Abstract
Parrots are exceptional among birds for their high levels of exploratory behaviour and manipulatory abilities. It has been argued that foraging method is the prime determinant of a bird's visual field configuration. However, here we argue that the topography of visual fields in parrots is related to their playful dexterity, unique anatomy and particularly the tactile information that is gained through their bill tip organ during object manipulation. We measured the visual fields of Senegal parrots Poicephalus senegalus using the ophthalmoscopic reflex technique and also report some preliminary observations on the bill tip organ in this species. We found that the visual fields of Senegal parrots are unlike those described hitherto in any other bird species, with both a relatively broad frontal binocular field and a near comprehensive field of view around the head. The behavioural implications are discussed and we consider how extractive foraging and object exploration, mediated in part by tactile cues from the bill, has led to the absence of visual coverage of the region below the bill in favour of more comprehensive visual coverage above the head.
Keywords: psittacines, visual field, exploration, extractive foraging, bill tip organ, tactile cues
1. Introduction
For many animals, vision is the main sense used for gaining information about the position and physical properties of objects in the surrounding environment [1]. In conjunction with head and body movements, the visual field of an animal governs what can influence an animal's behaviour from moment to moment [2–4]. Among birds, parrots and cockatoos (order Psittaciformes, collectively known as psittacines [5]) are exceptional for their high levels of exploratory behaviour and manipulatory abilities [6,7]. These are made possible by distinctive morphological adaptations of the bill, feet and tongue [8], and highly developed cognitive abilities [9–11]. We ask whether these are complemented by sensory adaptations, principally vision and tactile cues perceived by the bill tip organ [12]. Additionally, we ask whether parrot visual fields differ from those of other birds.
It has been proposed that avian visual field configuration is primarily determined by foraging technique [13,14]. Birds that peck or lunge for their food (e.g. starlings, Sturnidae; or herons, Ardeidae [15,16]), or take prey in their feet (e.g. eagles, Accipitridae [17]) require accurate visual control of the bill or foot position. This has evolutionarily favoured relatively narrow (20°–30°) but vertically long (120°–180°) binocular fields centred about the bill. However, this results in large blind areas to the rear, requiring increased vigilance behaviour against predators [18]. This is overcome in birds that do not need accurate visual guidance of bill position, such as tactile probers (e.g. woodcocks Scolopax rusticola [19]) and filter feeders (e.g. mallards Anas platyrhynchos [20]). Here, the bill falls at the periphery of the frontal binocular field, which is very narrow (approx. 10°) and stretches above the head, providing comprehensive coverage of the celestial hemisphere.
Nothing is known, however, about the visual fields of extractive foraging birds. Psittacines display dextrous manipulatory abilities not only in extracting embedded food items, but also in exploring non-food items. Such manipulation is achieved by coordination of zygodactyl feet (allowing a secure grasp of objects), and a highly curved maxilla and a muscular tongue [6,7,21–23]. Furthermore, the maxilla is joined to the skull by a synovial joint (in all other birds the maxilla is fused to the skull), enabling independent movement of both upper and lower jaws [8,24–26]. Object manipulation in psittacines is supported by a comparatively large mesopallium and a highly investigative nature [9–11,27,28]. While in most birds neophilia is restricted to juvenescence, in psittacines it continues throughout life, even in situations not directly motivated by food [29]. This suggests that exploration and object play are important for continually updating information within a dynamic environment (summarized in [4]). Therefore, we investigated whether parrot visual fields have an additional function to the traditional drivers of avian visual field configuration: foraging and predator detection [13,14].
Psittacine eyes are positioned laterally and high in the skull, suggesting their visual fields are unlikely to resemble those of visually guided foragers. Availability of somatosensory information from the bill tip organ [12,30–35] during object manipulation may have allowed the visual field to extend above the head, providing extensive coverage of the celestial hemisphere. The somatosensory area of the brains of parrots predominantly represents the bill and tongue, followed by the feet [36–39]. The psittacine bill tip organ probably consists of groups of mechanoreceptors embedded in pits at the tip and along the inner ventral edges of the hard keratin (rhamphotheca) of the bill, as well as in the tongue [12]. By contrast, the bill tip organs of tactile guided foragers (e.g. ducks and geese, Anatidae; or shorebirds, Scolopacidae) are embedded in the bone of the maxilla and mandible beneath the keratin [31,32,40]. Psittacine bill tip organs, it seems, have not been studied since the initial descriptions by Goujon [12], so here we provide further description and consider how tactile information may complement visual information during object manipulation and extractive foraging.
