Abstract
Overexpression of phytochrome B (phyB) in Arabidopsis has previously been demonstrated to result in dominant negative interference of phytochrome A (phyA)-mediated hypocotyl growth inhibition in far-red (FR) light. This phenomenon has been examined further in this study and has been found to be dependent on the FR fluence rate and on the availability of metabolizable sugars in the growth medium. Poorly metabolized sugars capable of activating the putative hexokinase sensory function were not effective in eliciting the phytochrome interference response. Overexpressed phyB lacking the chromophore-binding site was also effective at inhibiting the phyA response, especially at higher fluence rates of FR. Overexpressed phyB produces the dominant negative phenotype without any apparent effect on phyA abundance or degradation. It is possible that phyA and phyB interact with a common reaction partner but that either the energy state of the cell or a separate sugar-signaling mechanism modulates the phytochrome-signaling interactions.
Light is among the most important environmental factors influencing plant growth and development. In the dark Arabidopsis seedlings exhibit a typical etiolated growth habit, with an elongated hypocotyl, an apical hook, and small, pale, closed cotyledons. Seedlings grown under white light progress through photomorphogenic development, which results in decreased hypocotyl-elongation rates, no apical hook, and expanded green cotyledons. The developmental changes are reflected in altered patterns of gene expression, cellular activities, and biochemical properties. These responses are mediated through a variety of photoreceptors including the five-member phytochrome family, at least two blue/UV-A light receptors, and one or more UV-B photoreceptors.
The Arabidopsis phytochromes, a family of homologous proteins designated phyA, phyB, phyC, phyD, and phyE (Sharrock and Quail, 1989; Clack et al., 1994), absorb primarily in the red and FR regions of the spectrum. The functions of each member of this gene family have not yet been fully determined, but analysis of mutation and overexpression of different phytochromes indicates that there are both overlapping and distinct processes controlled by each gene product (Boylan and Quail, 1989, 1991; Nagatani et al., 1991, 1993; Somers et al., 1991; Wagner et al., 1991; Parks and Quail, 1993; Reed et al., 1993, 1994; Whitelam and Harberd, 1994; Quail et al., 1995; Aukerman et al., 1997).
Type I phytochrome, now known to consist primarily of phyA in Arabidopsis (Hirschfeld et al., 1998), is by far the most abundant phytochrome in etiolated tissues and is produced in the Pr form in vivo (Mancinelli, 1994). Upon phototransformation to the Pfr form, phyA becomes degraded rapidly via a ubiquitin-mediated process (Clough and Vierstra, 1997). This instability results in a very low abundance of phyA during extended growth in normal light conditions. However, under FR conditions the photoconversion to the Pfr form is considerably less efficient, and the photoequilibrium between the Pr and Pfr forms strongly favors the more stable Pr form of phyA (Mancinelli, 1994). The remaining phytochromes of Arabidopsis do not undergo rapid degradation in the Pr form, although there is recent evidence that some light regulation of other phytochromes, especially phyC, occurs in vivo (Hirschfeld et al., 1998).
The phytochrome holoproteins consist of an apoprotein of roughly 125 kD, with a linear tetrapyrrole chromophore (phytochromobilin) covalently attached to a specific Cys in the NH2-terminal domain (Quail, 1991; Furuya, 1993; Furuya and Song, 1994). In vitro, and probably in vivo as well, phytochrome is active as a homodimer (Cherry and Vierstra, 1994), but it is not yet clear whether heterodimers form (and, if so, whether they are active) among different members of the phytochrome family in vivo. One or more dimerization domains are thought to reside in the COOH-terminal domain (Edgerton and Jones, 1992; Cherry et al., 1993; Wagner et al., 1996b). A defined region of the COOH-terminal half of phyA and phyB has also been implicated as critical for the signaling function of the molecules (Quail et al., 1995, 1996; Wagner and Quail, 1995; Wagner et al., 1996a).
Analysis of phyA and phyB mutants has indicated that the so-called FR high-irradiance responses in seedlings are primarily mediated through phyA (Dehesh et al., 1993; Nagatani et al., 1993; Parks and Quail, 1993; Reed et al., 1994; Xu et al., 1995), whereas the majority of red high-irradiance responses and the so-called shade-avoidance response are regulated primarily through phyB (Somers et al., 1991; Dehesh et al., 1993; Reed et al., 1993; Smith and Whitelam, 1997). Recent discovery and analysis of null phyD mutants—the closest homolog to the PHYB gene—also indicate that phyD is active and performs specific regulatory functions in Arabidopsis that only partially duplicate those of phyB (Aukerman et al., 1997). Careful analysis of hypocotyl elongation in seedlings of the wild type and of phytochrome mutants indicate no detectable effect of phyB or phyD under continuous FR light, whereas phyA has little or no effect on elongation under continuous red light (Dehesh et al., 1993; Parks and Quail, 1993; Reed et al., 1993; Quail et al., 1995, 1996).
These analyses are complicated by data showing that accumulation of some phytochromes is regulated at least in part by the levels of other members of the phytochrome family (Hirschfeld et al., 1998). Studies of mutants deficient in one or more phytochromes have been complemented by the development of transgenic Arabidopsis plants expressing complete or partial phytochrome gene products (Boylan and Quail, 1991; Wagner et al., 1991, 1996a, 1996b; Boylan et al., 1994; Qin et al., 1997). Transgenic expression of certain Arabidopsis phyA and phyB fragments, especially those with products lacking the COOH-terminal region of the molecule, have shown dominant negative effects on primarily phyA-mediated hypocotyl elongation (Boylan et al., 1994; Wagner et al., 1996b). However, others studying the same or similar transgenic plants did not report this dominant negative response in the phyB-overexpressing lines (McCormac et al., 1993).
