Abstract
Little is known about the olfactory capabilities of extinct basal (non-neornithine) birds or the evolutionary changes in olfaction that occurred from non-avian theropods through modern birds. Although modern birds are known to have diverse olfactory capabilities, olfaction is generally considered to have declined during avian evolution as visual and vestibular sensory enhancements occurred in association with flight. To test the hypothesis that olfaction diminished through avian evolution, we assessed relative olfactory bulb size, here used as a neuroanatomical proxy for olfactory capabilities, in 157 species of non-avian theropods, fossil birds and living birds. We show that relative olfactory bulb size increased during non-avian maniraptoriform evolution, remained stable across the non-avian theropod/bird transition, and increased during basal bird and early neornithine evolution. From early neornithines through a major part of neornithine evolution, the relative size of the olfactory bulbs remained stable before decreasing in derived neoavian clades. Our results show that, rather than decreasing, the importance of olfaction actually increased during early bird evolution, representing a previously unrecognized sensory enhancement. The relatively larger olfactory bulbs of earliest neornithines, compared with those of basal birds, may have endowed neornithines with improved olfaction for more effective foraging or navigation skills, which in turn may have been a factor allowing them to survive the end-Cretaceous mass extinction.
Keywords: Theropoda, Aves, olfactory bulb, Archaeopteryx, basal birds, olfactory ratio
1. Introduction
Several anatomical, physiological and behavioural traits of living birds are known to have first evolved among non-avian theropods [1–14]. However, little is known about the extent to which birds inherited their sensory modalities from their non-avian theropod ancestors. Aspects of visual, auditory and vestibular senses have been investigated in some extinct birds in order to understand the sensory changes associated with the origin of flight during the non-avian theropod/bird transition [5,15–17]. In comparison, changes in olfaction (i.e. sense of smell) have received limited attention, which may be due, in part, to the preconceived notion that olfaction was a declining sensory modality during bird evolution [18–21] and to the historical misconception that birds have a poor sense of smell [22]. Birds are now known to have a wide range of olfactory capabilities, which are used for a variety of purposes, such as foraging, orientation and social interactions [22–24].
Among birds and extinct theropods, investigations of the olfactory system have emphasized the role of the olfactory bulbs, anterior projections of the forebrain, in olfaction. The relative size of the olfactory bulbs has been suggested to be related to the olfactory capabilities of living birds [25–29] and extinct theropods [5,16,30–33]. A recent study of olfactory bulb size in non-avian theropods and Archaeopteryx led to the inference that the oldest known bird had olfactory capabilities typical of a similar-sized non-avian theropod [33]. Although this suggests that olfaction remained unchanged during the non-avian theropod/bird transition [33], a large-scale study of relative olfactory bulb size in extinct and extant theropods is necessary to shed light on the hypothesis that olfaction declined progressively through avian evolution. Here, we present the most inclusive study of early avian olfactory evolution by analysing relative olfactory bulb size in 157 species of non-avian theropods, fossil birds and living birds, while taking into consideration the effects of body mass and phylogeny, in order to assess changes in olfaction through theropod evolution.
2. Olfactory bulb size and olfactory capabilities
Olfactory bulb size has long been suggested to be correlated with olfactory capabilities in vertebrates [34] based on the well-established principle of proper mass [35], which states that the relative size of a brain region reflects the relative importance of the neural function of that region to the biology of the animal. Various studies have demonstrated that olfactory bulb size is correlated with olfactory ability in birds and mammals [36–38]. The relationship between olfactory bulb size and olfactory ability may be related to: (i) the number and size of mitral cells in the bulb [29,39]; (ii) the number of glomeruli in the bulb [40]; and (iii) the number of olfactory receptor genes [41,42].
In birds, relative olfactory bulb size has long been considered a neuroanatomical proxy for olfactory capabilities [25–27,37,41,42] and is quantified in a metric termed the olfactory ratio, defined as the ratio between the greatest linear dimension of the bulb and the greatest linear dimension of the cerebral hemispheres, regardless of their orientation [25,27]. Recent studies have supported the validity of this proxy because olfactory ratios are: (i) positively correlated with the number of olfactory receptor genes (i.e. the larger the olfactory ratio, the greater the number of olfactory receptor genes) [41,42], and (ii) negatively correlated with the odour detection threshold (lowest detectable odorant concentration) across bird orders [37], both suggesting that larger ratios indicate better olfactory capabilities. Given these correlations, olfactory ratios are an appropriate measure of the relative importance of olfaction through large-scale evolution of non-avian and avian theropods. Furthermore, defining the olfactory ratio in terms of the longest linear dimensions of the olfactory bulbs and cerebral hemispheres regardless of orientation, rather than the measurement of a standardized orientation (e.g. rostrocaudal diameter), allows for the documented changes in shape or orientation of these brain components that occurred during theropod evolution [16,20,31,35,43,44] to be taken into consideration. Thus, the olfactory ratio is a useful comparative statistic to reflect the relative importance of olfaction in comparison with other sensory modalities, even when, for example, the cerebral hemispheres were undergoing substantial evolution throughout the non-avian theropod/bird transition.
3. Material and methods
(a). Olfactory ratio calculations
Olfactory ratios, calculated from the greatest linear measurements of the olfactory bulb and cerebral hemisphere, have been the standard measure of the relative size of these features in extant birds [25,27]. Although complex parameters (e.g. mass or volume of brain components) may seem to be more appropriate measurements for size, single linear measurements are often used to estimate complex parameters that are otherwise not readily obtainable [45–50]. Furthermore, for fossil specimens, linear measurements of endocranial features are often the most appropriate, if not the only, measurement possible because of incomplete preservation or ossification of bones (e.g. the sphenethmoid [51]).
The olfactory ratios of 20 species of non-avian theropod dinosaurs representing eight families/superfamilies, seven species of fossil birds representing six orders, and 130 species of living birds representing 26 orders were considered in this study (see the electronic supplementary material). Olfactory ratios were calculated as the ratio between the longest diameter of the olfactory bulb and the longest diameter of the cerebral hemisphere, regardless of orientation, multiplied by 100 [27,33] (figure 1).
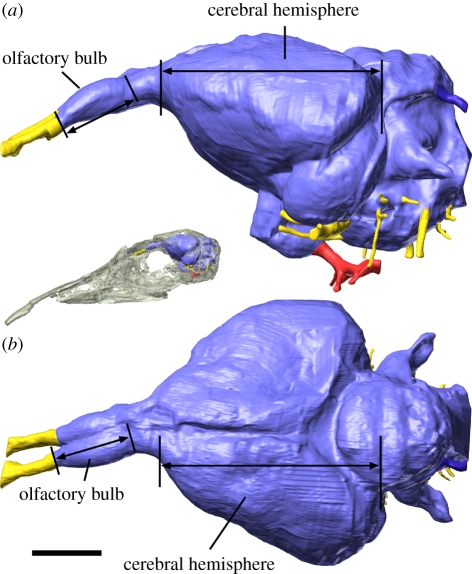
Figure 1.
Virtual brain endocast of Lithornis plebius, (a) in left lateral and (b) dorsal view, showing the location of the olfactory bulbs and cerebral hemispheres. The greatest linear dimension of the olfactory bulb and cerebral hemisphere, regardless of orientation, was used to calculate olfactory ratios (only rostrocaudal dimensions illustrated). Red features represent blood vessels, yellow features represent cranial nerves. Scale bar, 5 mm. Inset (not to scale) shows the position of the brain endocast within the skull of Lithornis promiscuus.
