Abstract
The ability to distinguish actions and effects caused by oneself from events occurring in the external environment is a fundamental aspect of human cognition. Underlying such distinctions, self-monitoring processes are often assumed, in which predicted events accompanied by one's own volitional action are compared with actual events observed in the external environment. Although many studies have examined the absence or presence of a certain type of self-recognition (i.e. mirror self-recognition) in non-human animals, the underlying cognitive mechanisms remain unclear. Here, we provide, to our knowledge, the first behavioural evidence that chimpanzees can perform self/other distinction for external events on the basis of self-monitoring processes. Three chimpanzees were presented with two cursors on a computer display. One cursor was manipulated by a chimpanzee using a trackball, while the other displayed motion that had been produced previously by the same chimpanzee. Chimpanzees successfully identified which cursor they were able to control. A follow-up experiment revealed that their performance could not be explained by simple associative responses. A further experiment with one chimpanzee showed that the monitoring process occurred in both temporal and spatial dimensions. These findings indicate that chimpanzees and humans share the fundamental cognitive processes underlying the sense of being an independent agent.
Keywords: agency, chimpanzee, self recognition, voluntary action, self-monitoring
1. Introduction
The concept of self is one of the most intriguing aspects of the human mind. However, there is currently little knowledge concerning the evolutionary origin of the sense of self [1]. Researchers have paid considerable attention to mirror self-recognition (MSR) in non-human animals [2]. The typical evidence for MSR is that an animal is capable of using its image reflected in a mirror to wipe off marks painted on its body that would not have been visible to the animal without the mirror (‘mirror mark test’). Multiple studies have reported MSR capabilities in great apes such as chimpanzees [2–4], but negative findings and controversies regarding this ability have emerged in studies of other taxa [5–12]. Although MSR reflects a certain level of self-recognition and is important in understanding the evolutionary origin of self, the underlying cognitive mechanisms of this phenomenon remain controversial [13–17].
Importantly, it is currently unclear what aspects of cognitive processing humans and apes share in terms of sense of self. To elucidate this issue it may be helpful to consider recent advances in human cognitive science. In these human studies, the concept of self is typically divided into several components to explore the underlying cognitive mechanisms of each component [18,19]. Some studies have addressed self-recognition within the context of voluntary action. When humans act with volition, they report a subjective feeling of controlling or initiating the movement of their body parts and the effect of that movement on the external world [20–23]. This feeling is known as the sense of ‘self-agency’ [18], which facilitates the concept of the self as an independent agent who has an effect on the external environment. For example, in multi-player videogames in which each person has a controller, it can be confusing which character is being controlled on the screen. However, a person will typically come to gradually recognize which character they are controlling once the game has started, accompanied by the subjective feeling ‘I am controlling that’. This feeling constitutes the sense of self-agency.
Possibly underlying this subjective experience, humans are thought to possess specific cognitive mechanisms enabling the distinction of events caused by oneself from externally generated events [20–23]. It has been postulated that such a distinction is accomplished by the monitoring of congruency between the prediction of the effect of one's own action and the actual feedback from the external environment. When performing voluntary action, the human mind can produce predictions about what will happen in the environment as a result of their behaviour, before the results are actually perceived. Such predictions could be raised in several ways and recent studies have shown the important roles of prior intention [21,24] or motor commands [25]. At the moment the actual results are perceived, these predictions are compared with the actual outcome. If information from these two sources corresponds, the observed action will be regarded as caused by one's self, and self-agency will be subjectively perceived. Using the videogame example above, the self-agency will be experienced as the feeling that ‘I am controlling a character’, because the character's actions correspond exactly with one's own intentional action.
Interestingly, action may also play a critical role in establishing MSR. For example, a contingency behaviour in which animals repeatedly move their bodies in the same manner while looking at a mirror is typically observed before they exhibit self-directed behaviour, a sign of MSR [7]. It was often inferred that the animal might check to which degree the mirror image is coupled with its own action. In another example, Kitchen et al. showed chimpanzees recognize a mirror self-image even when it is distorted by concave/convex mirrors [26]. This study indicates the importance of the action rather than a clear visual image of the animal itself. Thus, it could be argued that subjects attribute the mirror image to themselves because the action viewed in the mirror image corresponds with their intended actions, leading to the perception of agency.
However, the interpretation of MSR remains controversial, with no broad consensus. Gallup [13] suggests that MSR abilities imply the potential to imagine oneself as an entity that can be viewed by others, an ability related to theory of mind. On the other hand, Heyes [15] argued that demonstrations of MSR are no different from showing that an animal can learn a novel association between a particular response and a particular outcome. In a subsequent study, Povinelli et al. [27] claimed that passing the normal mark test may reflect subjects' experience of the sense of self-agency. They showed that 2–3 year-old human infants passed a normal mark test but not the delayed mark test in which infants viewed video images of themselves recorded 3 min previously, meaning there is no concurrent correspondence between their action and the video. By contrast, 3–4 years olds passed both types of task. However, Suddendorf [28] claims that such developmental asynchrony regarding live and delayed conditions is also found in infants' ability to find hidden objects, not only marks on their own body. Thus, he argues that the task is not relevant for discussing the developmental shift of self-recognition, reflecting more general cognitive abilities. As mentioned above, it remains controversial what information subjects in this task actually exploit from mirror/video images, and what cognitive processes are involved in these behaviours.
This study sought to directly test whether chimpanzees are able to experience the sense of self-agency through the proper self/other distinction for the origin of action or event. To this end, we tested whether chimpanzees could differentiate events caused by themselves from externally caused events under a condition where it is confusing which events were caused by the self, but could be identified on the basis of congruency between their own intended results of action and the observed results. The experimental paradigm was essentially the same as that used by Daprati et al. [29], modified for use with chimpanzees. Chimpanzees were trained to move a cursor on a computer monitor using a trackball (functionally equivalent to a computer mouse). We presented two cursors on the monitor: a self-controlled cursor (‘self-cursor’) and a ‘distractor’ cursor. The chimpanzees could control the self-cursor using the trackball. By contrast, the distractor cursor was moved by predefined computer algorithms. The cursors were identical in shape and colour.