Physiological and molecular studies have begun to yield information about the interaction between light cues and their modulation by other environmental stimuli (Cheng et al., 1992; Mohr, 1994; Short and Briggs, 1994). Carbohydrates have long been recognized as important regulators of growth, development, and gene expression in a variety of systems (for review, see Madore and Lucas, 1995; Pontis et al., 1995; Koch, 1996). Among these functions, carbohydrates have been found to alter responsiveness to light, particularly with respect to specific gene expression (Tsukaya et al., 1991; Cheng et al., 1992; Harter et al., 1993; Dijkwel et al., 1996, 1997). Recent data have shown that metabolizable sugars can overcome the phyA-specific repression of protochlorophyllide oxidoreductases (Barnes et al., 1996; T.W. Short, unpublished data), that Suc represses light-inducible plastocyanin production (Dijkwel et al., 1996), and that Suc can specifically affect seedling growth in FR light (Whitelam et al., 1993; Dijkwel et al., 1997). However, it is unclear how carbohydrates and phyA-signaling mechanisms interact to regulate these functions. A series of mutants in which Suc and light responses are uncoupled (sun mutants) have been isolated and characterized (Dijkwel et al., 1997), and analysis of several of them suggests that gene-expression responses to Suc and light follow separate, but linked, signaling pathways.
Results from several laboratories suggest that carbohydrate status may be sensed through metabolic modification of sugars. In particular, sugar phosphates (Sadka et al., 1994) may regulate gene expression. The primary sensors and transducers for this mechanism are likely the Arabidopsis hexokinases (Jang et al., 1997). Although this transduction system may act on expression of many sugar-responsive genes, it remains uncertain whether the hexokinase-signaling pathway is the major mechanism involved in carbohydrate modulation of light responses. Data from the sun mutants support the likelihood of multiple signaling pathways (Dijkwel et al., 1997). Whereas the sun6 mutation decreased the capacity of FR-light-grown seedlings to green after transfer to white light in the presence or absence of Suc, the sun7 mutation had the opposite effect. Furthermore, sun7 alters the Suc effect on cotyledon opening and hypocotyl elongation, whereas sun6 does not appear to modulate these responses.
In this study we used Arabidopsis overexpressing a PHYB transgene to examine further the role of sugars in modulating phytochrome signaling. We have addressed the seemingly discrepant reports of phyB-overexpression studies and the dominant negative effect on growth, and we have investigated possible mechanisms of Suc/light interactions and placed them in the context of previous studies.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The transgenic ABO lines were originally produced in the No-O ecotypic background (Wagner et al., 1991) and were introgressed three times into ecotype RLD. Mutant phyB-expressing lines with chromophore-binding Cys-357 converted to Ser (Cys-357-Ser) were also constructed in the No-O ecotype, and phyA-101 mutants were isolated from the RLD ecotype. The original ABO(RLD) and Cys-357-Ser(No-O)-overexpressing transgenics and phyA-101 mutants were generously provided by Drs. D. Wagner and P. Quail.
Seeds were surface-sterilized for 20 min in 20% commercial bleach (final sodium hypochlorite concentration, 1.05% [w/v]) and 0.01% SDS, and then rinsed three times in sterile water. Seeds were sown on 100-mm Petri plates with sterile medium containing 0.8% (w/v) agar, MS nutrient salts (Murashige and Skoog, 1962), and 2% (w/v) Suc, unless otherwise noted. Plates were kept in the dark at 4°C for 2 to 7 d and were then induced to germinate by exposure to white fluorescent light for 3 h before placement in the dark at 23°C ± 1°C for 21 h. In experiments testing the effects of different sugars and analogs, sterilized seeds were instead plated onto 50-mm circles of fine nylon mesh and floated on a liquid MS salt solution, and the sugars were added to the medium at the same time the plates were transferred to FR light. Except where noted, seedlings were allowed to grow under continuous FR light (8.7 μmol m−2 s−1) for 72 h before they were measured. FR light was obtained from a high-output, 735-nm LED source (Q-Beam 2001, Quantum Devices, Barneveld, WI). Where indicated, light was filtered through plexiglass (FRF700, Westlake Plastics, Lenni Mills, PA) to eliminate minor red-light emissions from the LED source. Light intensity and spectral output were measured with a spectroradiometer (LI-1800, Li-Cor, Lincoln, NE).
Analytical Measurements
Following light treatments, seedlings were laid flat on 1% agar plates and photographed on slide film beside a reference ruler. Slides were projected onto a digitizing tablet (model 1212, Kurta, Banska Bystrica, Slovakia), and hypocotyls, petioles, and roots were measured directly using SigmaScan software (Jandel Scientific, San Rafael, CA). Unless otherwise indicated, growth data represent results from two or three independent replicates containing 30 to 100 seedlings per treatment. Within each experiment the total number of seedlings was approximately equivalent for each treatment, and all of the seedlings were measured to avoid potential selection bias.
For western blotting, seeds were sterilized and grown as indicated above, except they were kept for 3 d in darkness and then exposed to continuous FR or red light before harvesting at the times indicated. The phytochrome extractions were performed by grinding 1.5 g of frozen tissue for 3 min in 1.5 mL of extraction buffer (100 mm Tris, pH 8.3, 140 mm [NH4]2SO4, 50% ethylene glycol, 10 mm Na2EDTA, 2 μg/mL aprotinin, 1 μg/mL leupeptin, 1 μg/mL pepstatin, 2 mm phenylmethylsulfonyl fluoride, 18 mm iodoacetamide, and 12 mm sodium metabisulfate) and centrifuging at 25,000g for 15 min at 4°C. Supernatants were brought to 36% (NH4)2SO4 with a cold, saturated solution of (NH4)2SO4 and centrifuged at 25,000g for 20 min at 4°C. Pellets were resuspended in 100 μL of resuspension buffer (50 mm Tris, pH 7.8, 5.0 mm EDTA, 25% ethylene glycol, and 10 mm iodoacetamide). Two 5-μL samples were removed for protein assay by a modified Lowry assay (Peterson, 1977), and the remainder was combined with 20 μL of sample buffer (350 mm Tris, pH 6.8, 20% SDS, 4 mm β-mercaptoethanol, 30% [w/v] glycerol, and 15 mg/mL bromphenol blue). SDS-PAGE and immunoblotting on nitrocellulose were carried out according to methods described previously (Xu et al., 1995). Following staining with 1 mg/mL Ponceau S in 1% acetic acid to confirm equivalent protein loads, visualization of blots was performed with the PHYA apoprotein-specific monoclonal antibody 073D (developed by Dr. J. Shanklin and generously provided by Dr. P. Quail) and an antibody-detection system (Vectastain ABC-AP, Vector Laboratories, Burlingame, CA) using a colorimetric substrate as previously described (Short et al., 1992).