In summary, we measured the visual fields of Senegal parrots (Psittacidae, Poicephalus senegalus) and ask whether the features of these visual fields can be related to extractive foraging and/or the acquisition of information associated with exploration. Additionally, since parrots could also gain tactile information about objects from their bill, we report some preliminary observations of the Senegal parrot bill tip organ.
2. Material and methods
(a). Subjects
Our subjects were two adult captive Senegal parrots (male and female, 5 years old). Senegal parrots are resident across West Africa, inhabiting woodland and savannah. Their diet, like the majority of other psittacines, consists of seeds, nuts, blossoms and fruit [41,42]. Senegal parrots also show the characteristic psittacine exploratory tendency, which lasts throughout their long life (approx. 30 years) [6]. Their hook-like maxilla is used both for climbing and object manipulation (Z. P. Demery, J. Chappell & G. R. Martin 2011, personal observation). They use the same method of extracting seeds as found in nearly all Psittaciformes [6].
(b). Visual fields
Visual field parameters (monocular, binocular and cyclopean fields) and eye movement amplitudes were measured in the two subjects using the ophthalmoscopic reflex technique. This is non-invasive and well established as the standard procedure for measuring avian visual fields [13]. The ethical guidelines of the UK Animals (Scientific Procedures) Act 1986 were followed.
For a detailed description of the apparatus and methods, see [13,43]. Briefly, each subject was securely fastened into a foam cradle with Velcro straps. The head was held at the centre of a visual perimeter by a custom-made holder moulded from hardened Fimo (Eberhard Faber GmbH), mounted on an adjustable steel brace to allow for the unique shape and manoeuvrability of the parrot bill. The back of the head was supported by a Fimo brace. The eye-to-bill tip angle projected at 50° below the horizontal, which was the approximate head position adopted by the birds when held in the hand or in a resting perched position.
The perimeter's coordinate system follows conventional latitude and longitude with the equator aligned vertically in the median-sagittal plane of the head. The eyes of the alert bird were examined using an ophthalmoscope mounted on a perimeter arm and its latitudinal position read to ± 0.5°. Alignment of the bird's head in the perimeter was such that the ophthalmoscope viewing aperture was essentially moved over the surface of a sphere (radius 320 mm) centred on the mid-point of the line joining the centres of the pupils; the cyclopean projection centre. In each eye, the limits of the retinal visual field were determined as a function of elevation in the median-sagittal plane at 10° ± 1.0° intervals. The non-conjugate eye movements at each elevation were induced and determined by the difference between these maximum and minimum values. The projections of the edges of the pecten were also recorded. The direction of the optic axis of each eye was determined by recording the positions at which the first and second Purkinje images (reflections from the cornea and the lens anterior surface) of a discrete source of light, held close to the line of sight on the perimeter arm, were most closely aligned.
A topographical map of the visual field and its principal features was constructed based on the retinal margin projections as a function of elevation, corrected for viewing from a hypothetical viewpoint placed at infinity [44].
(c). Bill tip organ
If a bill tip organ is present, tactile pits (in which mechanoreceptors are likely to be embedded in bone or in keratin) can be observed with the naked eye [12,31,32]. Four skulls of Senegal parrots held in the collections of the Natural History Museum (Tring, UK) both with and without intact keratin were inspected with the naked eye and photographed. The bill tip organs of the two live Senegal parrots were also photographed.
3. Results
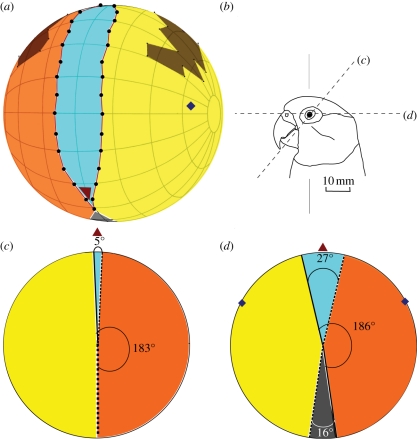
Four complete sets of visual field measurements (within 2° of each other at each elevation) were made in the male Senegal parrot. Three sets of measurements were made in the female, but we were not able to complete a comprehensive series of measurements at all elevations for this bird. However, in both birds, we recorded unobstructed measurements at elevations from directly behind the head through an arc to below the bill tip. When the birds were mounted in the apparatus, the bill holder prevented observation of the visual field boundaries below the bill tip. Nonetheless, when the birds were held in the hand we were able to verify from casual observations that binocularity ended at or just below the bill tip. Therefore, we combined data from the two birds to describe a mean visual field. Figure 1 shows a map of the mean frontal visual field, as well as horizontal sections through the visual field in the horizontal plane, and in the plane of the eye–bill tip projection (50° below the horizontal). The width of the binocular field as a function of elevation in the median–sagittal plane in the male bird is shown in figure 2.