Olfactory ratios for extant and extinct birds were derived from the literature and from computed tomographic (CT) data of skull material. The olfactory ratios for most living birds studied were obtained from the literature and are based on linear measurements of soft tissue [28,52]. However, the olfactory ratios for two species (Struthio camelus and Eudromia elegans) were obtained from virtual endocasts (i.e. a representation of the endocranial cavity produced using CT data; see below). The fact that endocast-derived and soft tissue-derived olfactory ratios are comparable (see the electronic supplementary material) and that endocranial volume and brain mass are highly correlated in birds [53] supports the inclusion of virtual endocast data in our study. Olfactory ratios for extinct birds were derived from virtual endocasts, except for Confuciusornis sanctus, which was calculated from measurements made on a CT-scanned skull using the software Amira v. 5.3, and Archaeopteryx lithographica, which was obtained from the literature [33]. Because the posterior portion of the cerebral hemispheres in the Hesperornis endocast is crushed, both minimum and maximum cerebral hemisphere lengths were estimated, resulting in a maximum and a minimum olfactory ratio. The mean of these two values was used for ancestral state reconstructions. The Cretaceous bird Cerebavis cenomanica [5] was not included in our study owing to uncertainties related to the dimensions of key endocranial features and to its taxonomic affinity.
Olfactory ratios of most non-avian theropod species were obtained from the literature [33]. Some published non-avian theropod specimens (e.g. Gorgosaurus, Albertosaurus, subadult Tyrannosaurus rex) were excluded owing to uncertainties in their olfactory ratios [33]. We augmented the dataset from Zelenitsky et al. [33] with olfactory ratios of additional non-avian theropod species (Deinonychus antirrhopus and Tsaagan mangas) and additional specimens of the previously studied species (Allosaurus fragilis, Tyrannosaurus rex and Tarbosaurus bataar); all new olfactory ratios are based on virtual endocasts. Mean olfactory ratios for each non-avian theropod species were calculated prior to bivariate analysis and ancestral state reconstruction.
All virtual endocasts used in this study were produced using CT data in the WitmerLab at the Ohio University College of Osteopathic Medicine following a previously published method [54]. To ensure consistency in structure identification and to eliminate inter-observer error in the measurement of the olfactory bulb and cerebral hemisphere dimensions, all measurements taken on virtual endocasts and on fossil specimens were made by a single individual (F.T.).
(b). Body mass estimates
Body masses for extant bird species are calculated as the mean of mean female and mean male body masses published in Dunning [55] (see the electronic supplementary material).
Body masses for fossil birds were estimated from regressions for living birds with comparable lifestyles or body plans (electronic supplementary material). Body masses for volant fossil birds (except Archaeopteryx) were estimated from a least-squares regression of log-transformed humerus length versus body mass (log(body mass, in kilogram) = 0.4822 × log(humerus length, in millimetres) + 2.0722, r2 = 0.97) derived from published data for 17 living volant birds [56]. Body mass for the non-volant diver Hesperornis was estimated from a published regression of femur length versus body mass for extant diving birds [57].
Body mass estimates for Archaeopteryx and non-avian theropod species, except Deinonychus and Tsaagan, were obtained from the literature [33] (see the electronic supplementary material). The body mass estimate for Deinonychus is based on a three-dimensional virtual model [58], whereas that of the dromaeosaurid Tsaagan is considered equivalent to the dromaeosaurid Velociraptor owing to the similarity in skull size.
(c). Regression analysis
A log-transformed bivariate plot of olfactory ratio versus body mass was produced for non-avian theropods and birds in order to assess the influence of body size on olfactory ratio. Least-squares regressions, rather than reduced major axis regressions, were used to quantify the relationship between olfactory ratio and body mass because these regressions are considered more accurate when plotting a ratio as a function of a direct measurement [59]. The influence of phylogenetic relationships among taxa was accounted for by producing phylogenetically corrected regressions through the method of phylogenetically independent contrasts [60] using the PDAP module v. 1.14 [61] of the software Mesquite v. 2.72 [62] (see the electronic supplementary material). It was not possible to determine individual branch lengths for the calculation of phylogenetically independent contrasts owing to the large number of taxa considered, the fact that not all of these species have been subjected to a molecular phylogenetic analysis, and the uncertainty of the divergence time between some taxa. Consequently, the alternative, but equally valid method of assigning a branch length of one was used, which in effect assumes that all evolutionary changes took place during speciation events [63].
(d). Phylogenetic hypotheses
Two recent hypotheses for the high-order phylogenetic relationships of extant neornithines were considered in our analyses, one based on molecular data [64] and one based on morphological data [65]. Each of these high-order phylogenetic hypotheses was combined with several phylogenetic hypotheses below the ordinal level [65–82] to establish the phylogenetic relationships of all 130 extant species considered in this study (see the electronic supplementary material).
Inclusion of extinct birds in our analysis based on the molecular phylogeny [64] was problematic because this phylogeny did not include fossil taxa. Consequently, the phylogenetic position of the extinct birds had to be inferred from previous morphological phylogenetic analyses. The phylogenetic position of Presbyornis and basal birds was readily determined using published morphological analyses [83,84]. The phylogenetic placement of the extinct genus Lithornis, however, was problematic owing to differences in tree topology between morphological and molecular phylogenies for palaeognaths and because of the variable position of Lithornis in morphological phylogenies (i.e. as a basal palaeognath or as the sister taxon to Neornithes). In order to include Lithornis in the analysis based on the molecular phylogeny, we used the inter-relationships of palaeognaths and Lithornis from the morphological phylogenetic hypothesis of Dyke & Van Tuinen [85].
The phylogenetic relationships among non-avian theropods follow the cladogram compiled from the literature by Zelenitsky et al. [33], supplemented by the dromaeosaurid phylogeny of Csiki et al. [86].
(e). Ancestral state reconstruction
Changes in relative olfactory bulb size through higher order nodes of theropod (including bird) phylogeny were examined via maximum-parsimony ancestral state reconstructions using Mesquite v. 2.72 [62]. Ancestral states of olfactory ratios were reconstructed for the phylogeny of Aves (electronic supplementary material). Ancestral states of olfactory ratio residuals (relative to the non-avian theropod regression), rather than of olfactory ratios, were reconstructed for the phylogeny of the non-avian theropod/bird transition in order to take into consideration the influence of body mass on olfactory ratios because a strong correlation exists between these two variables in non-avian theropods [33] (also see §4). This approach permits the comparison of relative olfactory bulb size between early birds and non-avian theropods.
Major changes in olfactory ratios through avian evolution were identified when the reconstructed ancestral state at a given higher order node fell outside of the 95% confidence interval of the mean of the ancestral states for the four preceding higher order nodes.
(f). Statistical analyses
Statistical analyses were conducted with the software PASW Statistics v. 17.0.2 and GraphPad Prism v. 5.0.