A pioneering study by Jorgensen et al. [30] used a similar experimental paradigm with chimpanzees and capuchin monkeys, reporting that both species could successfully direct the self-cursor to the target using the joystick before the computer cursor reached it. This finding indicates that the subject could somehow differentiate the self-cursor from the computer cursor. However, their study could not determine whether the discrimination was based on self-monitoring or simple visual discrimination using a particular visual cue. For example, the clumsier (or smoother) movement of the computer cursor might provide a valuable cue for recognizing the cursor to which they should respond.
Thus, in the current study, we used recorded action that was performed by the subject in the past as the action of the distractor cursor. In this situation, it is expected that simple visual discrimination cannot solve the task, because the physical movement properties of cursor actions should be comparable. In other words, a third observer passively watching the experiment would have difficulty identifying which cursor the subject was controlling. However, the subject who actually controlled the cursor should be able to recognize the self-cursor on the basis of congruency between their intended action and the observed action.
Experiment 1 investigated whether chimpanzees were capable of discriminating a self-cursor from a distractor cursor when the visual properties of the two cursor movements were comparable. In experiment 2, we tested whether the performance observed in experiment 1 involved a self-monitoring process, or whether it could be explained by simple associative responses based on particular visual cues. In experiment 3, we further addressed the role of spatio-temporal factors in the perception of self-agency by introducing temporal delay and distortion in spatial mapping. These factors are known to have a crucial impact on the performance of self-agency judgments in humans [31].
2. General methods
(a). Participants
Three female adult chimpanzees (Pan troglodytes; Ai, Chloe, and Pendesa; mean age, 28.8 years; s.d. = 3.1) living in a social group of 14 individuals at the Primate Research Institute, Kyoto University, Japan, took part in the study. Ai, Chloe and Pendesa took part in experiments 1 and 2. Chloe took part in experiment 3. All animals had previously taken part in various perceptual/cognitive tasks [32,33].
(b). Apparatus
The chimpanzees were tested in an experimental booth (160 × 180 × 210 cm) with acrylic panel walls. A 17 inch liquid crystal display (LCD) touch-panel monitor (Gunze AV10226N02W, 1280 × 1024 resolution, 60 Hz refresh rate) was used for stimulus presentation. A trackball (Sanwa H55-0300-SET) 12 cm in diameter was attached 34 cm below the monitor. The distance between the monitor and the chimpanzees was approximately 37 cm so that the visual angle between the centre of monitor and the trackball was approximately 49°. This configuration prevented the chimpanzees from viewing the monitor and the trackball simultaneously. Small fruit rewards (8 mm pieces of apple or 5 mm raisins) were delivered using a universal feeder (Biomedica, BUF-310), which was connected to a Windows-based computer.
3. Experiment 1
(a). Procedure
(i). Task
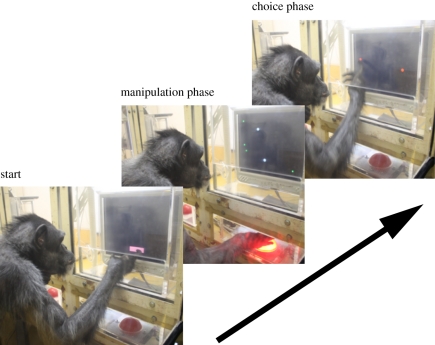
A variant of the two-choice simultaneous discrimination task was used (figure 1; see also the electronic supplementary material, movie S1). The trial consisted of manipulation and choice phases. Each trial was initiated when the chimpanzees touched a warning stimulus located at the bottom centre of the monitor. This was followed by the manipulation phase, in which two identical cursors (white-filled circles, 12 mm in diameter) and three targets (green-filled rectangles, 6.3 × 6.3 mm) were presented. The initial positions of the cursors and targets differed across trials and were pseudo-randomly distributed in a 3 × 2 matrix with a cell size of 84 × 90 mm. One of the cursors (self-cursor) could be controlled by the chimpanzee using the trackball. The other cursor was the distractor, controlled by the predefined computer algorithm. When the chimpanzee hits one of the three targets using the self-cursor, or when a certain duration had passed regardless of the manipulation by the chimpanzees (see below for details), the manipulation was terminated and the choice phase began. During the choice phase, all targets disappeared, and the two cursors turned red at their final locations at the end of the manipulation phase. At this moment, both cursors could not be moved and the chimpanzee was required to touch one of the cursors on the touch-sensitive monitor. If the chimpanzee touched the self-cursor, a chime sounded, and a food reward was delivered (correct choice). If the chimpanzee touched the distractor cursor, a buzzer sounded, and a 3 s time-out was added to the usual intertrial interval. When the two cursors collided during the manipulation phase, the trial was terminated, and was followed by the buzzer and a 3 s time-out. This prevented the possibility of the cursors overlapping in the choice phase, which would make it difficult to tell which cursor the chimpanzees had touched.
Figure 1.
Procedure of the cursor discrimination task. One chimpanzee (Pendesa) is shown performing the task. Each trial was initiated by touching the warning stimulus on a touch-sensitive LCD display. This was followed by the manipulation phase, in which the chimpanzee could move one of the cursors using a trackball attached below the monitor. The other distractor cursor was moved by a computer algorithm. During the test trials, the manipulation phase ended after a pre-defined duration had passed, followed by a choice phase in which the chimpanzees were required to touch the cursor they had previously been able to control.