Figure 1.
Visual fields of Senegal parrots P. senegalus. (a) Perspective view of an orthographic projection of the frontal retinal field (grid at 20° intervals). It shows the region of binocular overlap (blue) between the monocular portion of the left eye (yellow) and right eye (orange) visual fields, as well as the projection of the pectens (brown). The blind area is in dark grey and shows as just a small segment below the bill. The projections of the optic axes are indicated by the diamond points. The bird's head may be imagined to be at the centre of the sphere with its bill tip projecting towards the triangular point in the same posture as depicted in (b). This is the typical resting posture, in which measurements were also taken. Lines (c) and (d) through the eye refer to the respective diagrams of (c) and (d). (c) The section through the visual field in the plane (50° below the horizontal) that passes through the eye and the bill tip (bill tip direction is indicated by a red triangle). In this plane, the binocular field (blue) has a width of only 5°. (d) The section through the visual field in the horizontal plane. This is the region where the binocular field has its maximum width (27°). In this plane, there is a blind area behind the head (grey) of 16°. The yellow and orange sectors show the monocular portions of the visual fields of each eye and the full widths of the monocular fields of each eye in these two planes are indicated.
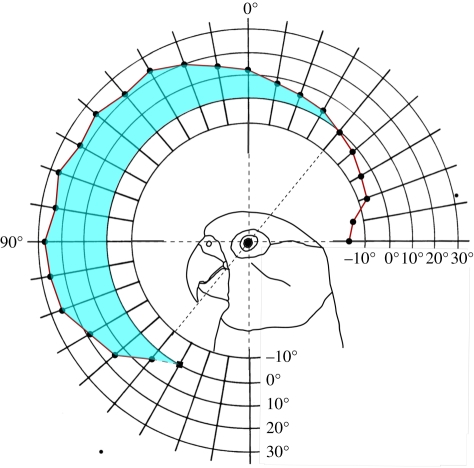
Figure 2.
Binocular field width in Senegal parrots P. senegalus as a function of elevation in the median-sagittal plane. The orientation of the bird's head is shown diagrammatically. The last point at 150° elevation has been extrapolated. Note that where the binocular field (shaded in blue) ends, the blind areas below and behind the head begin.
(a). Binocular field
The region where binocularity occurs is vertically long (190°) and narrow, and extends from just below the bill tip to behind the head about 40° from the vertical plane (figure 2). The maximum width is 27° and occurs at the horizontal plane. The binocular area covers approximately 8 per cent of the total horizontal width of the visual field, or 6 per cent of the total visual field sphere. It is of a similar width (approx. 20°) throughout most of its vertical extent. Where the bill tip projects, the binocular width is only 5° and there is a blind area directly below the bill.
(b). Monocular fields
Each eye has a monocular retinal field of 186° in the horizontal plane (figure 1d) and approximately 88 per cent of the total visual field in the horizontal plane has monocular visual coverage. This is true nearly throughout the vertical extent of the visual fields, as illustrated in figure 1c in the plane of the bill tip projection, where each monocular field is 183°.
(c). Cyclopean fields and blind areas
The cyclopean field covers approximately 98 per cent of the celestial hemisphere (figure 1); 344° in horizontal plane. A blind sector begins approximately 50° beyond the vertical plane to the back of the head, and widens to 16° at the horizontal. The pecten in each eye projects from 40° above the horizontal to 10° beyond the vertical plane. The parrot is technically blind in these areas, which cover approximately 10 per cent of the celestial hemisphere, but they can be abolished effectively by the large amplitude of eye movement.
(d). Eye movements
Maximum eye movement amplitude is 24° and eye movements of about this amplitude were found through a range of 60° of elevation in the region of the binocular frontal field. Owing to the small degree of maximum binocular overlap, these eye movements mean parrots can abolish binocularity. Eye movements are non-conjugate and can, therefore, produce asymmetrical visual fields.