4. Results/discussion
(a). Comparison of olfactory ratios and olfactory abilities among non-avian theropods and birds
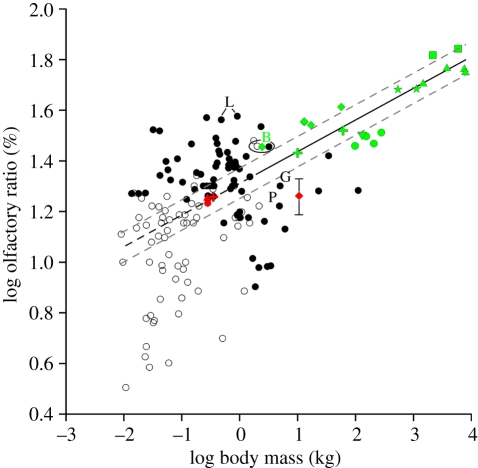
A bivariate analysis reveals that a strong positive correlation exists between olfactory ratio and body mass among non-avian theropods (r2 = 0.8, p < 1.27e − 7), whereas the data for Aves are uncorrelated (r2 = 0.009, p = 0.26) and widely scattered (figure 2). When basal birds are investigated alone, the slope of the regression between olfactory ratio and body mass in these taxa is not significantly different from zero (p = 0.4). These results indicate that olfactory ratios can be compared among avian taxa regardless of body mass differences, whereas the effect of body mass must be taken into consideration when comparing olfactory ratios between non-avian theropods and birds.
Figure 2.
Plot of log-transformed olfactory ratio versus log-transformed body mass in avian and non-avian theropods. No significant correlation is observed between olfactory ratio and body mass among birds when phylogeny is considered (r2 = 0.009, p = 0.26). In contrast, a significant positive correlation is observed between olfactory ratio and body mass among non-avian theropods (r2 = 0.8, p < 1.27e − 7), indicating that olfactory ratios increase with body mass among non-avian theropods. The non-avian theropod regression (solid black line) and its extrapolation (black dashed line) bisect the distribution of olfactory ratios for birds. The majority of neornithine species basal to the common ancestor of Charadriiformes and Passeriformes have higher olfactory ratios than more derived taxa. Most basal birds fall near the non-avian theropod regression. The fossil diving bird Hesperornis plots near the extant divers Gavia immer (loon, G) and Pygoscelis adeliae (Adelie penguin, P). The error associated with Hesperornis reflects the uncertainty of its cerebral hemisphere length (see §3). The dromaeosaurid Bambiraptor (B) plots near Cathartes aura (turkey vulture, open circle) and Phoebastria nigripes (black-footed albatross, solid circle). The extinct palaeognath Lithornis (L) has high olfactory ratios. Green diamonds, dromaeosaurids; green squares, tyrannosaurids; green triangles, allosauroids; green stars, ceratosaurs; green circles, ornithomimosaurs; inverted green triangle, Citipati; green crosses, Dilong and Troodon; red circle, Archaeopteryx; red triangle, Confuciusornis; red cross, Ichthyornis; red diamond, Hesperornis; black circles, neornithines basal to charadriiform–passeriform common ancestor (based on molecular phylogeny); white circles, neornithines more derived than charadriiform–passeriform common ancestor (based on molecular phylogeny). Dashed grey lines represent the 95% confidence interval of the non-avian theropod regression.
The data for Aves are evenly distributed about the non-avian theropod regression with 53.3 per cent of avian species plotting above the regression and 46.7 per cent below it (figure 2). Olfactory ratios of most basal birds (i.e. Archaeopteryx, Confuciusornis, Ichthyornis) fall near the regression line, suggesting that they had olfactory capabilities expected for non-avian theropods of their respective sizes (figure 2). One exception is Hesperornis, which had a lower olfactory ratio than that predicted for a non-avian theropod of its size (figure 2), suggestive of weaker olfactory capabilities than a similar-sized non-avian theropod. Neornithine birds display a greater range of olfactory ratios about the regression line than either non-avian theropods or basal birds (figure 2; see residuals in the electronic supplementary material), indicative of a greater diversity of olfactory abilities than their ancestors. At large body sizes (greater than 4.85 kg), neornithines tend to have lower olfactory ratios than predicted for non-avian theropods, whereas at smaller body sizes neornithines are more evenly distributed about the regression (figure 2).
Comparison of neornithine olfactory ratios with those of similar-sized non-avian theropods can elucidate aspects of olfaction and behaviour in these extinct taxa (see the electronic supplementary material). Relative to extant birds of similar size, the small dromaeosaurid Bambiraptor has an olfactory ratio similar to that of Cathartes aura (turkey vulture) and Phoebastria nigripes (black-footed albatross) (figure 2), carnivorous birds with high olfactory ratios (greater than 28%) known to rely heavily on olfactory cues while foraging (i.e. olfactory foraging) [28,87–89]. Given the correlation that exists between high olfactory ratios and olfactory foraging among birds (for statistical test, see the electronic supplementary material), it is possible that Bambiraptor also relied considerably on olfaction to locate food, supporting the previous behavioural interpretations made for non-avian theropods with high olfactory ratios [33].
Similarly, inferences can be made about the olfactory capabilities and behaviours of extinct birds through comparison of their olfactory ratios with those of living neornithines. Basal birds had olfactory ratios that are just above average for neornithines (figure 3), but significantly lower than those of olfactory foraging neornithines (p < 0.004; see the electronic supplementary material). Consequently, vision must have played a more important role than olfaction while foraging in these taxa. Nevertheless, olfaction was probably important in basal bird ecology as these birds possessed olfactory capabilities similar to domestic pigeons (olfactory ratio = 18.2%), birds that have been reported to use olfactory cues for aerial navigation and homing [90–94]. The basal bird Hesperornis is a specialized non-volant diver [57] that plots near extant divers Pygoscelis adeliae (Adelie penguin) and Gavia immer (loon), birds that are primarily visual foragers [95,96] (figure 2). The similarity between Hesperornis and these extant divers probably reflects evolutionary convergence related to comparable lifestyles. Among extinct neornithines, the volant palaeognath Lithornis has a high olfactory ratio (mean = 37.1%), which is not significantly different (p = 0.051) from those of known olfactory foraging taxa (mean = 30.6%), such as Procellariiformes (tube-nosed seabirds) [88,97–101], birds that also use olfaction to navigate over open seas [98,102–104], and Apteryx (kiwi) [105–107] (figure 2). This result suggests that olfaction was a key sense for food location in Lithornis and could also have played a role in navigation.
Figure 3.
Higher order phylogeny of Aves showing maximum-parsimony ancestral state reconstruction of olfactory ratios. (a) Molecular phylogeny based primarily on Hackett et al. [64]. (b) Morphological phylogeny based on Livezey & Zusi [65]. Major increases or decreases in olfactory ratio ancestral states are denoted with (+) and (−), respectively. Through avian evolution, major increases in olfactory ratios have occurred independently in many different lineages, primarily in clades basal to the common ancestor of Charadriiformes and Passeriformes. Significant decreases in olfactory ratios are prevalent in clades more derived than this common ancestor. Numbers between parentheses represent mean olfactory ratios for clades. See the electronic supplementary material for details.
(b). Evolution of olfaction among non-avian theropods and birds
Ancestral state reconstruction of olfactory ratios and olfactory ratio residuals was used to document changes in olfactory capabilities through non-avian theropod and bird evolution. Olfactory ratio residuals initially decrease in the Maniraptoriformes common ancestor and subsequently increase in the Eumaniraptora common ancestor (figure 4). Interestingly, the residuals of the Eumaniraptora, Aves and Pygostylia common ancestors remain the same (figure 4), supporting the previous suggestion that olfactory capabilities were unchanged across the non-avian theropod/bird transition [33]. Olfactory ratios and residuals show an increase through basal birds, with a major increase occurring at the common ancestor of neornithines (figures 3 and 4). These results indicate that the relative size of the olfactory bulbs increased through the evolution of non-avian maniraptoriforms and basal birds. Olfaction was therefore not a declining modality, as previously suggested [19,20,31], but rather became relatively more important during early bird evolution. This suggests that olfaction likely played a significant ecological role during the evolution of basal birds, as it is doubtful that olfactory bulb size would have continued to increase without conferring a selective advantage.