RESULTS
Overexpression of phyB and Suc Alter Growth in FR Light
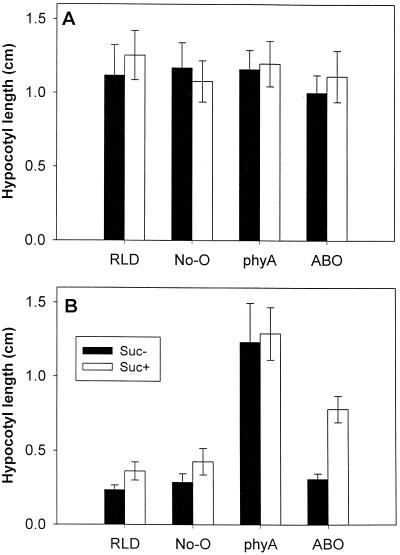
To address seemingly contradictory data on the effects of constitutive high expression of phyB, we explored the response of ABO, phyA, and the parental wild types under a variety of conditions. Examination of the hypocotyl-elongation response indicated that mutations in phyA result in a loss of normal responsivity to FR light regardless of the presence of 2% Suc in the growth medium (Fig. 1), as has been indicated previously (Nagatani et al., 1993; Parks and Quail, 1993; Reed et al., 1994; Whitelam and Harberd, 1994). Similarly, Suc had a relatively small effect on the growth of wild-type seedlings grown in darkness (Fig. 1A) or in 8.7 μmol m−2 s−1 FR light (Fig. 1B), although the light-grown seedlings did have a slightly enhanced growth response with added Suc. ABO yielded markedly different rates of growth in FR light depending on the presence of exogenous Suc. Suc resulted in growth approximately double that of ABO seedlings without Suc or that of wild-type seedlings on Suc-containing medium. Wild-type, phyA, and ABO seedlings in all cases exhibited similar levels of growth after 3 d in darkness with or without Suc.
Figure 1.
Light-regulated hypocotyl elongation in ABO seedlings is dependent on Suc. Seedlings from wild type (RLD and No-O ecotypes), phyA(RLD) mutants, and phyB(No-O) overexpressers introgressed three times into RLD were grown as described in Methods with (white bars) or without (black bars) 2% Suc either in darkness (A) or in 35 μmol m−2 s−1 FR light (B). No-O is included to control for possible residual effects of the original phyB ecotype after introgression. Error bars represent ±1 sd of 75 to 120 seedlings from replicate experiments.
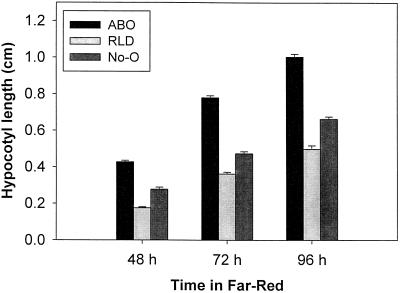
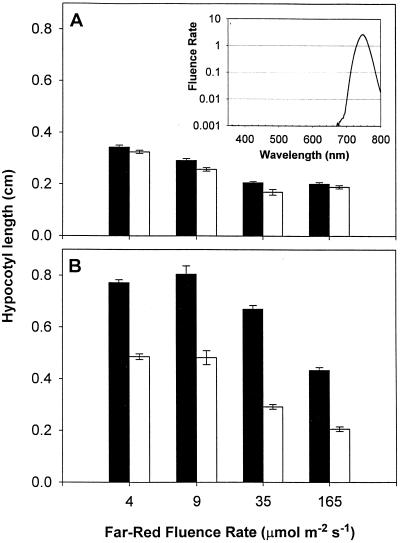
The effects of Suc and FR light on differential growth rates in wild-type and ABO plants were maintained throughout the period of 48 to 96 h following transfer to light (Fig. 2), with relative hypocotyl-length ratios remaining almost identical throughout the period under examination. The differential growth rate was also maintained over a range of FR fluence rates (Fig. 3). Within the fluence rate window investigated, the accelerated growth in ABO plants was only observed in the presence of Suc. These disparate rates of elongation resulted in a 58% increase in growth over wild type at 4 μmol m−2 s−1 to a 130% increase at 35 μmol m−2 s−1.
Figure 2.
Dominant negative interference by overexpressed phyB affects growth throughout early seedling development. Hypocotyl elongation on 2% Suc medium was measured 2, 3, or 4 d following transfer of the seedlings to 8.7 μmol m−2 s−1 FR light. Growth of original parental (No-O) and introgressed parental (RLD) wild types are compared with ABO in two independent experiments representing approximately 100 seedlings per treatment. Error bars represent ±1 se.
Figure 3.
Effect of FR fluence rate on dominant negative interference. Hypocotyl elongation of ABO (black bars) and wild-type (RLD, white bars) plants was measured after growth for 3 d at the indicated fluence rate of continuous FR light. Results are from plants grown on medium without (A) or with (B) 2% Suc. Error bars represent ±1 se of 100 to 150 seedlings from independent replicate experiments. The inset in A shows the light output from the filtered LED sources on a logarithmic scale.
Effects of Metabolically Active Sugars and Inactive Analogs
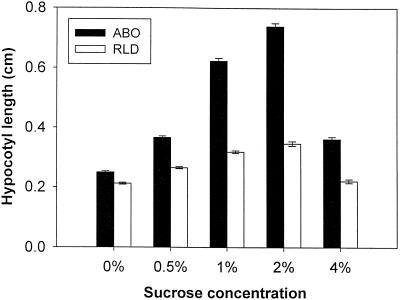
To study possible mechanisms of Suc modulation of phytochrome interactions, we examined further the role of Suc and other sugars in mediating dominant negative phyB suppression of phyA-mediated growth inhibition. Wild-type and ABO seedlings were grown on a series of MS-agar plates containing different Suc concentrations. Although small increases in growth were observed in wild-type RLD as the concentration of exogenous Suc was increased (Fig. 4), a much greater growth response was seen in the corresponding ABO seedlings. Transgenic and wild-type lines were significantly inhibited at Suc concentrations of 4% and above. At 6% Suc germination was inhibited, and the few seedlings that did germinate grew very slowly and died before producing primary leaves (data not shown).