(ii). Pretraining
The chimpanzees were already experienced in manipulating the trackball (see the electronic supplementary material, figure S1), and had prior opportunities to perceive self-agency when manipulating the cursor using the devices implemented here. Prior to the test sessions, the chimpanzees were trained on the general task procedure (i.e. touching the warning stimulus, manipulating the trackball and touching one of the cursors). In particular, the use of the trackball was a novel task, so we shaped this behaviour using the successive approximation technique. During pretraining, a regular action was used as the action of the distractor cursor. The distractor cursor moved 105 mm along either a horizontal or a vertical axis at a velocity of 65 mm s−1. Once performance exceeded 80 per cent correct for three consecutive 48-trial sessions, chimpanzees were shifted to the test trials. Chimpanzees performed an average of 22.3 pretraining sessions. Several simple visual cues could potentially be used for discrimination in pretraining, since we used a regular distractor action. The choice of criteria used to determine the correct cursor was made by the chimpanzee. That is, they could solve the pretraining task either using simple visual discrimination (for example, choosing the cursor exhibiting irregular action rather than ordinal regular action), or agency discrimination (choosing the cursor under their control).
(iii). Test sessions
Three types of conditions were used in the manipulation phase in the test sessions: hit trials, fixed-duration trials and manipulation–hit trials. For the hit trials, the manipulation phase finished when the self-cursor hits either of the targets. During these trials, the self-cursor was always at a location where the target was shown, whereas the distractor was not close to the target locations. In this condition, the chimpanzee could choose the correct cursor by remembering the target position, even if the self-cursor accidentally hits the target and the chimpanzee was not actually able to recognize which was the self-cursor. To eliminate this possibility, fixed-duration trials were introduced, and, in these trials, the cursor could not reach the target. If the cursor moved closer than 26 mm from the target, the target disappeared and reappeared in a different position. The replacement of the targets occurred equally for the self-cursor and the distractor. Thus, the minimum distance to the target from the cursor and the distractor was equivalent. For this trial type, the manipulation phase ended after a predetermined duration (2000 ms) regardless of the action of the cursors. If only these two types of manipulation phases had been used, the chimpanzee might have learned that the manipulation was not necessary, given that targets were unreachable; therefore, they would have stopped manipulating the trackball. To prevent this possibility, manipulation–hit trials were introduced; in these trials, the target was unreachable until the chimpanzee manipulated the cursor at a predefined distance (106–370 mm) in any direction, at which point, the target became reachable. These three types of manipulation phase were conducted in a pseudo-random order. Note that the fixed-duration trials were the important test trials, in which the target position was not available as a cue for the choice. The other trial types were regarded as ‘catch trials’ to maintain the chimpanzee's manipulation of the trackball.
During each of the test trials, a recording of a previous self-cursor motion was used as the distractor trajectory. That is, the distractor paths reflected movements performed more than 1 day earlier by the same chimpanzee on trials that lasted more than 2000 ms and had been completed successfully. Each of these paths was used only once. Hence, the distractor trajectory was unique for each trial.
The chimpanzee received no feedback (i.e. rewards or sounds) for the fixed-duration test trials. During the other trials, in which differential reinforcement was applied, regular motion was used, such as repeated and ordinal motion from one position to the other, as in pretraining. The vertical or horizontal axis of the distractor trajectory was counterbalanced across trials. Each test session consisted of seven fixed-duration trials (test trials), 21 hit trials and 21 manipulation–hit trials. Each chimpanzee completed five sessions for a total of 35 test trials. The ratio of test trial types was arbitrarily defined at the level where chimpanzees did not stop the task owing to the absence of reward.
(b). Results and discussion
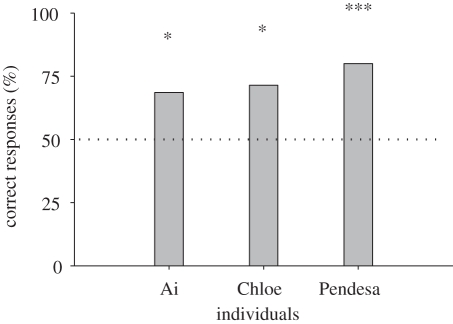
The chimpanzees showed a 93.7 per cent correct rate for hit trials and a 93.7 per cent correct rate for manipulation–hit trials. The accuracy rate of the probe test trials (fixed-duration trials) was 73.3 per cent averaged across subjects (figure 2). Individual analyses (binomial tests, two-tailed, α = 0.05) revealed that the performance of all three chimpanzees was significantly better than chance (24/35, p = 0.041 for Ai; 25/35, p = 0.017 for Chloe; 28/35, p < 0.001 for Pendesa). These results indicate that the chimpanzees were able to distinguish the cursor actions controlled by themselves from those caused by other factors, even when the physical properties of those actions were almost identical. Because both the self- and distractor cursor movements were produced by the same individual, the movements were presumably indistinguishable to a third person (and to the experimenters), who passively observed the display. Thus, successful generalization to this test condition indicates that the congruence between internally generated predictions and the observed external outcomes is fundamental to recognition of the self.
Figure 2.
Performance on the probe test using past actions that were performed by themselves. Percentages of correct responses (35 trials per individual) compared with the expected value if the response had been random (50%, dashed line). Asterisks indicate significance at *p < 0.05 and ***p < .001 on a binomial test (two-tailed, n = 35).
However, it could still be argued that other external discriminative cues were present, and that these cues, rather than perceived agency, were responsible for the chimpanzees' behaviour. For example, if the chimpanzees manipulated the trackball to the bottom of the screen during each trial, such a biased pattern of responses would produce a simple associative response to touch the cursor located at the bottom of the screen, regardless of whether they recognized that they had controlled the cursor. Thus, experiment 2 was designed to rule out the possibility of an associative response to a particular external cue.