Figure 4.
Phylogeny of non-avian theropods and early birds showing maximum-parsimony ancestral state reconstruction of olfactory ratio residuals relative to the non-avian theropod regression. Residuals increase from the Maniraptoriformes common ancestor (node 1) to the Eumaniraptora common ancestor (node 2). Residuals then remain constant across the non-avian theropod/bird transition, between the Eumaniraptora common ancestor (node 2) and the Pygostylia common ancestor (node 3). Within Aves, residuals increase from negative values in basal birds to strongly positive values in neornithines, indicating that olfactory ratios increase and surpass values predicted by the regression for non-avian theropods. These results reveal that olfactory capabilities improved during the evolution from non-avian theropods to modern birds. Yellow box, Aves; blue box, Neornithes. Skulls with endocasts are, from top to bottom, Majungasaurus crenatissimus, Allosaurus fragilis, Tyrannosaurus rex, Struthiomimus altus, Bambiraptor feinbergi, Archaeopteryx lithographica, Ichthyornis dispar, Lithornis sp. and Presbyornis sp. Skulls are not to scale.
Analysis of both molecular and morphological phylogenies reveals similar trends in olfactory ratios among Neornithes, despite differences in tree topology. Olfactory ratios remained relatively high (usually greater than 20%) well into neornithine evolution, until the most recent common ancestor of Passeriformes and Charadriiformes (figure 3). A continual decrease in olfactory ratios occurs from this common ancestor to the common ancestor of Passerida (olfactory ratio ancestral state approx. 10.5%) (figure 3). Olfactory ratios are generally well below 20 per cent among clades more derived than the charadriiform–passeriform common ancestor. These results indicate that relative olfactory bulb size remained large during a major part of neornithine evolution before a reduction occurred in derived Neoaves clades.
Among palaeognaths, relatively larger olfactory bulbs evolved in basal forms, such as Lithornis and Apteryx, and olfactory bulb size then decreased continuously towards the more derived Struthioniformes (figure 3a); the same trend is observed if Lithornis is treated as the sister taxon to Neornithes (figure 3b). This trend may reflect behavioural changes among palaeognaths, from olfactory foraging in more basal forms (e.g. Apteryx [107]), to non-olfactory or visual foraging in more derived taxa (e.g. Struthio, Dromaius [108,109]). If the molecular tree topology for palaeognaths is analysed and Lithornis is excluded from the study, the opposite trend is observed, where olfactory bulb size increased continually through palaeognath evolution, from basal Struthio to derived Apteryx (not illustrated).
Among neognaths, increases in the relative size of the olfactory bulb occur mainly among clades basal to the common ancestor of Charadriiformes and Passeriformes, whereas reductions in size are prevalent among more derived clades (figures 2 and 3a). This pattern suggests that olfaction is a relatively more important modality in taxa basal to the charadriiform–passeriform common ancestor, which is consistent with reports on the use of olfaction in such taxa [22,24,88,90,91,93,97–102,104,105,107,110–113]. Increases in olfactory ratio are more frequent in clades more derived than the charadriiform–passeriform common ancestor in the morphological analysis than in the molecular analysis owing to differences in tree topologies (i.e. some clades that are more basal in the molecular phylogeny are more derived in the morphological phylogeny; figure 3).
The greatest reduction in relative olfactory bulb size among neognaths occurs at the common ancestor of Passeriformes (in the molecular phylogeny, figure 3a) or among Passeriformes (in the morphological phylogeny, figure 3b). If the highly divergent psittaciform Strigops habroptila is removed from the ancestral state reconstruction, major decreases in olfactory bulb size are then observed in the common ancestor of Psittaciformes and Passeriformes as well as in the Psittaciformes common ancestor in both molecular- and morphological-based analyses (not illustrated). Passeriformes and Psittaciformes are clades noted for advanced cognitive abilities [114], such as true tool use and high frequency of foraging innovations [115–117]. The coincidence between increased cognitive abilities and reduced olfactory capabilities may indicate that enhanced cognition reduced selective pressures for the use of olfaction at a late stage in neornithine evolution.
5. Conclusion
Early avian sensory evolution has been characterized previously by enhancements to visual, auditory and vestibular senses [15], while olfaction was considered to have been a deteriorating modality [19,20,31]. In contrast, our results show that olfaction continued to become relatively more important during the transition from non-avian theropods to early neornithines, thus indicating that olfaction was another significant sensory modality during early avian evolution. The diversification of olfactory abilities among neornithines and the enhancement of olfaction in several basal neornithine and neoavian clades suggest that olfaction retained its significance well into neornithine evolution. The heightened olfactory abilities of ancestral and early neornithines may have provided these birds with a competitive advantage, in the form of increased efficiency at foraging and navigation, over other Cretaceous bird lineages and increased their survivability through the end-Cretaceous extinction.
Acknowledgements
This project was funded by a Natural Sciences and Engineering Research Council of Canada Discovery Grant (D.K.Z.) and National Science Foundation Grant IOB-0517257 (L.M.W. and R.C.R.). The skull models of T. rex and Archaeopteryx used in figure 4 were generated by S. Pinney and S. Hoeger, respectively. We thank the curatorial and collections staff from the various institutions for access to specimens used in this project. We are also grateful to Drs Michael Newbrey, Christopher DeBuhr and Sheryl Zelenitsky for discussions about various aspects of this research. Finally, we wish to thank the reviewers, Drs Gareth Dyke and Andrew Fidler, for constructive comments that improved the article.