Figure 4.
The dominant negative growth response is dependent on Suc concentration. ABO(RLD) and RLD seedlings were placed on plates with MS salts supplemented with the indicated concentrations of Suc. Standard growth conditions of 1 d in darkness followed by 3 d in FR light at 8.7 μmol m−2 s−1 FR light were used, and hypocotyls were measured as described in the text. Each measurement is the result of three independent experiments with 40 to 75 seedlings per treatment per experiment. Error bars represent ±1 se.
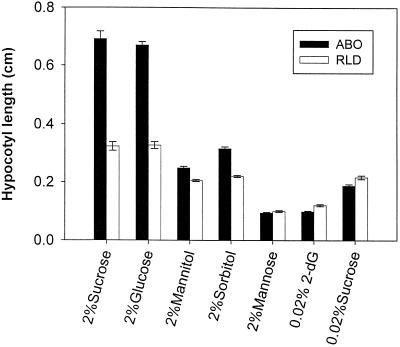
Because of the apparent existence of more than one sugar-signaling pathway in higher plants and because of the possibility that Suc acts through osmotic effects, we examined the influences of alternate sugars on mediating the dominant negative phytochrome response. The results of growth with sugars known to be transported and easily utilized in plant cells (Suc and Glc), sugars that are osmotically active but nonmetabolizable (sorbitol and mannitol), and sugars that can be phosphorylated and activate the hexokinase-based sensory system but cannot be readily metabolized (Man and 2-dG; Jang et al., 1997) are shown in Figure 5. The disaccharide Suc and the monosaccharide Glc gave essentially indistinguishable results in these experiments, although in other experiments a slightly lower effectiveness of Glc was noted (not shown). Neither sorbitol nor mannitol was able to elicit the strong dominant negative response in ABO at the concentrations expected to provide comparable osmotic conditions. Man at 2% and 2-dG at 0.02%—concentrations sufficient to activate the hexokinase sensory/regulatory system—partially inhibited growth and eliminated the differential between ABO and RLD wild type. Additionally, Fru, maltose, and raffinose, but not Gal, were nearly as effective as Suc and Glc at eliciting the ABO response (data not shown).
Figure 5.
Effects of different sugars on the dominant negative interference response. Dominant negative hypocotyl growth was measured for ABO and wild-type (RLD) seedlings sown on liquid MS salts medium (see details in text). Following stratification, seeds were induced to germinate with a 3-h white-light treatment followed by 21 h in darkness. At that time seeds were transferred to FR light (8.7 μmol m−2 s−1), and the medium was supplemented with a 10× stock of the indicated sugars in MS medium (2-dG). Hypocotyl measurements were taken after 3 d in FR light. Error bars indicate ±1 se of 60 to 120 seedlings from independent replicate experiments.
Suc and phyB Effects on phyA Abundance
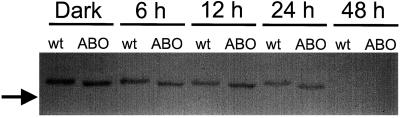
In attempting to explain the mechanism by which Suc and ectopically expressed phyB act to inhibit a response normally attributed to phyA, we used western-blot analysis to examine possible changes in phyA accumulation and degradation. After 3 d of dark growth on Suc-containing medium, wild-type and ABO plants were exposed to FR light for varying times or were kept in the dark prior to protein extraction and SDS-PAGE. After transfer to nitrocellulose, western blots were probed with monoclonal antibodies—shown previously to be phyA specific (Hirschfeld et al., 1998)—to determine relative initial levels of phyA in Arabidopsis tissues and the comparative rates of degradation upon exposure to FR light. Figure 6 shows that, within the limits of quantitation for immunoblotting, the level of PHYA apoprotein in dark-grown ABO was indistinguishable from that in wild-type seedlings under our growth conditions.
Figure 6.
PhyA accumulation and degradation under FR light. Seedlings from the transgenic ABO and RLD wild-type (wt) lines were grown on MS medium supplemented with 2% Suc as described in the text. Following 2 d of growth in darkness, plates were transferred to FR light (35 μmol m−2 s−1) for the indicated times or were left in darkness for an additional 24 h (Dark). Seedlings were harvested, and phytochrome-enriched extracts were subjected to western-blot analysis and probed with a phyA-specific antibody. Equivalent total proteins (90 μg/gel lane) were loaded onto gels, and the loading and electroblot transfer was confirmed by Ponceau S staining. The blot was scanned for the addition of labels and printing.
Furthermore, the rate of phyA degradation between 6 and 48 h in FR light was similar in both lines. By 48 h the amount of phyA (Fig. 6) was essentially undetectable by this method, although there was a faint signal present in one of the replicate experiments for wild-type and ABO extracts at 48 h (not shown). We have attributed the slight shift in the size of the phyA signal to displacement by the excess phyB present in the ABO samples, rather than to nonspecific recognition of phyB by the antibodies or degradation of phyA. The 073D antibodies have been shown to have high specificity for phyA (see Hirschfeld et al., 1998, and refs. therein), and phyB has a slightly higher molecular mass than phyA. Furthermore, immunoblots with lower protein loads do not exhibit this apparent shift in mobility (not shown).