(e). Optic axes
The eyes project laterally, with the optic axes oriented slightly above the horizontal plane (13°), and 28° forward. The optic axis of an eye is likely to be the direction of highest optical quality within a visual field and is therefore also likely to coincide with the direction of highest visual acuity in each eye.
(f). Bill tip organ
Figure 3 shows photographs of the Senegal parrot maxilla. Seven pairs of pits within the rhamphotheca can be seen along the edges, with a single pit at the bill tip. These pits probably contain clusters of mechanoreceptors [12,31,32]. No trace of structures associated with the bill tip organ was found in the bones (maxilla and mandible) of any specimens that we inspected. We observed irregular grooves leading down towards the bill tip, which may reveal the course of the blood vessels and/or nerve fibres that supply mechanoreceptors embedded in the keratin sheath. Figure 3b indicates the hard keratin sheath grows to extend beyond the bone. Also clearly visible are periodic grooves within the keratin, lying distal to the smooth mouth palate, which Collar [6] suggests aids grip. The junction between these grooved and smoothed sections of keratin lies approximately where the mandible meets the maxilla when the bill is closed. A similar arrangement of tactile pits in the maxilla is found in the mandible but in smaller numbers. This is contrary to Goujon's descriptions [12], who found the mandible has a significantly larger number of tactile pits than the maxilla.
Figure 3.
Location of the bill tip organ in a Senegal parrot P. senegalus. (a) A ventral view and (b) lateral view of a Senegal parrot skull superimposed on a photograph of a live bird to illustrate how the keratin, in which the bill tip organ is embedded, extends the bill into a hook-like structure beyond the maxilla bone. The blue line in both diagrams indicates approximately where the bone ends.
4. Discussion
We have shown that Senegal parrots have a visual field topography unlike those described hitherto in any other birds. Here, we discuss these differences and whether there are features of the visual fields that can be related to extractive foraging and/or the acquisition of information associated with exploration.
(a). General topography of the psittacine visual field
In birds, two main arrangements of visual fields have been described: those associated with visually guided foraging for food taken directly in the bill or feet and those associated with tactile probing or filter feeding [13]. In both types, the binocular field is relatively long (up to approx. 180°) than it is broad, but its location with respect to bill position differs. Visually guided foragers have a broad frontal binocular field (20°–30°), within which the bill is centrally placed and there is a blind area above and behind the head. By contrast, in tactile and filter feeders, the bill is at the periphery of the binocular field and there is comprehensive coverage of the celestial hemisphere. Additionally, the binocular field width in filter feeders and tactile probers is narrower (approx. 10°) than in visually guided foragers.
In Senegal parrots a different arrangement is found, which can be viewed as a compromise between the two main visual field types (see the electronic supplementary material, figure SA). The binocular field is relatively broad (approx, 27°), but there is nearly comprehensive coverage of the celestial hemisphere. As in tactile probers and filter feeders, the bill tip projects at the periphery of the visual field (so parrots can just see below their bill tip), but because it projects at a steep angle from the horizontal, absolute coverage of the celestial hemisphere is not achieved. However, by pitching the head back only 40°–50°, parrots could achieve comprehensive visual coverage, enabling them to see predators around the entire horizon.
As another consequence of where the bill tip projects, if Senegal parrots wish to inspect visually objects that lie below the bill, they must either pitch their head forwards to view them binocularly, or turn their head to use the lateral, monocular portion of their visual field. This probably prohibits the rapid and accurate control of the bill towards objects, which is achieved by birds that peck or lunge for their food [14]. However, like visually guided foragers, Senegal parrots do have a relatively broad frontal binocular field, which could aid inspection of objects held up in the foot. Parrots are often seen bringing their food items or novel objects up into their field of view with their feet [8,29]. Psittacines exhibit lateralization of visual function (e.g. one eye is often used preferentially for certain tasks) and motor function (e.g. ‘footedness’ in grasping objects) [45–50]. Highest acuity in all avian taxa is thought to occur in the lateral field [14].