References
- 1.Britt B. B., Makovicky P. J., Gauthier J., Bond N. 1998. Postcranial pneumatization in Archaeopteryx. Nature 395, 374–376 10.1038/26469 (doi:10.1038/26469) [DOI] [Google Scholar]
- 2.Currie P. J. 1987. Bird-like characteristics of the jaws and teeth of troodontid theropods (Dinosauria, Saurischia). J. Vertebr. Paleontol. 7, 72–81 10.1080/02724634.1987.10011638 (doi:10.1080/02724634.1987.10011638) [DOI] [Google Scholar]
- 3.Erickson G. M., Rauhut O. W. M., Zhou Z., Turner A. H., Inouye B. D., Hu D., Norell M. A. 2009. Was dinosaurian physiology inherited by birds? Reconciling slow growth in Archaeopteryx. PLoS ONE 4, e7390. 10.1371/journal.pone.0007390 (doi:10.1371/journal.pone.0007390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji Q., Currie P. J., Norell M. A., Ji A. 1998. Two feathered dinosaurs from northeastern China. Nature 393, 753–761 10.1038/31635 (doi:10.1038/31635) [DOI] [Google Scholar]
- 5.Kurochkin E. N., Dyke G. J., Saveliev S. V., Pervushov E. M., Popov E. V. 2007. A fossil brain from the Cretaceous of European Russia and avian sensory evolution. Biol. Lett. 3, 309–313 10.1098/rsbl.2006.0617 (doi:10.1098/rsbl.2006.0617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norell M. A., Clark J. M., Chiappe L. M., Dashzeveg D. 1995. A nesting dinosaur. Nature 378, 774–776 10.1038/378774a0 (doi:10.1038/378774a0) [DOI] [Google Scholar]
- 7.Norell M. A., Makovicky P. J., Clark J. M. 1997. A Velociraptor wishbone. Nature 389, 447. 10.1038/38918 (doi:10.1038/38918) [DOI] [Google Scholar]
- 8.O'Connor P. M., Claessens L. P. A. M. 2005. Basic avian pulmonary design and flow-through ventilation in non-avian theropod dinosaurs. Nature 436, 253–256 10.1038/nature03716 (doi:10.1038/nature03716) [DOI] [PubMed] [Google Scholar]
- 9.Ostrom J. H. 1976. Archaeopteryx and the origin of birds. Biol. J. Linn. Soc. 8, 91–182 10.1111/j.1095-8312.1976.tb00244.x (doi:10.1111/j.1095-8312.1976.tb00244.x) [DOI] [Google Scholar]
- 10.Sato T., Cheng Y., Wu X., Zelenitsky D. K., Hsiao Y. 2005. A pair of shelled eggs inside mother dinosaur. Science 308, 375. 10.1126/science.1110578 (doi:10.1126/science.1110578) [DOI] [PubMed] [Google Scholar]
- 11.Varricchio D. J., Jackson F., Borkowski J. J., Horner J. R. 1997. Nest and egg clutches of the dinosaur Troodon formosus and the evolution of avian reproductive traits. Nature 385, 247–250 10.1038/385247a0 (doi:10.1038/385247a0) [DOI] [Google Scholar]
- 12.Varricchio D. J., Moore J. R., Erickson G. M., Norell M. A., Jackson F. D., Borkowski J. J. 2008. Avian paternal care had dinosaur origin. Science 322, 1826–1828 10.1126/science.1163245 (doi:10.1126/science.1163245) [DOI] [PubMed] [Google Scholar]
- 13.Xu X., Norell M. A. 2004. A new troodontid dinosaur from China with avian-like sleeping posture. Nature 431, 838–841 10.1038/nature02898 (doi:10.1038/nature02898) [DOI] [PubMed] [Google Scholar]
- 14.Xu X., Zhou Z.-H., Prum R. O. 2001. Branched integumental structures in Sinornithosaurus and the origin of feathers. Nature 410, 200–204 10.1038/35065589 (doi:10.1038/35065589) [DOI] [PubMed] [Google Scholar]
- 15.Domínguez Alonso P., Milner A. C., Ketcham R. A., Cookson M. J., Rowe T. B. 2004. The avian nature of the brain and inner ear of Archaeopteryx. Nature 430, 666–669 10.1038/nature02706 (doi:10.1038/nature02706) [DOI] [PubMed] [Google Scholar]
- 16.Milner A. C., Walsh S. A. 2009. Avian brain evolution: new data from palaeogene birds (Lower Eocene) from England. Zool. J. Linn. Soc. 155, 198–219 10.1111/j.1096-3642.2008.00443.x (doi:10.1111/j.1096-3642.2008.00443.x) [DOI] [Google Scholar]
- 17.Walsh S., Milner A. 2010. Halcyornis toliapicus (Aves: Lower Eocene, England) indicates advanced neuromorphology in Mesozoic Neornithes. J. Syst. Paleontol. 9, 173–181 [Google Scholar]
- 18.Turner C. H. 1892. A few characteristics of the avian brain. Science 19, 16–17 10.1126/science.ns-19.466.16 (doi:10.1126/science.ns-19.466.16) [DOI] [PubMed] [Google Scholar]
- 19.Edinger T. 1951. The brains of the Odontognathae. Evolution 5, 6–24 10.2307/2405427 (doi:10.2307/2405427) [DOI] [Google Scholar]
- 20.Pearson R. 1972. The avian brain. London, UK: Academic Press [Google Scholar]
- 21.Wenzel B. M. 1971. Olfaction in birds. In Handbook of sensory physiology (ed. Beidler L. M.), pp. 432–448 New York, NY: Springer-Verlag [Google Scholar]
- 22.Caro S. P., Balthazart J. 2010. Pheromones in birds: myth or reality? J. Comp. Physiol. A 196, 751–766 10.1007/s00359-010-0534-4 (doi:10.1007/s00359-010-0534-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagelin J. C., Jones I. L. 2007. Bird odors and other chemical substances: a defense mechanism or overlooked mode of intraspecific communication? Auk 124, 741–761 10.1642/0004-8038(2007)124[741:BOAOCS]2.0.CO;2 (doi:10.1642/0004-8038(2007)124[741:BOAOCS]2.0.CO;2) [DOI] [Google Scholar]
- 24.Roper T. J. 1999. Olfaction in birds. In Advances in the study of behavior (eds Slater P. J. B., Rosenblatt J. S., Snowden C. T., Roper T. J.), pp. 247–332 New York, NY: Academic Press [Google Scholar]
- 25.Bang B. G., Cobb S. 1968. The size of the olfactory bulb in 108 species of birds. Auk 85, 55–61 [Google Scholar]
- 26.Bang B. G., Wenzel B. M. 1985. Nasal cavity and olfactory system. In Form and function in birds (eds King A. S., McLelland J.), pp. 195–225 New York, NY: Academic Press [Google Scholar]
- 27.Cobb S. 1960. A note on the size of the avian olfactory bulbs. Epilepsia 1, 394–402 10.1111/j.1528-1157.1959.tb04276.x (doi:10.1111/j.1528-1157.1959.tb04276.x) [DOI] [PubMed] [Google Scholar]
- 28.Bang B. G. 1971. Functional anatomy of the olfactory system in 23 orders of birds. Acta Anat. 58, 1–76 [DOI] [PubMed] [Google Scholar]
- 29.Wenzel B. M., Meisami E. 1987. Number, size, and density of mitral cells in the olfactory bulbs of the northern fulmar and rock dove. In Olfaction and taste (eds Roper S., Atema J.), pp. 700–702 New York, NY: Annals of the New York Academy of Sciences [Google Scholar]
- 30.Brochu C. A. 2000. A digitally-rendered endocast for Tyrannosaurus rex. J. Vertebr. Paleontol. 20, 1–6 10.1671/0272-4634(2000)020[0001:ADREFT]2.0.CO;2 (doi:10.1671/0272-4634(2000)020[0001:ADREFT]2.0.CO;2) [DOI] [Google Scholar]
- 31.Franzosa J. W. 2004. Evolution of the brain in Theropoda (Dinosauria). PhD thesis, University of Texas at Austin, Austin, TX, USA [Google Scholar]