Dominant Negative Regulation by Photobiologically Inactive phyB
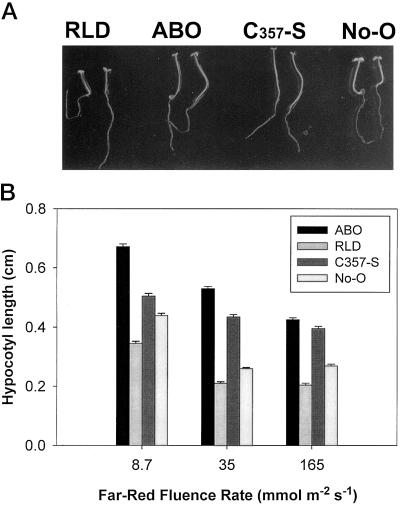
Among the possible explanations for the dominant negative effects seen in the ABO line are the following: (a) the excess PHYB apoprotein competes for limiting chromophore, or (b) phyB-induced signals actively suppress phyA transduction pathways. To address these possibilities, the transgenic Cys-357-Ser(No-O) line was analyzed. This line expresses a PHYB apoprotein in which the Cys chromophore attachment site has been mutated to a Ser and is known to be photochemically inactive (Wagner et al., 1996b). Growth of the Cys-357-Ser(No-O) line on Suc-containing medium was examined at three different fluence rates of FR light (Fig. 7B). At the higher intensities, these experiments yielded results comparable to those seen in ABO. However, at the lowest fluence rate tested there was little difference observed between wild-type No-O and Cys-357-Ser(No-O) seedlings. A photograph of these seedlings grown at 150 μmol m−2 s−1 FR light shows that under these conditions the ABO(RLD) and Cys-357-Ser(No-O) lines appeared identical to each other but were significantly different from their respective wild types in hypocotyl length, petiole length, and cotyledon expansion (Fig. 7A). Quantitation of petiole lengths yielded variable results. Although the ABO and Cys-357-Ser petioles consistently appeared slightly longer than those of wild-type plants, technical difficulties with these measurements resulted in large sds (not shown). In the ABO and Cys-357-Ser plants, the cotyledons appeared somewhat smaller than those of the wild type, similar to previous observations of phyA (Parks and Quail, 1993). Because of the strong curling observed in cotyledons of FR-light-grown seedlings, quantitation of this phenotype proved unreliable. Root lengths did not vary significantly as a function of phyB overexpression compared with wild type grown under comparable conditions (data not shown).
Figure 7.
Overexpressed phyB with and without the chromophore-binding site yield similar dominant negative phenotypes. Seedlings of parental wild types (RLD and No-O) and transgenics ABO or phyB with the chromophore-binding site Cys-357 mutated to Ser (C357-S) were grown for 3 d on MS medium supplemented with 2% Suc at 150 μmol m−2 s−1 FR light (A) or at the indicated fluence rates for quantitation of elongation (B). Cys-357-Ser is in the No-O background, and ABO was introgressed into the RLD ecotype. Quantitative measurements were made on replicate experiments consisting of 30 to 50 seedlings per treatment per experiment. Error bars represent ±1 se.
DISCUSSION
Suc Is Necessary for the Dominant Negative Phenotype of Transgenic phyB
Studies of mutant Arabidopsis plants deficient in one or more phytochromes indicate that the strong FR-light suppression of hypocotyl growth is almost exclusively mediated through the phyA protein (Dehesh et al., 1993; Nagatani et al., 1993; Parks and Quail, 1993; Reed et al., 1994). Previously, hypocotyl elongation of phyB-overexpressing seedlings in FR light has been documented as either identical to that of wild type (McCormac et al., 1993; Quail et al., 1996) or eliciting a dominant negative effect on the normal suppression of growth (Wagner et al., 1996a, 1996b). Among the most probable differences that could explain this apparent discrepancy was the medium on which the seedlings were grown. In the former, seedlings were plated on agar containing MS salts with no added carbohydrates, whereas in the latter, growth medium containing Suc (Valvekens et al., 1988) was used.
Research demonstrating that carbohydrates alter hypocotyl elongation (Whitelam and Harberd, 1994) and other phytochrome-mediated responses (Tsukaya et al., 1991; Barnes et al., 1996; Dijkwel et al., 1997) also indicate a strong interaction between light- and sugar-signaling pathways. Therefore, we tested the effects of sugar on the phyB-mediated inhibition of the phyA growth response. As demonstrated in Figure 1, Suc is a necessary component for obtaining the dominant negative suppression of phyA-dependent growth inhibition by constitutively expressed transgenic phyB. Suc was not sufficient to overcome growth suppression by FR light in the absence of ectopically expressed phyB, nor was the excess phyB sufficient to block growth inhibition in the absence of an appropriate sugar. This result implies interplay, whether direct or indirect, of usable sugars with phyA/phyB regulatory pathway interactions.
These interference phenomena can be seen throughout the early growth of the seedlings. At 2, 3, and 4 d after transfer to FR light (Fig. 2), the growth rate appears linear for the wild types and the ABO line. In contrast, other responses dependent on sugars and phyA show a more complex relationship. For example, anthocyanin accumulation varies throughout early development and is dependent on the fluence rate, on the quality of light, and on the concentration of exogenous sugars (Mancinelli, 1983; Batschauer et al., 1991; T.W. Short, unpublished observations). However, FR-light-dependent anthocyanin accumulation is also decreased in ABO compared with the wild type (T.W. Short, unpublished observations).
Prior work by Wagner and coworkers (1996b) demonstrated that the dominant negative phenotype correlates directly with the level of phyB accumulation in various transgenic lines. This response is fluence rate dependent (Fig. 3), suggesting that Suc probably acts on the light-signaling pathway(s) rather than acting solely on the elongation response. This interpretation is consistent with data from other groups studying Suc/phytochrome interactions, such as the ability of Suc to overcome the FR-light-mediated block of greening and expression of the plastocyanin gene (Dijkwel et al., 1997).
Suc concentration, phyB content, and fluence rate are important for obtaining a maximal dominant negative response. The growth rate increases steadily with Suc concentration in the ABO seedlings and, to a lesser degree, in the wild type. Again, this dosage-dependent result is more consistent with signal pathway interactions than mere additive effects on the final response. At higher concentrations of Suc, growth may be inhibited via a different mechanism. Cell toxicity may be responsible for the very low germination rates and poor growth at the highest concentrations tested.
Although the window of fluence rates and the degree of elongation response we observed differs slightly from those that Dijkwel and colleagues (1997) reported for wild-type plants harboring a transgenic plastocyanin-luciferase construct, these small quantitative discrepancies may be explained by differences in: (a) the transgene construct, which may bind to one or more limiting light-regulatory factors; (b) the ecotype background (C24) used in their studies; (c) the growth medium and Suc concentration used (germination medium [Valvekens et al., 1988] with or without 3% Suc); and/or (d) the light-source characteristics and sample size.