(b). Extractive foraging and exploration
The tactile pits of the Senegal parrot bill tip organ are arranged along the inside of the curve of the bill tip. This means that unlike other taxa, such as kiwi (Apterygidae), ibises (Threskiornithidae) and probing shorebirds (Scolopacidae) [34,35,40,51], the parrot bill tip organ can only provide information about objects within the bill. Moreover, the unique bill shape obstructs a clear view of an object held within it (see the electronic supplementary material, figure SBb for more information). This suggests items to be explored are typically detected visually first, and the bill tip organ provides tactile information only once an object is grasped. Thus, parrots can manipulate objects efficiently without further need for visual information. This may have allowed natural selection to favour eyes placed high and laterally within the skull, resulting in extensive visual coverage above and behind the head, presumably for detecting predators and conspecifics. Some birds species that are known to manipulate and position objects carefully between their mandibles, such as hornbills (Bucerotidae [52]) and cormorants (Phalacrocoracidae [53]), have more forward-facing eyes, allowing them to inspect visually items held in the bill. By contrast, parrots seem to have achieved control over objects held in the bill without the need for visual cues.
Both object exploration and extractive foraging are likely to have been important factors in the evolution of psittacine visual fields, as both activities set parrots apart from other birds. However, the visual fields could be seen as facilitating the exploratory approach towards an object, rather than visual exploration of the object once it has been grasped. Another important factor that may be associated with parrot visual field configuration is the mode of locomotion used during climbing, in which the hooked bill tip is used as a third appendage. When a Senegal parrot climbs, the maximum binocular field width lies forward and above, allowing it to determine the position of the next point it can grasp with its bill. Similarly, it would prove most efficient when walking towards a target object for the parrot to approach with its head pitched downwards and then swung up towards the object just before grasping it with the bill (see the electronic supplementary material, figure SBa).
What tentative behavioural predictions can be drawn from our description of the Senegal parrot visual field? During exploration, we hypothesize that a parrot would first attend to a novel object or food item using its monocular (left/right) visual field, then on approach pass visual control to the binocular field by orientating its head frontally. The parrot might then continue to tilt its head downwards in the approach towards the object, keeping it within the binocular field, close to the horizontal (as defined in figures 1 and 2). Then the individual may either pick up the object in a foot and bring it to a monocular field (left/right preference [45–50]), or grasp it directly in the bill where tactile exploration could proceed using the bill tip organ, allowing further understanding of the object's affordances [2,9].
(c). Conclusion
The Senegal parrot visual field is unlike those described previously in any other bird species. It has both a relatively broad frontal binocular field in the horizontal plane and a near comprehensive field of view around the head. Although this could be considered a compromise between features described previously in visually guided foragers and the features seen in tactile guided probers and filter feeders, we argue that parrot visual fields are actually associated with their unique anatomy, extractive foraging, exploratory play and their climbing mode of locomotion. The key to all of these behaviours probably lies in the somatosensory information provided by the bill tip organ, which seems to integrate tightly with information from the Senegal parrots' visual fields. This allows parrots to have foregone visual coverage of the region below the bill in favour of more comprehensive visual coverage above the head, presumably for greater predator and conspecific detection. The ability to manipulate objects with the foot and present them in the lateral visual field for inspection may also have facilitated the evolution of eye position to be high and lateral in the skull.
Acknowledgements
We would like to thank the National History Museum at Tring, UK, for access to the skeleton collection and permission to take measurements and photographs of the skulls. Dr Susan Cunningham, Dr Roland Brandstaetter and Catherine Jones provided valuable advice on avian bill morphology and histology. Prof. Martin Wild and Prof. Lesley Rogers were consulted on avian neuroanatomy. Simone Webber kindly translated Goujon [12]. Many thanks also to Dr Susannah Thorpe and Jolyon Troscianko for their comments on the early drafts of the manuscript. Dr Christopher Pringle provided advice on visual field calculations. This work was funded by a BBSRC doctoral training grant.
References
- 1.Hughes A. 1977. The topography of vision in mammals of contrasting lifestyle: comparative optics and retinal organisation. In Handbook of sensory physiology VII/5 (ed. Crescitelli F.), pp. 613–756 Berlin, Germany: Springer [Google Scholar]
- 2.Ficken M. S. 1977. Avian play. Auk 94, 573–582 [Google Scholar]
- 3.Fagen R. 1981. Animal play behavior. New York, NY: Oxford University Press [Google Scholar]
- 4.Power T. G. 2000. Play and exploration in children and animals, 1st edn. London, UK: Psychology Press [Google Scholar]
- 5.Birdlife International. 2011. See http://www.birdlife.org/datazone/species .