4. Experiment 2
Experiment 2 aimed to clarify whether the chimpanzee's performance observed in experiment 1 was owing to a self-agency judgement, or to mere visual discrimination. This experiment involved online and offline conditions. The online condition was identical to the fixed-duration trial in the previous experiment, whereas in the offline condition, the chimpanzee was presented with cursor movements that were identical to those in an online condition performed in the past. That is, in an offline trial, one of the cursors replayed the motion of the previous self-cursor controlled by the chimpanzee in a previous online trial, and the other cursor replayed the motion of the distractor in the same online trial. Thus, manipulating the trackball in the offline condition had no effect on the display. The target locations were also the same as those in the online trial. Therefore, all events in the offline condition were exactly the same as those in an original single trial of the online condition, but the chimpanzee could control neither of the cursors. The online trials used for the offline test were randomly chosen from the previous online tests performed more than 1 day before. If the chimpanzee's behaviour was based on simple associative responses to particular visual properties shared by actions of the correct cursor (or a common feature of the distractor cursor), they should still choose the correct cursor (which was formerly the self-cursor) over the other (which was formerly the distractor). However, if the behaviour was governed by the recognition that they were controlling the cursor, performance in the offline condition should be significantly lower than that in the online condition.
(a). Methods
(i). Task
The experimental task was identical to that in experiment 1, except for the following three features. First, the possible locations and the numbers of targets were increased to maintain variability in the chimpanzee's manipulation patterns. Each target and cursor was randomly distributed on a 9 × 7 square matrix with a cell size of 34 × 34 mm. Two to nine targets were shown simultaneously, and their number and distribution were pseudo-randomly determined according to the same algorithm throughout the experiment. Second, recorded past motion was used as the distractor in all trial types instead of computer-defined regular actions, and the chimpanzees were reinforced for all trials. These changes reduced the available cues, meaning that the chimpanzees had to rely more on self-agency than mere visual cues, except in the offline condition. Thus, this change was designed to maximize the contrast between the online and offline conditions. Each distractor motion was unique to the trial, and was chosen from a previous trial of the same type (for example, the distractor action of a hit trial was derived from a recorded self-action in a past hit trial). Third, the duration of the fixed-duration trial was adjusted for each individual to ensure stable performance (3700 ms for Chloe and Pendesa, 5400 ms for Ai.).
(ii). Pretraining
The chimpanzees received pretraining sessions before the test sessions to ensure consistent performance. The pretraining session consisted of 48 trials including 16 fixed-duration trials, 16 hit trials and 16 manipulation–hit trials. The chimpanzees were reinforced for all types of trials. The duration of the manipulation phase was gradually increased, and this preliminary training continued until the percentage of correct responses in the fixed-duration trials in each session exceeded 80 per cent for three successive sessions. Chimpanzees performed an average of 16.7 pretraining sessions.
(iii). Test design
Each test session consisted of 54 trials, including six online tests, six offline tests, 12 hit trials, 18 fixed-duration trials and 12 manipulation–hit trials. The ratio of trial type was different from the pretraining, owing to the introduction of the offline condition, which led the chimpanzees to use cues rather than self-agency. As such, we increased the fixed-duration trials, in which chimpanzees would be expected to rely more on recognizing that they were controlling the cursor. Note that the online test trials were identical to the fixed-duration trials, but were predetermined as online test trials. In all trials, the chimpanzees received differential reinforcement. In the offline condition, if a chimpanzee touched a cursor that had been correct in an original online condition, it was regarded as a correct response. Each chimpanzee completed a total of 36 sessions. The first four sessions did not include an offline test because no previous material was available to replay. To balance the total number of trials, the last four sessions did not include an online test. Therefore, we obtained data from 32 sessions for each individual.
(b). Results and discussion
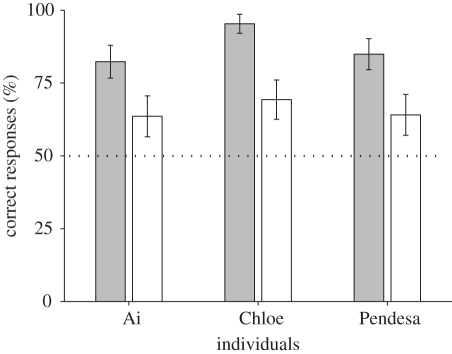
During the test sessions, the chimpanzees exhibited 87.9 per cent correct for fixed-duration trials, 99.2 per cent for hit trials and 99.1 per cent correct for manipulation–hit trials, averaged across participants. Figure 3 shows the results of the online and offline test trials for each chimpanzee. As shown in this figure, the chimpanzees exhibited better performance in the online compared with the offline trials (χ2-test, α = 0.05; χ21 = 16.2, n = 384, p < 0.001 for Ai; χ21 = 42.9, n = 384, p < 0.001 for Chloe; χ21 = 20.8, n = 384, p < 0.001 for Pendesa). The offline and the online tests were visually identical, so if the chimpanzees based their responses on a particular feature of the correct cursor, the performance under the offline condition should not differ from that under the online condition. The observation that the chimpanzees performed better in the online than the offline condition strongly suggests that they relied on information that was available when they were actually controlling the cursor.
Figure 3.
Mean performance under the online and offline conditions. Percentages of correct responses in the online test, in which chimpanzees could move one of the cursors, and in the offline test, in which both cursors were replays of recorded motion from a previous online test. Each bar shows the mean of all trials. The dashed line represents the expected chance level. Error bars show 95% confidence intervals. Grey bars, online; white bars, offline.
However, it should be noted that all three chimpanzees performed significantly better than chance even in the offline condition (65.6% correct on average, binomial test, α = 0.05; 122/192, p < 0.001 for Ai; 133/192, p < 0.001 for Chloe; 123/192, p < 0.001 for Pendesa), suggesting that potential cues other than self-agency might be available for the task, and that their performance was based on these cues to some extent.