Other Metabolizable Sugars Substitute for Suc
Recently, efforts at isolating sugar sensory receptors and potential signaling pathway genes have been very fruitful. Among the most promising findings has been the discovery that Arabidopsis hexokinases fulfill metabolic and sensory functions within the plant (Jang et al., 1997). These hexokinases generally have a broad substrate specificity, and their regulatory functions are activated by sugars and sugar analogs that can be phosphorylated (see Koch, 1996). Monosaccharides such as Man and the Glc analog 2-dG can be phosphorylated by hexokinase and initiate sensory pathways but are not metabolized efficiently. This property makes them useful for separating specific hexokinase-mediated signaling from general carbohydrate status effects. Although numerous metabolizable sugars, including Glc, Fru, maltose, and raffinose, were nearly as effective as Suc in promoting the phyB-dominant negative effect, neither Man nor 2-dG elicited the response (Fig. 5 and T.W. Short, unpublished observations). Therefore, it is unlikely that phyA and phyB interactions are modulated by the hexokinase-signaling pathway. Because comparable concentrations of mannitol and sorbitol, which are not easily transported into the plant and are not phosphorylated by hexokinase, were also insufficient to activate the ABO response, it is unlikely to be a reflection of general osmotic stress. This conclusion is also consistent with the apparent specificity of the dominant negative effect. Therefore, the most likely mechanism of action in this response is either that phytochrome interactions respond to the general carbohydrate or energy availability within the plant, or that one or more additional sensory receptors detect and respond to sugars and have specificities distinct from those of hexokinase. Although the current experiments cannot distinguish between these possibilities, evidence from the sun mutants (Dijkwel et al., 1997) and transgenic sense and antisense expressors of hexokinases (Jang et al., 1997) indicate that more than one pathway exists for sugar signaling at the receptor and downstream transduction steps.
Mechanisms of phyB-Dominant Negative Inhibition of phyA Signaling
There are many possible explanations for the dominant negative regulation of phyA pathways by phyB. First, phyB may down-regulate expression or otherwise reduce accumulation of phyA in plant cells. Although phyA has been shown to be present at normal concentrations in 7-d-old etiolated tissue of ABO (Wagner et al., 1996b), its dark accumulation level and its FR-light-mediated degradation kinetics have not, to our knowledge, been reported previously for younger tissue. In the present study we showed that, within the limits of quantitation by western blotting, the rate of phyA degradation in ABO was indistinguishable from that of wild-type plants under conditions similar to those in which the dominant negative response was apparent (Fig. 6). These results, supported by previous studies showing comparable phyA levels and photoreversibility under somewhat different growth conditions, indicate that phyB probably does not repress phyA production or abundance but, rather, acts by an alternative mechanism.
Second, the findings shown in Figure 6 imply that excess phyB does not simply sequester limiting amounts of chromophore. Because degradation of phyA is dependent on photoconversion to the Pfr form of the molecule (Vierstra and Quail, 1986; Clough and Vierstra, 1997), comparable kinetics of phyA turnover between ABO and wild-type seedlings suggest that the same proportion of PHYA apoprotein molecules binds chromophore in both lines (Xu et al., 1995). Also consistent with this result, a mutant ABO with very high levels of photoconvertible phyB but lacking dominant negative interference of phyA was described by Wagner and Quail (1995), which shows that binding of chromophore is unlikely to be the mechanism of interference. Expressed deletion constructs lacking portions of either the COOH-terminal domain (Boylan et al., 1994; Cherry and Vierstra, 1994; Wagner et al., 1996b) or the NH2-terminal domain (including some lacking the entire chromophore-binding region; Wagner et al., 1996b) have been found to exhibit a similar dominant negative interference of phyA. Therefore, either a short region common to NH2-terminal and COOH-terminal deletion constructs is responsible for the dominant negative response, or there are active regions at each end of the molecule, either of which can give the dominant negative phenotype independently.
Wagner and colleagues (1996b) did not find that the Cys-356-Ser transgenics caused a dominant negative response. However, closer examination of the response at different light intensities indicates that the Cys-356-Ser line does exhibit the phenotype (Fig. 7). At lower fluence rates, similar to those used in the previous study (6 μmol m−2 s−1; Wagner et al., 1996b), there was essentially no dominant negative effect, but at higher fluence rates the Cys-356-Ser effect equaled that of ABO. Together, these data provide evidence that sequestration of chromophore by ectopic phyB expression is not the primary cause of the ABO FR-light phenotype.
The above results also suggest a third possibility for explaining the dominant negative interaction: increased phyB (by virtue of the 18- to 30-fold increase in ABO lines; Wagner et al., 1991) may allow FR-light activation of phyB pathways that actively inhibit phyA signaling. Because the photochemically inactive phyB construct—as well as numerous deletion constructs—is able to elicit the response, a photoconvertible product is apparently not requisite, so the effect is likely passive rather than active in nature.
There are at least two remaining possibilities that represent the most likely candidates for the observed dominant negative interference phenotype. Overexpressed phyB may form nonproductive heterodimers with native phyA protein, effectively reducing the pool of active phyA, or excess phyB may compete for a common interaction partner, preventing active phyA homodimers from initiating signaling events. Although the data presented in this paper cannot differentiate between these possibilities, there is considerable evidence supporting the second mechanism over the first. Native gel electrophoresis indicates that several of the dominant negative constructs are monomeric (Wagner et al., 1996b). Mutant analysis (Quail et al., 1995; Xu et al., 1995; Wagner et al., 1996b) and domain-swapping experiments (Wagner et al., 1996a) have suggested a common signaling mechanism for at least a subset of phyA and phyB responses. Furthermore, recent results from two hybrid screens have yielded a gene designated PIF3, whose product binds to COOH-terminal domains of phyA and phyB (Quail, 1998).