- 6.Collar N. 1997. Family Psittacidae (Parrots). In Handbook of the birds of the world, sandgrouse to cuckoos, vol. 4 (eds del Hoyo J., Elliott A., Sargatal J.), pp. 286–324 Barcelona: Lynx Editions [Google Scholar]
- 7.Rowley I. 1997. Family Cacatuidae (Cockatoos). In Handbook of the birds of the world, sandgrouse to cuckoos, vol. 4 (eds del Hoyo J., Elliott A., Sargatal J.), pp. 246–269 Barcelona: Lynx Editions [Google Scholar]
- 8.Whittow G. C., Sturkie P. D. 1999. Sturkie's avian physiology. New York, NY: Academic Press [Google Scholar]
- 9.Gibson E. J. 1988. Exploratory behavior in the development of perceiving, acting, and the acquiring of knowledge. Annu. Rev. Psychol. 39, 1–42 10.1146/annurev.ps.39.020188.000245 (doi:10.1146/annurev.ps.39.020188.000245) [DOI] [Google Scholar]
- 10.Jarvis E. D., et al. 2005. Avian brains and a new understanding of vertebrate brain evolution. Nat. Rev. Neurosci. 6, 151–159 10.1038/nrn1606 (doi:10.1038/nrn1606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber L., Gajdon G. K. 2006. Technical intelligence in animals: the kea model. Anim. Cogn. 9, 295–305 10.1007/s10071-006-0033-8 (doi:10.1007/s10071-006-0033-8) [DOI] [PubMed] [Google Scholar]
- 12.Goujon D. E. 1869. Sur un appareil de corpuscules tactiles situe dans le bec des perroquets. J. de L'Anat. et de La Physiol. 6, 449–455 [Google Scholar]
- 13.Martin G. R. 2007. Visual fields and their functions in birds. J. Ornithol. 148, S547–S562 10.1007/s10336-007-0213-6 (doi:10.1007/s10336-007-0213-6) [DOI] [Google Scholar]
- 14.Martin G. R. 2009. What is binocular vision for? A bird' s eye view. J. Vis. 9, 1–19 10.1167/9.11.14 (doi:10.1167/9.11.14) [DOI] [PubMed] [Google Scholar]
- 15.Martin G. R. 1986. The eye of a passeriform bird, the European starling (Sturnus vulgaris): eye movement amplitude, visual fields and schematic optics. J. Comp. Physiol. A 159, 545–557 10.1007/BF00604174 (doi:10.1007/BF00604174) [DOI] [Google Scholar]
- 16.Martin G. R., Katzir G. 1994. Visual fields and eye movements in herons (Ardeidae). Brain Behav. Evol. 44, 74–85 10.1159/000113571 (doi:10.1159/000113571) [DOI] [PubMed] [Google Scholar]
- 17.Martin G. R., Katzir G. 1999. Visual fields in short-toed eagles, Circaetus gallicus (Accipitridae), and the function of binocularity in birds. Brain Behav. Evol. 53, 55–66 10.1159/000006582 (doi:10.1159/000006582) [DOI] [PubMed] [Google Scholar]
- 18.Guillemain M., Martin G. R., Fritz H. 2002. Feeding methods, visual fields and vigilance in dabbling ducks (Anatidae). Funct. Ecol. 16, 522–529 10.1046/j.1365-2435.2002.00652.x (doi:10.1046/j.1365-2435.2002.00652.x) [DOI] [Google Scholar]
- 19.Martin G. R. 1994. Visual fields in woodcocks Scolopaxrusticola (Scolopacidae; Charadriiformes). J. Comp. Physiol. A 174, 787–793 [Google Scholar]
- 20.Martin G. R. 1986. Total panoramic vision in the mallard duck, Anas platyrhynchos. Vis. Res. 26, 1303–1305 10.1016/0042-6989(86)90112-4 (doi:10.1016/0042-6989(86)90112-4) [DOI] [PubMed] [Google Scholar]
- 21.Homberger D. G. 1980. Functional morphology and evolution of the feeding apparatus in parrots, with special reference to the Pesquet's Parrot, Psittrichas fulgidus (lesson). In Conservation of New World parrots: Proceedings of the ICBP Parrot Working Group Meeting, St. Lucia, 1980 (ed. Pasquier R. F.), p. 471 Washington, DC: Smithsonian Institution Press [Google Scholar]