There are three possible candidates as cues for visual discrimination. One possible cue is a position bias, as mentioned in the discussion of experiment 1. Another possible cue is the difference in ‘goal-directedness’ of each cursor (i.e. how efficiently the cursor approaches the targets). In the online condition, the chimpanzees attempted to hit the target once they detected the self-cursor, meaning that the actions of the self-cursor tended to be directed towards the target. On the other hand, the movements of the distractor were independent of the current location of the target because the location of the target was not a replication of the trial in which the distractor action was recorded. Thus, there was a visual difference in the two cursor actions in terms of how efficiently the cursor was directed towards the targets. The offline conditions were an exact replay of an online trial, including the cursor actions and target locations, meaning that a difference in goal-directedness was still present. Chimpanzees are known to attribute another's action to the apparent goals of an action [34]. In addition, it has been reported in human studies that the subjective feeling of causing an action to occur increases when task performance is experimentally manipulated so that it appears to achieve the task goal more efficiently than was actually the case [35]. Therefore, the difference in ‘goal-directedness’ of the cursor actions might bias the choice of chimpanzees in the offline condition. Another possibility arises from the termination of trials when the two cursors collided to prevent the cursors overlapping at the choice phase, which would have made it difficult to determine which cursor the chimpanzees were trying to touch. However, once chimpanzees identified the correct cursor and attended to it during the manipulation phase, they attempted to keep the correct cursor away from the distractor to avoid the termination of the trial. This motion pattern was also a possible visual cue for distinguishing the correct cursor. In future studies, it would be interesting to manipulate the goal-directedness of the distractor, and to test how this manipulation may affect the agency discrimination in chimpanzees.
The offline condition was the most critical control condition for determining whether chimpanzees responded to particular external information properties or based their choice on judgments of self-agency. Although their above-chance performance under the offline condition indicates that other factors also contributed to their discrimination performance, the chimpanzees' better performance under the online condition suggests that they used judgments of self-agency as a discriminative cue. In the following experiment, we further explored which stimulus properties affected the chimpanzees' performance.
5. Experiment 3
To further explore the strategy used by the chimpanzees, we investigated the effect of experimentally induced temporal delay and/or spatial distortion in the relationship between the trackball manipulation and cursor motion. If the chimpanzees chose the cursor based on the congruence between the predicted cursor action and the actual cursor action, their performance would be expected to deteriorate under conditions of delay or distortion.
(a). Methods
(i). Design and procedure
One chimpanzee, Chloe, who showed the most robust and consistent performance in the previous experiments, participated. We used a 2 × 2 within-subject design. By combining two spatio-temporal factors (0 and 300 ms temporal delays and 0° and 135° of spatial rotational mapping), we prepared four test conditions. The baseline condition (0 ms delay and 0° distortion) kept the same contingency between chimpanzee's manipulation of the trackball and the output action of the self-cursor. In the delay condition, the cursor moved following a 300 ms delay after manipulation of the trackball. In the distortion condition, the direction of cursor motion was deviated 135° in a clockwise direction from the manipulation of the trackball. In the delay-distortion condition, the action of the self-cursor was delayed by 300 ms and distorted by 135°. Each test session consisted of 56 trials including 16 test trials (four trials per condition), 24 fixed-duration trials, eight hit trials and eight manipulation–hit trials. Only the cursor movements from fixed-duration trials or 0 ms × 0° test trials were used as distractors. Chloe completed 32 test sessions.
(b). Results and discussion
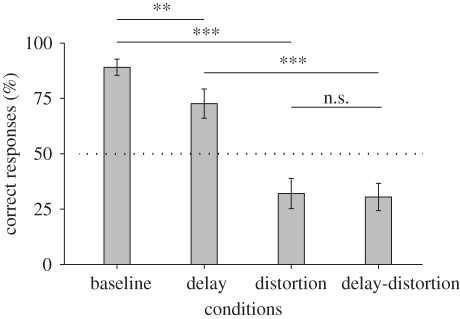
Chloe exhibited an 85.5 per cent correct response rate for fixed-duration trials, 94.6 per cent for hit trials and 96.2 per cent for manipulation–hit trials. Figure 4 shows Chloe's accuracy in each test condition. The results showed that either delay or distortion decreased the performance relative to that under the baseline condition. A χ2-test revealed significant differences between the four conditions (α = 0.05; χ23 = 134.9, n = 512, p < 0.001). We further performed multiple comparisons for each pair of test conditions using a χ2-test with a Bonferroni correction (α' = 0.0125). The temporal delay significantly decreased performance when the spatial relation was not distorted, χ21 = 10.1, n = 256, p = 0.001, for the comparison of baseline versus delay conditions. However, this difference was not significant when the spatial relation was distorted by 135°, χ21 = 0.02, n = 256, p = 0.89 for the comparison of distortion versus delay-distortion conditions. By contrast, the spatial distortion effect was significant regardless of the temporal manipulation, χ21 = 84.8, n = 256, p < 0.001, for the comparison of baseline versus distortion conditions, and χ21 = 43.9, n = 256, p < 0.001 for the comparison of delay versus delay-distortion conditions.
Figure 4.
The effect of temporal delay and spatial distortion. Bars show the mean percentages of correct trials for all test sessions. Performance declined following experimental adjustments of either the temporal or the spatial dimension. Asterisks indicate significance at *p < 0.01, **p < 0.005 and ***p < 0.001 in χ2-tests. The dashed line represents the expected chance level. Error bars show 95% confidence intervals.
The finding that performance declined with the introduction of either a temporal delay or a spatial distortion suggests that the chimpanzee's performance was based on monitoring in both the temporal and spatial domains. Self-agency attribution is thought to be based on the congruency between the predicted outcome of an action (preceding its execution) and feedback about the actual action outcome, after it has occurred [21–23]. The spatial distortion or temporal delay introduced in this experiment broke the default contingency between the trackball manipulation and the cursor action, meaning that the cursor action was no longer congruent with the chimpanzees' ‘aimed action’. Declining performance under conditions of delay or distortion suggests that the monitoring of congruency occurs in the domain of both temporal and spatial factors.