If the latter mechanism of dominant negative interference is accurate, it is not clear how metabolically active sugars are involved in this interaction. Among the simplest of the possibilities is that Suc status affects the relative preference of the phyA/phyB-signaling partner for one phytochrome versus another by modifying either phytochrome or the reaction partner. Alternatively, carbohydrates could down-regulate the overall abundance or binding efficiency of this putative reaction partner, such that the chance of a productive phyA interaction with the signaling pathway eventually falls below a threshold for activity. The coregulation of hypocotyl growth by phytochrome and sugars may be particularly important for modulating relative growth responsiveness to phyA and phyB in the context of available storage carbohydrates in the seed and during the initiation of photosynthesis.
In the presence of sufficient metabolizable sugars at low ratios of red to FR light, there may be an advantage to the plant in overriding the normal inhibition of growth (via phyA) and switching rapidly to the shade-avoidance response mediated through phyB to outcompete neighboring seedlings. Under lower carbohydrate conditions, the seedling requires use of existing sugars to produce maximal photosynthetically active surface area and therefore could be at a disadvantage if energy were expended in increased elongation.
ACKNOWLEDGMENTS
The author wishes to thank Michael Lin, Peter Altman, and Ana Maria Estela for their expert technical assistance in these experiments. Special thanks to Dr. Peter Quail for the generous gift of antibodies and to Drs. Doris Wagner and Peter Quail for transgenic seeds.
Abbreviations:
- 2-dG
2-deoxyglucose
- ABO
Arabidopsis phyB-overexpressing
- FR
far-red
- LED
light-emitting diode
- MS
Murashige and Skoog
- phyX
phytochrome X, where X is any letter
Footnotes
This work was supported in part by the National Science Foundation (grant nos. IBN 9421770 and IBN 9734527) and by grants from The City University of New York Professional Staff Congress-City University of New York Research Award Program.
LITERATURE CITED
- Aukerman MJ, Hirschfeld M, Wester L, Weaver M, Clack T, Amasino RM, Sharrock RA. A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. Plant Cell. 1997;9:1317–1326. doi: 10.1105/tpc.9.8.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Nishizawa NK, Quaggio RB, Whitelam GC, Chua N-H. Far-red light blocks greening of Arabidopsis seedlings via a phytochrome A-mediated change in plastid development. Plant Cell. 1996;8:601–615. doi: 10.1105/tpc.8.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batschauer A, Ehmann B, Schafer E. Cloning and characterization of a chalcone synthase gene from mustard and its light-dependent expression. Plant Mol Biol. 1991;16:175–185. doi: 10.1007/BF00020550. [DOI] [PubMed] [Google Scholar]
- Boylan MT, Douglas N, Quail PH. Dominant negative suppression of Arabidopsis photoresponses by mutant phytochrome A sequences identifies spatially discrete regulatory domains in the photoreceptor. Plant Cell. 1994;6:449–460. doi: 10.1105/tpc.6.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan MT, Quail PH. Oat phytochrome is biologically active in transgenic tomatoes. Plant Cell. 1989;1:765–773. doi: 10.1105/tpc.1.8.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan MT, Quail PH. Phytochrome A overexpression inhibits hypocotyl elongation in transgenic Arabidopsis. Proc Natl Acad Sci USA. 1991;88:10806–10810. doi: 10.1073/pnas.88.23.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C-L, Acedo GN, Cristinsin M, Conkling MA. Sucrose mimics the light induction of Arabidopsis nitrate reductase gene transcription. Proc Natl Acad Sci USA. 1992;89:1861–1864. doi: 10.1073/pnas.89.5.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JR, Hondred D, Walker JM, Keller JM, Hershey HP, Vierstra RD. Carboxy-terminal deletion analysis of oat phytochrome A reveals the presence of separate domains required for structure and biological activity. Plant Cell. 1993;5:565–575. doi: 10.1105/tpc.5.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JR, Vierstra RD. The use of transgenic plants to examine phytochrome structure/function. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants, Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 271–297. [Google Scholar]
- Clack T, Mathews S, Sharrock RA. Plant Mol Biol. 1994;25:413–427. doi: 10.1007/BF00043870. [DOI] [PubMed] [Google Scholar]
- Clough RC, Vierstra RD. Phytochrome degradation. Plant Cell Environ. 1997;20:713–721. [Google Scholar]
- Dehesh K, Franci C, Parks BM, Seeley KA, Short TW, Tepperman JM, Quail PH. Arabidopsis HY8 locus encodes phytochrome A. Plant Cell. 1993;5:1081–1088. doi: 10.1105/tpc.5.9.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkwel PP, Huijser C, Weisbeek PJ, Chua NH, Smeekens SCM. Sucrose control of phytochrome A signaling in Arabidopsis. Plant Cell. 1997;9:583–595. doi: 10.1105/tpc.9.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkwel PP, Kock PAM, Bezemer R, Weisbeek PJ, Smeekens SCM. Sucrose represses the developmentally controlled transient activation of the plastocyanin gene in Arabidopsis thaliana seedlings. Plant Physiol. 1996;110:455–463. doi: 10.1104/pp.110.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton MD, Jones AM. Localization of protein-protein interactions between subunits of phytochrome. Plant Cell. 1992;4:161–171. doi: 10.1105/tpc.4.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M. Phytochromes: their molecular species, gene families, and functions. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:617–645. [Google Scholar]
- Furuya M, Song P-S. Assembly and properties of holophytochrome. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants, Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 105–140. [Google Scholar]
- Harter K, Talke-Messerer C, Barz W, Schaefer E. Light- and sucrose-dependent gene expression in photomixotrophic cell suspension cultures and protoplasts of rape (Brassica napus L.) Plant J. 1993;4:507–516. [Google Scholar]
- Hirschfeld M, Tepperman JM, Clack T, Quail PH, Sharrock RA. Coordination of phytochrome levels in phyB mutants of Arabidopsis as revealed by apoprotein-specific monoclonal antibodies. Genetics. 1998;149:523–535. doi: 10.1093/genetics/149.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J-C, León P, Zhou L, Sheen J. Hexokinase as a sugar sensor in higher plants. Plant Cell. 1997;9:5–19. doi: 10.1105/tpc.9.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Madore MM, Lucas WL (1995) Carbon partitioning and source sink interactions in plants. In Current Topics in Plant Physiology, Vol 13. American Society of Plant Physiologists, Rockville, MD