- 22.Homberger D. G. 2003. The comparative biomechanics of a prey-predator relationship: the adaptive morphologies of the feeding apparatus of Australian Black-Cockatoos and their foods as a basis for the reconstruction of the evolutionary history of the Psittaciformes. In Vertebrate biomechanics and evolution (eds Bells V. A., Gasc J. P., Casinos A.), pp. 203–228 Oxford, UK: BIOS Scientific Publishers [Google Scholar]
- 23.Zweers G. A., Berkhoudt H., van den Berge J. C. 1994. Behavioral mechanisms of avian feeding. In Biomechanics of feeding in vertebrates, advances in comparative environmental physiology (eds Bels V. L., Chardon M., van de Walle P.), pp. 241–279 Berlin, Germany: Springer [Google Scholar]
- 24.King A. S., McLelland J. 1989. Form and function in birds. New York, NY: Academic Press [Google Scholar]
- 25.Tokita M. 2004. Morphogenesis of parrot jaw muscles: understanding the development of an evolutionary novelty. J. Morphol. 259, 69–81 10.1002/jmor.10172 (doi:10.1002/jmor.10172) [DOI] [PubMed] [Google Scholar]
- 26.Tokita M., Kiyoshi T., Armstrong K. N. 2007. Evolution of craniofacial novelty in parrots through developmental modularity and heterochrony. Evol. Dev. 9, 590–601 10.1111/j.1525-142X.2007.00199.x (doi:10.1111/j.1525-142X.2007.00199.x) [DOI] [PubMed] [Google Scholar]
- 27.Lefebvre L., Reader S. M., Sol D. 2004. Brains, innovations and evolution in birds and primates. Brain Behav. Evol. 63, 233–246 10.1159/000076784 (doi:10.1159/000076784) [DOI] [PubMed] [Google Scholar]
- 28.Iwaniuk A. N., Hurd P. L. 2005. The evolution of cerebrotypes in birds. Brain Behav. Evol. 65, 215–230 10.1159/000084313 (doi:10.1159/000084313) [DOI] [PubMed] [Google Scholar]
- 29.Luescher A. U. 2006. Manual of parrot behavior. San Francisco, CA: Wiley-Blackwell [Google Scholar]
- 30.Necker R. 1972. Response of trigeminal ganglion neurons to thermal stimulation of the beak in pigeons. J. Comp. Physiol. A 78, 307–314 10.1007/BF00697660 (doi:10.1007/BF00697660) [DOI] [Google Scholar]
- 31.Gottschaldt K. M., Lausmann S. 1974. The peripheral morphological basis of tactile sensibility in the beak of geese. Cell Tissue Res. 153, 477–496 10.1007/BF00231542 (doi:10.1007/BF00231542) [DOI] [PubMed] [Google Scholar]
- 32.Berkhoudt H. 1979. The morphology and distribution of cutaneous mechanoreceptors (Herbst and Grandry corpuscles) in bill and tongue of the mallard (Anas platyrhynchos L.). Neth. J. Zool. 30, 1–34 10.1163/002829680X00014 (doi:10.1163/002829680X00014) [DOI] [Google Scholar]
- 33.Gentle M. J., Breward J. 1986. The bill tip organ of the chicken (Gallus gallus var. domesticus). J. Anat. 145, 79–85 [PMC free article] [PubMed] [Google Scholar]
- 34.Cunningham S., Castro I., Alley M. 2007. A new prey-detection mechanism for kiwi (Apteryx spp.) suggests convergent evolution between paleognathous and neognathous birds. J. Anat. 211, 493–502 10.1111/j.1469-7580.2007.00786.x (doi:10.1111/j.1469-7580.2007.00786.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunningham S. J., Alley M. R., Castro I., Potter M. A., Cunningham M., Pyne M. J. 2010. Bill morphology of ibises suggests a remote-tactile sensory system for prey detection. Auk 127, 308–316 10.1525/auk.2009.09117 (doi:10.1525/auk.2009.09117) [DOI] [Google Scholar]
- 36.Stingelin W. 1965. Qualitative und quantitative Untersuchungen an Kerngebieten der Medulla oblongata bei Vögeln. Bibl. Anat. 6, 1–116 10.1159/000270904 (doi:10.1159/000270904) [DOI] [PubMed] [Google Scholar]
- 37.Wild J. M. 1981. Identification and localization of the motor nuclei and sensory projections of the glossopharyngeal, vagus, and hypoglossal nerves of the cockatoo (Cacatua roseicapilla), Cacatuidae. J. Comp. Neurol. 203, 351–377 10.1002/cne.902030304 (doi:10.1002/cne.902030304) [DOI] [PubMed] [Google Scholar]