However, the disruptive effects were stronger when spatial distortion was applied relative to when the temporal delay was applied. Performance in the condition with temporal delay but without spatial distortion was still above chance levels (binomial test, 93/128, p < 0.001). By contrast, performance with spatial distortion was below chance levels (41/128, p < 0.001) for the distortion condition, and the delay-distortion condition (39/128, p < 0.001). We speculate that the strong effect of spatial distortion in the current study may be explained by the observation that the manipulations performed by this chimpanzee were biased along the vertical axis to some extent. The motion of the correct cursor in the trials with 135° of distortion tended not to be vertical, but became more horizontal, whereas the motion of the distractor remained biased towards the vertical axis; hence, the chimpanzee might have avoided attending to the less familiar motion. Thus, the unfamiliar action, in addition to the absence of congruency between intended motion and feedback in the spatial domain, might have caused the significant deterioration in performance to below chance levels. Based on these experimental results and those of the offline condition in experiment 2, we conclude that the chimpanzees' performance in the current task resulted from perceived self-agency, but also from an influence of a variety of external factors.
6. General discussion
In a series of experiments, we investigated the cognitive capacity of self-agency judgement in chimpanzees on a cursor discrimination task. In experiment 1, we demonstrated that chimpanzees were able to recognize which cursor was under their control, even when the appearance and movement properties of the cursor were comparable to those of another cursor. In experiment 2, we confirmed that this performance cannot be attributed solely to simple associations. The results indicated that chimpanzees used information only available when they actually controlled the cursor, which is the congruence between an internally generated prediction about the cursor's action, and the actual observed action. The results of experiment 3 revealed that these monitoring processes were conducted within both spatial and temporal dimensions. Taken together, these three experiments provide clear evidence for self-agency recognition in chimpanzees.
Our results extend the findings of previous MSR studies in chimpanzees, to the context of voluntary action. MSR is considered to be a crucial step in self-recognition, but its underlying cognitive mechanisms remain unclear [13,15–17]. Our present aim was to specifically focus on the self-monitoring process. Here, we showed that chimpanzees performed self/other attribution about external events based on the monitoring of intended action and actual feedback observed in the external environment. Thus, the results suggest that chimpanzees and humans share fundamental cognitive processes underlying the sense of being an independent agent.
Our results also have implications for the discontinuity between chimpanzees and humans. All chimpanzees in this study showed performance that was above chance in the offline condition. In addition, Chloe's performance in experiment 3 was significantly below chance in a spatial distortion condition. These results suggest that the chimpanzees' behaviour was governed by multiple cues, not self-agency alone. The current task is intuitive for humans, easily choosing which cursor ‘they were controlling’. Why were chimpanzees so influenced by other factors? Chimpanzees may not possess a categorical representation of self-agency, and may have less opportunity to explicitly label whether the observed event was caused by self or others in their daily lives. Categorical representation results in sharp boundaries in responses to physically similar stimuli belonging to different categories [36]. We showed that chimpanzees are capable of agency discrimination based on congruency/incongruence between their internal aims and observed feedback. However, this does not necessarily imply categorical representation of self-agency. Our current results are not enough to conclude this view and it would be valuable for future studies to investigate the categorical representation of self-agency in chimpanzees.
It should be noted that the current findings differ from the mere goal-directed action using some input devices. Goal-directed aiming actions using joysticks, mirrors or video recordings in non-human primates have been reported in several studies [37–40]. However, these reports do not necessarily imply that subject animals performed self/other discrimination on the basis of a monitoring process. Several human cognitive studies have suggested a dissociation between self-agency judgments and goal-directed actions [41,42], indicating the existence of independent features of the underlying processes involved in each.
The present experimental task required chimpanzees to make explicit judgments of self-agency. Processing self-agency is considered to occur at multiple levels of cognition, and some authors have discussed the distinction between explicit self-agency judgments and implicit aspects of self-agency [23,43–45]. Explicit judgments of self-agency refer to the explicit self/other distinction and judgments about the cause of actions or effects, for example, when participants are required to report subjective feelings of controlling or initiating observed events [46], or judge whether they caused an action shown on a monitor or not [29]. By contrast, implicit measurements of self-agency refer to measurements where some self/other distinction processes are involved, but participants are not directly asked to judge the agent of the action, such as sensory attenuation [47] or intentional binding [48]. The implicit aspects of self-agency, especially sensory attenuation, are reported in non-human animals as well as in humans, and their neural mechanisms have been increasingly investigated [49,50]. However, no studies have directly addressed the explicit judgement of self-agency in non-human animals. The present study is, to our knowledge, the first demonstration of this cognitive function in chimpanzees.
The implicit aspects of self-agency may occur in many animals, while the explicit judgement of self-agency may only be observed in a limited number of species. Comparative studies including broader animal taxa would be useful for examining this question. The present study focused on chimpanzees, a species for which there is evidence of MSR. We suggest the recognition of agency might be the fundamental element to achieve MSR. Thus, it is particularly interesting to test with macaques [5,39] which did not show clear evidence of MSR, but show contingency behaviour [7] and can learn to use a mirror to guide their hands to reach an object [39]. Furthermore, many species belonging to a wide range of taxa have exhibited such mirror-guided reaching [6,51,52], and positive reports of MSR in non-primates are currently accumulating [8–10,12]. These situations raise the question of whether all observations of MSR in different taxa reflect the same underlying cognitive mechanisms [11]. As mentioned above, there is increasing evidence for the absence/presence of particular behaviours regarding mirror/video image presentation across many species of animals. However, our understanding of the psychological mechanisms underlying these behaviours is lacking. Study of self-agency recognition in other species will help to elucidate the types of cognitive functions that MSR does or does not reflect, and the different stages of self-recognition in non-human animals.