- 32.Witmer L. M., Ridgely R. C. 2009. New insights into the brain, braincase, and ear region of tyrannosaurs, with implications for sensory organization and behavior. Anat. Rec. 292, 1266–1296 10.1002/ar.20983 (doi:10.1002/ar.20983) [DOI] [PubMed] [Google Scholar]
- 33.Zelenitsky D. K., Therrien F., Kobayashi Y. 2009. Olfactory acuity in theropods: palaeobiological and evolutionary implications. Proc. R. Soc. B 276, 667–673 10.1098/rspb.2008.1075 (doi:10.1098/rspb.2008.1075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edinger L. 1908. The relations of comparative anatomy to comparative psychology. J. Comp. Neurol. 18, 437–457 10.1002/cne.920180502 (doi:10.1002/cne.920180502) [DOI] [Google Scholar]
- 35.Jerison H. J. 1973. Evolution of the brain and intelligence. New York, NY: Academic Press [Google Scholar]
- 36.Buschhüter D., Smitka M., Puschmann S., Gerber J. C., Witt M., Abolmaali N. D., Hummel T. 2008. Correlation between olfactory bulb volume and olfactory function. Neuroimage 42, 498–502 10.1016/j.neuroimage.2008.05.004 (doi:10.1016/j.neuroimage.2008.05.004) [DOI] [PubMed] [Google Scholar]
- 37.Clark L., Avilova K. V., Beans N. J. 1993. Odor thresholds in passerines. Comp. Biochem. Physiol. A 104, 305–312 10.1016/0300-9629(93)90322-U (doi:10.1016/0300-9629(93)90322-U) [DOI] [Google Scholar]
- 38.Hammock J. 2005. Structure, function and context: the impact of morphometry and ecology on olfactory sensitivity. PhD thesis, Massachusetts Institutie of Technology, Boston, MA, USA [Google Scholar]
- 39.Mackay-Sim A., Royet J. P. 2006. Structure and function of the olfactory system. In Olfaction and the brain (eds Brewer W., Castle D., Pantelis C.), pp. 3–27 Cambridge, UK: Cambridge University Press [Google Scholar]
- 40.Mori K., Nagao H., Yoshihara Y. 1999. The olfactory bulb: coding and processing of odor molecule information. Science 286, 711–715 10.1126/science.286.5440.711 (doi:10.1126/science.286.5440.711) [DOI] [PubMed] [Google Scholar]
- 41.Steiger S. S., Fidler A. E., Kempenaers B. 2009. Evidence for increased olfactory receptor gene repertoire size in two nocturnal bird species with well-developed olfactory ability. BMC Evol. Biol. 9, 117. 10.1186/1471-2148-9-117 (doi:10.1186/1471-2148-9-117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steiger S. S., Fidler A. E., Valcu M., Kempenaers B. 2008. Avian olfactory receptor gene repertoires: evidence for a well-developed sense of smell in birds? Proc. R. Soc. B 275, 2309–2317 10.1098/rspb.2008.0607 (doi:10.1098/rspb.2008.0607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larsson H. C. E., Sereno P. C., Wilson J. A. 2000. Forebrain enlargement among nonavian theropod dinosaurs. J. Vertebr. Paleontol. 20, 615–618 10.1671/0272-4634(2000)020[0615:FEANTD]2.0.CO;2 (doi:10.1671/0272-4634(2000)020[0615:FEANTD]2.0.CO;2) [DOI] [Google Scholar]
- 44.Portmann A. 1947. Etudes sur la cérébralisation chez les oiseaux. Alauda 15, 1–15 [Google Scholar]
- 45.Pontzer H. 2007. Effective limb length and the scaling of locomotor cost in terrestrial animals. J. Exp. Biol. 210, 1752–1761 10.1242/jeb.002246 (doi:10.1242/jeb.002246) [DOI] [PubMed] [Google Scholar]
- 46.Tickle P., Nudds R., Codd J. 2009. Uncinate process length in birds scales with resting metabolic rate. PLoS ONE 4, e5667. 10.1371/journal.pone.0005667 (doi:10.1371/journal.pone.0005667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Head J. J., Bloch J. I., Hastings A. K., Bourque J. R., Cadena E. A., Herrera F. A., Polly P. D., Jaramillo C. A. 2009. Giant boid snake from the Palaeocene neotropics reveals hotter past equatorial temperatures. Nature 457, 715–717 10.1038/nature07671 (doi:10.1038/nature07671) [DOI] [PubMed] [Google Scholar]
- 48.Sánchez-Villagra M. R., Aguilera O., Horovitz I. 2003. The anatomy of the world's largest extinct rodent. Science 301, 1708–1710 10.1126/science.1089332 (doi:10.1126/science.1089332) [DOI] [PubMed] [Google Scholar]
- 49.Hamano K., Iwasaki N., Takeya T., Takita H. 1993. A comparative study of linear measurement of the brain and three-dimensional measurement of brain volume using CT scans. Pediatr. Radiol. 23, 165–168 10.1007/BF02013822 (doi:10.1007/BF02013822) [DOI] [PubMed] [Google Scholar]
- 50.Whalley H. C., Wardlaw J. M. 2001. Accuracy and reproducibility of simple cross-sectional linear and area measurements of brain structures and their comparison with volume measurements. Neuroradiology 43, 263–271 10.1007/s002340000437 (doi:10.1007/s002340000437) [DOI] [PubMed] [Google Scholar]
- 51.Ali F., Zelenitsky D. K., Therrien F., Weishampel D. B. 2008. Homology of the ‘ethmoid complex’ of tyrannosaurids and its implications for the reconstruction of the olfactory apparatus of non-avian theropods. J. Vertebr. Paleontol. 28, 123–133 10.1671/0272-4634(2008)28[123:HOTECO]2.0.CO;2 (doi:10.1671/0272-4634(2008)28[123:HOTECO]2.0.CO;2) [DOI] [Google Scholar]
- 52.Hagelin J. C. 2004. Observations on the olfactory ability of the Kakapo Strigops habroptilus, the critically endangered parrot of New Zealand. Ibis 146, 161–164 10.1111/j.1474-919X.2004.00212.x (doi:10.1111/j.1474-919X.2004.00212.x) [DOI] [Google Scholar]
- 53.Iwaniuk A. N., Nelson J. E. 2002. Can endocranial volume be used as an estimate of brain size in birds? Can. J. Zool. 80, 16–23 10.1139/z01-204 (doi:10.1139/z01-204) [DOI] [Google Scholar]
- 54.Witmer L. M., Chatterjee S., Franzosa J., Rowe T. 2003. Neuroanatomy of flying reptiles and implications for flight, posture and behaviour. Nature 425, 950–953 10.1038/nature02048 (doi:10.1038/nature02048) [DOI] [PubMed] [Google Scholar]
- 55.Dunning J. B., Jr 2008. CRC handbook of avian body masses, 2nd edn. Boca Raton, FL: CRC Press [Google Scholar]
- 56.Paige H. D., Anderson J. F., Rahn H. 1979. Scaling of skeletal mass to body mass in birds and mammals. Am. Nat. 113, 103–122 10.1086/283367 (doi:10.1086/283367) [DOI] [Google Scholar]
- 57.Hinić-Frlog S., Motani R. 2010. Relationship between osteology and aquatic locomotion in birds: determining modes of locomotion in extinct Ornithurae. J. Evol. Biol. 23, 372–385 10.1111/j.1420-9101.2009.01909.x (doi:10.1111/j.1420-9101.2009.01909.x) [DOI] [PubMed] [Google Scholar]