- 38.Wild J. M. 1997. A non-thalamic pathway contributes to a whole body map in the brain of the budgerigar. Brain Res. 755, 137–141 10.1016/S0006-8993(97)00026-7 (doi:10.1016/S0006-8993(97)00026-7) [DOI] [PubMed] [Google Scholar]
- 39.Gutiérrez-Ibáñez C., Iwaniuk A. N., Wylie D. R. 2009. The independent evolution of the enlargement of the principal sensory nucleus of the trigeminal nerve in three different groups of birds. Brain Behav. Evol. 74, 280–294 10.1159/000270904 (doi:10.1159/000270904) [DOI] [PubMed] [Google Scholar]
- 40.Piersma T., Aelst R., Kurk K., Berkhoudt H., Maas L. R. M. 1998. A new pressure sensory mechanism for prey detection in birds: the use of principles of seabed dynamics? Proc. R. Soc. Lond. B 265, 1377–1383 10.1098/rspb.1998.0445 (doi:10.1098/rspb.1998.0445) [DOI] [Google Scholar]
- 41.Athan M. S., Deter D. 1998. Guide to the Senegal parrot and its family. Hauppauge, NY: Barron's Educational Series [Google Scholar]
- 42.Alderton D. 2005. The ultimate encyclopedia of caged and aviary birds. Horsham, UK: Southwater [Google Scholar]
- 43.Martin G. R., Shaw J. M. 2010. Bird collisions with power lines: failing to see the way ahead? Biol. Conserv. 143, 2695–2702 10.1016/j.biocon.2010.07.014 (doi:10.1016/j.biocon.2010.07.014) [DOI] [Google Scholar]
- 44.Martin G. R. 1984. The visual fields of the tawny owl, Strix aluco L. Vis. Res. 24, 1739–1741, 1743–1751. (doi:10.1016/0042-6989(84)90005-1) [DOI] [PubMed] [Google Scholar]
- 45.Harris L. J. 1989. Footedness in parrots: three centuries of research, theory, and mere surmise. Can. J. Psychol. 43, 369–396 10.1037/h0084228 (doi:10.1037/h0084228) [DOI] [PubMed] [Google Scholar]
- 46.Snyder P. J., Harris L. J. 1997. Lexicon size and its relation to foot preference in the African grey parrot (Psittacus erithacus). Neuropsychologia 35, 919–926 10.1016/S0028-3932(97)00010-9 (doi:10.1016/S0028-3932(97)00010-9) [DOI] [PubMed] [Google Scholar]
- 47.Casey M. B., Martino C. M. 2000. Asymmetrical hatching behaviors influence the development of postnatal laterality in domestic chicks (Gallus gallus). Dev. Psychobiol. 37, 13–24 (doi:10.1002/1098-2302(200007)37:1<13::AID-DEV3>3.0.CO;2-M) [DOI] [PubMed] [Google Scholar]
- 48.Rogers L. J. 2008. Development and function of lateralization in the avian brain. Brain Res. Bull. 76, 235–244 10.1016/j.brainresbull.2008.02.001 (doi:10.1016/j.brainresbull.2008.02.001) [DOI] [PubMed] [Google Scholar]
- 49.Magat M., Brown C. 2009. Laterality enhances cognition in Australian parrots. Proc. R. Soc. B 276, 4155–4162 10.1098/rspb.2009.1397 (doi:10.1098/rspb.2009.1397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown C., Magat M. 2011. Cerebral lateralization determines hand preferences in Australian parrots. Biol. Lett. (doi:10.1098/rsbl.2010.1121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin G. R., Wilson K. J., Wild J. M., Parsons S., Kubke M. F., Corfield J. 2007. Kiwi forego vision in the guidance of their nocturnal activities. PLoS ONE 2, e198. 10.1371/journal.pone.0000198 (doi:10.1371/journal.pone.0000198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin G. R., Coetzee H. C. 2004. Visual fields in hornbills: precision-grasping and sunshades. Ibis 146, 18–26 10.1111/j.1474-919X.2004.00211.x (doi:10.1111/j.1474-919X.2004.00211.x) [DOI] [Google Scholar]
- 53.Martin G. R., White C. R., Butler P. J. 2008. Vision and the foraging technique of Great Cormorants Phalacrocorax carbo: pursuit or close-quarter foraging? Ibis 150, 485–494 10.1111/j.1474-919X.2008.00808.x (doi:10.1111/j.1474-919X.2008.00808.x) [DOI] [Google Scholar]