In summary, we developed a new comparative methodology to elucidate voluntary action involving the self-monitoring process and showed that chimpanzees and humans share fundamental cognitive processes underlying the sense of being an independent agent; that is, self-agency. The theoretical framework and the experimental paradigm proposed here provide new possibilities for studying self-recognition in non-human animals, and will inform future investigations of its underlying psychological mechanisms.
Acknowledgements
The care and use of chimpanzees complied with The Guide for the Care and Use of Laboratory Primates (2002) of the Primate Research Institute, Kyoto University.
We wish to thank T. Matsuzawa, M. Tanaka and I. Adachi for comments on the experimental design; T. Imura and H. Ito for their comments on the manuscripts; S. Hori and M. Nakashima for experimental assistance; and the staff of the Center for Human Evolution Modelling Research of the PRI for the care of the chimpanzees. The study was supported by the JSPS-MEXT Grants in Aid for Scientific Research (21 · 2668 to T.K., and 16002001, 19300091, 20002001 to M.T.) and Global COE Programmes (A06, D07).
References
- 1.Terrace H. S., Metcalfe J. 2005. The missing link in cognition. Origins of self-reflective consciousness. New York, NY: Oxford University Press [Google Scholar]
- 2.Gallup G. G. 1970. Chimpanzees: self-recognition. Science 167, 86–87 10.1126/science.167.3914.86 (doi:10.1126/science.167.3914.86) [DOI] [PubMed] [Google Scholar]
- 3.Povinelli D. J., Rulf A. B., Landau K. R., Bierschwale D. T. 1993. Self-recognition in chimpanzees (Pan troglodytes): distribution, ontogeny, and patterns of emergence. J. Comp. Psychol. 107, 347–372 10.1037/0735-7036.107.4.347 (doi:10.1037/0735-7036.107.4.347) [DOI] [PubMed] [Google Scholar]
- 4.Swartz K. B., Sarauw D., Evans S. 1999. Comparative aspects of mirror self-recognition in great apes. In The mentalities of gorillas and orangutans in comparative perspective (eds Parker S. T., Mitchell R. W., Miles H. L.), pp. 283–294 Cambridge, UK: Cambridge University Press [Google Scholar]
- 5.Gallup G. G. 1977. Absence of self-recognition in a monkey (Macaca fascicularis) following prolonged exposure to a mirror. Dev. Psychobiol. 10, 281–284 10.1002/dev.420100312 (doi:10.1002/dev.420100312) [DOI] [PubMed] [Google Scholar]
- 6.Povinelli D. J. 1989. Failure to find self-recognition in Asian elephants (Elephas maximus) in contrast to their use of mirror cues to discover hidden food. J. Comp. Psychol. 103, 122–131 10.1037/0735-7036.103.2.122 (doi:10.1037/0735-7036.103.2.122) [DOI] [Google Scholar]
- 7.Inoue-Nakamura N. 1997. Mirror self-recognition in nonhuman primates: a phylogenetic approach. Jpn Psychol. Res. 39, 266–275 10.1111/1468-5884.00059 (doi:10.1111/1468-5884.00059) [DOI] [Google Scholar]
- 8.Reiss D., Marino L. 2001. Mirror self-recognition in the bottlenose dolphin: a case of cognitive convergence. Proc. Natl Acad. Sci. USA 98, 5937–5942 10.1073/pnas.101086398 (doi:10.1073/pnas.101086398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plotnik J. M., de Waal F. B. M., Reiss D. 2006. Self-recognition in an Asian elephant. Proc. Natl Acad. Sci. USA 103, 17 053–17 057 10.1073/pnas.0608062103 (doi:10.1073/pnas.0608062103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prior H., Schwarz A., Güntürkün O. 2008. Mirror-induced behavior in the magpie (Pica pica): evidence of self-recognition. PLoS Biol. 6, 1642–1650 10.1371/journal.pbio.0060202 (doi:10.1371/journal.pbio.0060202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suddendorf T., Collier-Baker E. 2009. The evolution of primate visual self-recognition: evidence of absence in lesser apes. Proc. R. Soc. B 276, 1671–1677 10.1098/rspb.2008.1754 (doi:10.1098/rspb.2008.1754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajala A. Z., Reininger K. R., Lancaster K. M., Populin L. C. 2010. Rhesus monkeys (Macaca mulatta) do recognize themselves in the mirror: implications for the evolution of self-recognition. PLoS ONE 5, e12865. (doi:10.1371/journal.pone.0012865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallup G. G. 1982. Self-awareness and the emergence of mind in primates. Am. J. Primatol. 2, 237–248 10.1002/ajp.1350020302 (doi:10.1002/ajp.1350020302) [DOI] [PubMed] [Google Scholar]
- 14.Mitchell R. W. 1993. Mental models of mirror-self-recognition: two theories. New Ideas Psychol. 11, 295–325 10.1016/0732-118X(93)90002-U (doi:10.1016/0732-118X(93)90002-U) [DOI] [Google Scholar]
- 15.Heyes C. M. 1994. Reflections on self-recognition in primates. Anim. Behav. 47, 909–919 10.1006/anbe.1994.1123 (doi:10.1006/anbe.1994.1123) [DOI] [Google Scholar]
- 16.Gallup G. G., Povinelli D. J., Suarez S. D., Anderson J. R., Lethmate J., Menzel E. W. 1995. Further reflections on self-recognition in primates. Anim. Behav. 50, 1525–1532 10.1016/0003-3472(95)80009-3 (doi:10.1016/0003-3472(95)80009-3) [DOI] [Google Scholar]