- 58.Therrien F., Henderson D. M. 2007. My theropod is bigger than yours… or not: estimating body size from skull length in theropods. J. Vertebr. Paleontol. 27, 108–115 10.1671/0272-4634(2007)27[108:MTIBTY]2.0.CO;2 (doi:10.1671/0272-4634(2007)27[108:MTIBTY]2.0.CO;2) [DOI] [Google Scholar]
- 59.Smith R. J. 1999. Statistics of sexual size dimorphism. J. Hum. Evol. 36, 423–459 10.1006/jhev.1998.0281 (doi:10.1006/jhev.1998.0281) [DOI] [PubMed] [Google Scholar]
- 60.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15 10.1086/284325 (doi:10.1086/284325) [DOI] [Google Scholar]
- 61.Midford P. E., Garland T., Jr, Maddison W. P. 2008. PDAP package of MESQUITE, v. 1.13. See http://mesquiteproject.org/pdap_mesquite/index.html
- 62.Maddison W. P., Maddison D. R. 2008. MESQUITE: a modular system for evolutionary analysis, v. 2.5. See http://mesquiteproject.org
- 63.Garland T., Jr, Dickerman A. W., Janis C. M., Jones J. A. 1993. Phylogenetic analysis of covariance by computer simulation. Syst. Biol. 42, 265–292 [Google Scholar]
- 64.Hackett S. J., et al. 2008. A phylogenomic study of birds reveals their evolutionary history. Science 320, 1763–1768 10.1126/science.1157704 (doi:10.1126/science.1157704) [DOI] [PubMed] [Google Scholar]
- 65.Livezey B. C., Zusi R. L. 2007. Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy. II. Analysis and discussion. Zool. J. Linn. Soc. 149, 1–95 10.1111/j.1096-3642.2006.00293.x (doi:10.1111/j.1096-3642.2006.00293.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baker A. J., Pereira S. L., Paton T. A. 2007. Phylogenetic relationships and divergence times of Charadriiformes genera: multigene evidence for the Cretaceous origin of at least 14 clades of shorebirds. Biol. Lett. 3, 205–210 10.1098/rsbl.2006.0606 (doi:10.1098/rsbl.2006.0606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benz B. W., Robbins M. B., Peterson A. T. 2006. Evolutionary history of woodpeckers and allies (Aves: Picidae): placing key taxa on the phylogenetic tree. Mol. Phylogenet. Evol. 40, 389–399 10.1016/j.ympev.2006.02.021 (doi:10.1016/j.ympev.2006.02.021) [DOI] [PubMed] [Google Scholar]
- 68.Donne-Gousse C., Laudet V., Hanni C. 2002. A molecular phylogeny of Anseriformes based on mitochondrial DNA analysis. Mol. Phylogenet. Evol. 23, 339–356 10.1016/S1055-7903(02)00019-2 (doi:10.1016/S1055-7903(02)00019-2) [DOI] [PubMed] [Google Scholar]
- 69.Ericson P. G. P., et al. 2006. Diversification of Neoaves: integration of molecular sequence data and fossils. Biol. Lett. 2, 543–547 10.1098/rsbl.2006.0523 (doi:10.1098/rsbl.2006.0523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ericson P. G. P., Jansen A.-L., Johansson U. S., Ekman J. 2005. Inter-generic relationships of the crows, jays, magpies and allied groups (Aves: Corvidae) based on nucleotide sequence data. J. Avian Biol. 36, 222–234 10.1111/j.0908-8857.2001.03409.x (doi:10.1111/j.0908-8857.2001.03409.x) [DOI] [Google Scholar]
- 71.Ericson P. G. P., Johansson U. S. 2003. Phylogeny of Passerida (Aves: Passeriformes) based on nuclear and mitochondrial sequence data. Mol. Phylogenet. Evol. 29, 126–138 10.1016/S1055-7903(03)00067-8 (doi:10.1016/S1055-7903(03)00067-8) [DOI] [PubMed] [Google Scholar]
- 72.Griffiths C. S., Barrowclough G. F., Groth J. G., Mertz L. A. 2007. Phylogeny, diversity, and classification of the Accipitridae based on DNA sequences of the RAG-1 exon. J. Avian Biol. 38, 587–602 10.1111/j.2007.0908-8857.03971.x (doi:10.1111/j.2007.0908-8857.03971.x) [DOI] [Google Scholar]
- 73.Jonsson K. A., Fjeldsa J. 2006. A phylogenetic supertree of oscine passerine birds (Aves: Passeri). Zool. Scr. 35, 149–186 10.1111/j.1463-6409.2006.00221.x (doi:10.1111/j.1463-6409.2006.00221.x) [DOI] [Google Scholar]
- 74.Kennedy M., Page R. D. M. 2002. Seabird supertrees: combining partial estimates of procellariiform phylogeny. Auk 119, 88–108 10.1642/0004-8038(2002)119[0088:SSCPEO]2.0.CO;2 (doi:10.1642/0004-8038(2002)119[0088:SSCPEO]2.0.CO;2) [DOI] [Google Scholar]
- 75.Kennedy M., Valle C. A., Spencer H. G. 2009. The phylogenetic position of the Galápagos cormorant. Mol. Phylogenet. Evol. 53, 94–98 10.1016/j.ympev.2009.06.002 (doi:10.1016/j.ympev.2009.06.002) [DOI] [PubMed] [Google Scholar]
- 76.Lee P. L. M., Clayton D. H., Griffiths R., Page R. D. M. 1996. Does behavior reflect phylogeny in swiftlets (Aves: Apodidae)? A test using cytochrome b mitochondrial DNA sequences. Proc. Natl Acad. Sci. USA 96, 7091–7096 10.1073/pnas.93.14.7091 (doi:10.1073/pnas.93.14.7091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Livezey B. C. 1998. A phylogenetic analysis of the Gruiformes (Aves) based on morphological characters with an emphasis on the rails (Rallidae). Phil. Trans. R. Soc. Lond. B 353, 2077–2151 10.1098/rstb.1998.0353 (doi:10.1098/rstb.1998.0353) [DOI] [Google Scholar]
- 78.Nunn G. B., Stanley S. E. 1998. Body size effects and rates of cytochrome b evolution in tube-nosed seabirds. Mol. Biol. Evol. 15, 1360–1371 [DOI] [PubMed] [Google Scholar]
- 79.Payne R. B. 2005. The cuckoos. New York, NY: Oxford University Press [Google Scholar]
- 80.Reddy S., Cracraft J. 2007. Old World shrike-babblers (Pteruthius) belong with New World vireos (Vireonidae). Mol. Phylogenet. Evol. 44, 1352–1357 10.1016/j.ympev.2007.02.023 (doi:10.1016/j.ympev.2007.02.023) [DOI] [PubMed] [Google Scholar]
- 81.Trewick S. A. 1997. Flightlessness and phylogeny amongst endemic rails (Aves: Rallidae) of the New Zealand region. Phil. Trans. R. Soc. Lond. B 352, 429–446 10.1098/rstb.1997.0031 (doi:10.1098/rstb.1997.0031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wright T. F., et al. 2008. A multilocus molecular phylogeny of the parrots (Psittaciformes): support for a Gondwanan origin during the Cretaceous. Mol. Biol. Evol. 20, 2141–2156 10.1093/molbev/msn160 (doi:10.1093/molbev/msn160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ericson P. G. P. 2000. Systematic revision, skeletal anatomy, and paleoecology of the New World early Tertiary Presbyornithidae (Aves: Anseriformes). Paleobios 20, 1–23 [Google Scholar]
- 84.Zhou Z., Li F. Z. Z. 2010. A new Lower Cretaceous bird from China and tooth reduction in early avian evolution. Proc. R. Soc. B 277, 219–227 10.1098/rspb.2009.0885 (doi:10.1098/rspb.2009.0885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dyke G., Van Tuinen D. 2004. The evolutionary radiation of modern birds (Neornithes): reconciling molecules, morphology and the fossil record. Zool. J. Linn. Soc. 148, 153–177 10.1111/j.1096-3642.2004.00118.x (doi:1111/j.1096-3642.2004.00118.x) [DOI] [Google Scholar]