- Mancinelli AL. The photoregulation of anthocyanin synthesis. In: Shropshire WJ, Mohr H, editors. Encyclopedia of Plant Physiology. New York: Springer-Verlag; 1983. pp. 640–661. [Google Scholar]
- Mancinelli AL. The physiology of phytochrome action. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants, Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 211–269. [Google Scholar]
- McCormac AC, Wagner D, Boylan MT, Quail PH, Smith H. Photoresponses of transgenic Arabidopsis seedlings expressing introduced phytochrome-B encoding cDNAs: evidence that phytochrome A and phytochrome B have distinct photoregulatory functions. Plant J. 1993;4:19–27. [Google Scholar]
- Mohr H. Coaction between pigment systems. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants, Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 353–373. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–479. [Google Scholar]
- Nagatani A, Chory J, Furuya M. Phytochrome B is not detectable in the hy3 mutant of Arabidopsis, which is deficient in responding to end-of-day far-red light treatments. Plant Cell Physiol. 1991;32:1119–1122. [Google Scholar]
- Nagatani A, Reed JW, Chory J. Isolation and characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 1993;102:269–277. doi: 10.1104/pp.102.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Quail PH. Hy8, a new class of Arabidopsis long hypocotyl mutants deficient in functional phytochrome A. Plant Cell. 1993;5:39–48. doi: 10.1105/tpc.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pontis HG, Salerno GL, Echeverria EJ (1995) Sucrose metabolism, biochemistry, physiology, and molecular biology. In Current Topics in Plant Physiology, Vol 14. American Society of Plant Physiologists, Rockville, MD
- Qin M, Kuhn R, Moran S, Quail PH. Overexpressed phytochrome C has similar photosensory specificity to phytochrome B but a distinctive capacity to enhance primary leaf expansion. Plant J. 1997;12:1163–1172. doi: 10.1046/j.1365-313x.1997.12051163.x. [DOI] [PubMed] [Google Scholar]
- Quail PH. Phytochrome: a light-activated molecular switch that regulates plant gene expression. Annu Rev Genet. 1991;25:389–409. doi: 10.1146/annurev.ge.25.120191.002133. [DOI] [PubMed] [Google Scholar]
- Quail PH (1998) Photosensory perception and signal transduction by the phytochromes (abstract no. 11). 9th International Conference on Arabidopsis Research, June 24–28, Madison, WI
- Quail PH, Boylan MT, Parks BM, Short TW, Xu Y, Wagner D. Phytochromes: photosensory perception and signal transduction. Science. 1995;268:675–680. doi: 10.1126/science.7732376. [DOI] [PubMed] [Google Scholar]
- Quail PH, Parks BM, Short TW (1996) The phytochrome family: approaches to dissecting photosensory specificity and regulatory activity. In WR Briggs, RL Heath, EM Tobin, eds, Regulation of Plant Growth and Development by Light, Vol 17. American Society of Plant Physiologists, Rockville, MD, pp 42–56
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadka A, DeWald DB, May GD, Park WD, Mullet JE. Phosphate modulates transcription of soybean VspB and other sugar-inducible genes. Plant Cell. 1994;6:737–749. doi: 10.1105/tpc.6.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock RA, Quail PH. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989;3:1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- Short TW, Briggs WR. The transduction of blue light signals in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:143–171. [Google Scholar]
- Short TW, Porst M, Briggs WR. A photoreceptor system regulating in vivo and in vitro phosphorylation of a pea plasma membrane protein. Photochem Photobiol. 1992;55:773–781. [Google Scholar]
- Smith H, Whitelam GC. The shade-avoidance syndrome: multiple responses mediated by multiple phytochromes. Plant Cell Environ. 1997;20:840–844. [Google Scholar]
- Somers DE, Sharrock RA, Tepperman JM, Quail PH. The hy3 long hypocotyl mutant of Arabidopsis is deficient in phytochrome B. Plant Cell. 1991;3:1263–1274. doi: 10.1105/tpc.3.12.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi WB, Cashmore AR. Light-regulated transcription. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:445–474. [Google Scholar]
- Tsukaya H, Oshima T, Naito S, Chino M, Komeda Y. Sugar-dependent expression of the CHS-A gene for chalcone synthase from petunia in transgenic Arabidopsis. Plant Physiol. 1991;97:1414–1421. doi: 10.1104/pp.97.4.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvekens D, Van Montagu M, Van Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci USA. 1988;85:5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra RD, Quail PH. Phytochrome: the protein. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants. Dordrecht, The Netherlands: Martinus Nijhoff; 1986. pp. 35–60. [Google Scholar]
- Wagner D, Fairchild CD, Kuhn RM, Quail PH. Chromophore-bearing NH2-terminal domains of phytochromes A and B determine their photosensory specificity and differential light lability. Proc Natl Acad Sci USA. 1996a;93:4011–4015. doi: 10.1073/pnas.93.9.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, Koloszvari M, Quail PH. Two small spatially distinct regions of phytochrome B are required for efficient signaling rates. Plant Cell. 1996b;8:859–871. doi: 10.1105/tpc.8.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, Quail PH. Mutational analysis of phytochrome B identifies a small COOH terminal-domain region critical for regulatory activity. Proc Natl Acad Sci USA. 1995;92:8596–8600. doi: 10.1073/pnas.92.19.8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, Tepperman JM, Quail PH. Overexpression of phytochrome B induces a short hypocotyl phenotype in transgenic Arabidopsis. Plant Cell. 1991;3:1275–1288. doi: 10.1105/tpc.3.12.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam GC, Harberd NP. Action and function of phytochrome family members revealed through the study of mutant and transgenic plants. Plant Cell Environ. 1994;17:615–625. [Google Scholar]
- Whitelam GC, Johnson E, Peng J, Carol P, Anderson ML, Cowl JS, Harberd NP. Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell. 1993;5:757–768. doi: 10.1105/tpc.5.7.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Parks BM, Short TW, Quail PH. Missense mutations define a restricted segment in the COOH-terminal domain of phytochrome A critical to its regulatory activity. Plant Cell. 1995;7:1433–1443. doi: 10.1105/tpc.7.9.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]