/PKA regulates osteogenesis, adipogenesis and ratio of RANKL/OPG mRNA expression in mesenchymal stem cells by suppressing leptin. PLoS ONE 3, e1540. 10.1371/journal.pone.0001540 (doi:10.1371/journal.pone.0001540) [DOI] [PMC free article] [PubMed] [Google Scholar]