- 86.Csiki Z., Vremir M., Brusatte S. L., Norell M. A. 2010. An aberrant island-dwelling theropod dinosaur from the Late Cretaceous of Romania. Proc. Natl Acad. Sci. USA 107, 15 357–15 361 10.1073/pnas.1006970107 (doi:10.1073/pnas.1006970107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bang B. G. 1960. Anatomical evidence for olfactory function in some species of birds. Nature 188, 547–549 10.1038/188547a0 (doi:10.1038/188547a0) [DOI] [PubMed] [Google Scholar]
- 88.Hutchison L. V., Wenzel B. M. 1980. Olfactory guidance in foraging by procellariiforms. Condor 82, 314–319 10.2307/1367400 (doi:10.2307/1367400) [DOI] [Google Scholar]
- 89.Stager K. E. 1964. The role of olfaction in food location by the turkey vulture, Cathartes aura. Los Angeles Co. Mus. Contrib. Sci. 81, 1–63 [Google Scholar]
- 90.Gagliardo A., Ioalè P., Savini M., Wild M. 2008. Navigational abilities of homing pigeons deprived of olfactory or trigeminally mediated magnetic information when young. J. Exp. Biol. 211, 2046–2051 10.1242/jeb.017608 (doi:10.1242/jeb.017608) [DOI] [PubMed] [Google Scholar]
- 91.Jorge P. E., Marques P. A. M., Phillips J. B. 2009. Activational effects of odours on avian navigation. Proc. R. Soc. B 276, 45–49 10.1098/rspb.2009.1521 (doi:10.1098/rspb.2009.1521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Papi F. 1976. The olfactory navigation system of the homing pigeon. Verh. Dtsch. Zool. Ges. 69, 184–205 [Google Scholar]
- 93.Papi F. 1990. Olfactory navigation in birds. Experientia 46, 352–363 10.1007/BF01952168 (doi:10.1007/BF01952168) [DOI] [Google Scholar]
- 94.Wallraff H. G. 1982. The homing mechanism of pigeons. Nature 300, 293–294 10.1038/300293a0 (doi:10.1038/300293a0) [DOI] [Google Scholar]
- 95.Berger C. 2003. Sphenisciformes (Penguins). In Grzimek's animal life encyclopedia: birds I, vol. 8 (eds Hutchins M., Jackson J. A., Brock W. J., Olendorf D.), pp. 147–158, 2nd edn Farmington Hills, MI: Gale Group [Google Scholar]
- 96.Hosner P. 2003. Gaviiformes (Loons). In Grzimek's animal life encyclopedia: birds I vol. 8 (eds Hutchins M., Jackson J. A., Brock W. J., Olendorf D.), pp. 159–167, 2nd edn Farmington Hills, MI: Gale Group [Google Scholar]
- 97.Grubb T. C. 1972. Smell and foraging in shearwaters and petrels. Nature 237, 404–405 10.1038/237404a0 (doi:10.1038/237404a0) [DOI] [Google Scholar]
- 98.Grubb T. C. 1974. Olfactory navigation to the nesting burrow in Leach's petrel (Oceanodroma leucorhoa). Anim. Behav. 22, 192–202 10.1016/S0003-3472(74)80069-2 (doi:10.1016/S0003-3472(74)80069-2) [DOI] [PubMed] [Google Scholar]
- 99.Lequette B., Verheyden C., Jouventin P. 1989. Olfaction in subantarctic seabirds: its phylogenetic and ecological significance. Condor 91, 732–735 10.2307/1368131 (doi:10.2307/1368131) [DOI] [Google Scholar]
- 100.Nevitt G. A., Losekoot M., Weimerskirch H. 2008. Evidence for olfactory search in wandering albatross (Diomedea exulans). Proc. Natl Acad. Sci. USA 105, 4576–4581 10.1073/pnas.0709047105 (doi:10.1073/pnas.0709047105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Verheyden C., Jouventin P. 1994. Olfactory behavior of foraging procellariiforms. Auk 111, 285–291 [Google Scholar]
- 102.Nevitt G. A., Veit R. R., Kareiva P. 1995. Dimethyl sulphide as a foraging cue for Antarctic procellariiform seabirds. Nature 376, 681–682 10.1038/376680a0 (doi:10.1038/376680a0) [DOI] [Google Scholar]
- 103.Grubb T. C. 1973. Colony location by Leach's petrel. Auk 90, 78–82 [Google Scholar]
- 104.Grubb T. C. 1979. Olfactory guidance of Leach's storm petrel to the breeding island. Wilson Bull. 91, 141–143 [Google Scholar]
- 105.Benham W. B. 1906. The olfactory sense in Apteryx. Nature 74, 222–223 10.1038/074222b0 (doi:10.1038/074222b0) [DOI] [Google Scholar]
- 106.Cunningham S. J., Castro I., Potter M. A. 2009. The relative importance of olfaction and remote touch in prey detection by North Island brown kiwis. Anim. Behav. 78, 899–905 10.1016/j.anbehav.2009.07.015 (doi:10.1016/j.anbehav.2009.07.015) [DOI] [Google Scholar]
- 107.Wenzel B. M. 1968. Olfactory prowess of the kiwi. Nature 220, 1133–1134 10.1038/2201133a0 (doi:10.1038/2201133a0) [DOI] [PubMed] [Google Scholar]
- 108.Davies S. J. J. F. 2002. Ratites and tinamous. New York, NY: Oxford University Press [Google Scholar]
- 109.Martin G. R., Katzir G. 1995. Visual fields in ostriches. Nature 374, 19–20 10.1038/374019a0 (doi:10.1038/374019a0) [DOI] [Google Scholar]
- 110.Bonadonna F., Bretagnolle V. 2002. Smelling home: a good solution for burrow-finding in nocturnal petrels? J. Exp. Biol. 205, 2519–2523 [DOI] [PubMed] [Google Scholar]
- 111.Bonadonna F., Cunningham G. B., Jouventin P., Hesters F., Nevitt G. A. 2003. Evidence for nest-odour recognition in two species of diving petrel. J. Exp. Biol. 206, 3719–3722 10.1242/jeb.00610 (doi:10.1242/jeb.00610) [DOI] [PubMed] [Google Scholar]
- 112.Jouventin P., Mouret V., Bonadonna F. 2007. Wilson's storm petrels Oceanites oceanicus recognise the olfactory signature of their mate. Ethology 113, 1228–1232 10.1111/j.1439-0310.2007.01444.x (doi:10.1111/j.1439-0310.2007.01444.x) [DOI] [Google Scholar]
- 113.Martin G., Rojas L. M., Ramirez Y., McNeil R. 2004. The eyes of oilbirds (Steatornis caripensis): pushing at the limits of sensitivity. Naturwissenschaften 91, 26–29 10.1007/s00114-003-0495-3 (doi:10.1007/s00114-003-0495-3) [DOI] [PubMed] [Google Scholar]
- 114.Emery N. J. 2006. Cognitive ornithology: the evolution of avian intelligence. Phil. Trans. R. Soc. B 361, 23–43 10.1098/rstb.2005.1736 (doi:10.1098/rstb.2005.1736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lefebvre L., Nicolakakis N., Boire D. 2002. Tools and brains in birds. Behaviour 139, 939–973 10.1163/156853902320387918 (doi:10.1163/156853902320387918) [DOI] [Google Scholar]
- 116.Lefebvre L., Whittle P., Lascaris E., Finkelstein A. 1997. Feeding innovations and forebrain size in birds. Appl. Anim. Behav. Sci. 53, 549–560 [Google Scholar]
- 117.Timmermans S., Lefebvre L., Boire D., Basu P. 2000. Relative size of the hyperstriatum ventrale is the best predictor of feeding innovation rate in birds. Brain Behav. Evol. 56, 196–203 10.1159/000047204 (doi:10.1159/000047204) [DOI] [PubMed] [Google Scholar]