- 17.Itakura S. 2001. The level of self-knowledge in nonhuman primates: from the perspective of compartive cognitive science. In Primate origins of human cognition and behavior (ed. Matsuzawa T.), pp. 313–329 Tokyo, Japan: Springer [Google Scholar]
- 18.Gallagher S. 2000. Philosophical conceptions of the self: implications for cognitive science. Trends Cogn. Sci. 4, 14–21 10.1016/S1364-6613(99)01417-5 (doi:10.1016/S1364-6613(99)01417-5) [DOI] [PubMed] [Google Scholar]
- 19.Jeannerod M. 2003. The mechanism of self-recognition in humans. Behav. Brain Res. 142, 1–15 10.1016/S0166-4328(02)00384-4 (doi:10.1016/S0166-4328(02)00384-4) [DOI] [PubMed] [Google Scholar]
- 20.Georgieff N., Jeannerod M. 1998. Beyond consciousness of external reality: a ‘who’ system for consciousness of action and self-consciousness. Conscious. Cogn. 7, 465–477 10.1006/ccog.1998.0367 (doi:10.1006/ccog.1998.0367) [DOI] [PubMed] [Google Scholar]
- 21.Wegner D. M., Wheatley T. 1999. Apparent mental causation: sources of the experience of will. Am. Psychol. 54, 480–492 10.1037/0003-066X.54.7.480 (doi:10.1037/0003-066X.54.7.480) [DOI] [PubMed] [Google Scholar]
- 22.Blakemore S. J., Wolpert D. M., Frith C. D. 2002. Abnormalities in the awareness of action. Trends Cogn. Sci. 6, 237–242 10.1016/S1364-6613(02)01907-1 (doi:10.1016/S1364-6613(02)01907-1) [DOI] [PubMed] [Google Scholar]
- 23.Synofzik M., Vosgerau G., Newen A. 2008. Beyond the comparator model: a multifactorial two-step account of agency. Conscious. Cogn. 17, 219–239 10.1016/j.concog.2007.03.010 (doi:10.1016/j.concog.2007.03.010) [DOI] [PubMed] [Google Scholar]
- 24.Linser K., Goschke T. 2007. Unconscious modulation of the conscious experience of voluntary control. Cognition 104, 459–475 10.1016/j.cognition.2006.07.009 (doi:10.1016/j.cognition.2006.07.009) [DOI] [PubMed] [Google Scholar]
- 25.Tsakiris M., Haggard P., Franck N., Mainy N., Sirigu A. 2005. A specific role for efferent information in self-recognition. Cognition 96, 215–231 10.1016/j.cognition.2004.08.002 (doi:10.1016/j.cognition.2004.08.002) [DOI] [PubMed] [Google Scholar]
- 26.Kitchen A., Denton D., Brent L. 1996. Self-recognition and abstraction abilities in the common chimpanzee studied with distorting mirrors. Proc. Natl Acad. Sci. USA 93, 7405–7408 10.1073/pnas.93.14.7405 (doi:10.1073/pnas.93.14.7405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Povinelli D. J., Landau K. R., Perilloux H. K. 1996. Self-recognition in young children using delayed versus live feedback: evidence of a developmental asynchrony. Child Dev. 67, 1540–1554 10.1111/j.1467-8624.1996.tb01813.x (doi:10.1111/j.1467-8624.1996.tb01813.x) [DOI] [PubMed] [Google Scholar]
- 28.Suddendorf T. 1999. Children's understanding of the relation between delayed video representation and current reality: a test for self-awareness? J. Exp. Child Psychol. 72, 157–176 10.1006/jecp.1998.2485 (doi:10.1006/jecp.1998.2485) [DOI] [PubMed] [Google Scholar]
- 29.Daprati E., Franck N., Georgieff N., Proust J., Pacherie E., Dalery J., Jeannerod M. 1997. Looking for the agent: an investigation into consciousness of action and self-consciousness in schizophrenic patients. Cognition 65, 71–86 10.1016/S0010-0277(97)00039-5 (doi:10.1016/S0010-0277(97)00039-5) [DOI] [PubMed] [Google Scholar]
- 30.Jorgensen M. J., Suomi S. J., Hopkins W. D. 1995. Using a computerized testing system to investigate the pre-conceptual self in nonhuman primates and humans. In The self in infancy: theory and research (ed. Rochat P.), pp. 243–256 Amsterdam, The Netherlands: Elsevier Science [Google Scholar]
- 31.Franck N., Farrer C., Georgieff N., Marie-Cardine M., Dalery J., d'Amato T., Jeannerod M. 2001. Defective recognition of one's own actions in patients with schizophrenia. Am. J. Psychiatry 158, 454–459 10.1176/appi.ajp.158.3.454 (doi:10.1176/appi.ajp.158.3.454) [DOI] [PubMed] [Google Scholar]
- 32.Matsuzawa T. 2001. Primates origins of human cognition and behavior. Tokyo, Japan: Springer [Google Scholar]
- 33.Matsuzawa T., Tomonaga M., Tanaka M. 2006. Cognitive development in chimpanzees. Tokyo, Japan: Springer [Google Scholar]
- 34.Call J., Tomasello M. 2008. Does the chimpanzee have a theory of mind? 30 years later. Trends Cogn. Sci. 12, 187–192 10.1016/j.tics.2008.02.010 (doi:10.1016/j.tics.2008.02.010) [DOI] [PubMed] [Google Scholar]
- 35.Metcalfe J., Greene M. J. 2007. Metacognition of agency. J. Exp. Psychol. Gen. 136, 184–199 10.1037/0096-3445.136.2.184 (doi:10.1037/0096-3445.136.2.184) [DOI] [PubMed] [Google Scholar]
- 36.Bornstein M. H. 1987. Perceptual categories in vision and audition. In Categorical perception: the groundwork of cognition (ed. Harnand S.), pp. 287–300 New York, NY: Cambridge University Press [Google Scholar]
- 37.Epstein R., Lanza R. P., Skinner B. F. 1981. Self-awareness in the pigeon. Science 212, 695–696 10.1126/science.212.4495.695 (doi:10.1126/science.212.4495.695) [DOI] [PubMed] [Google Scholar]
- 38.Menzel E. W., Savagerumbaugh E. S., Lawson J. 1985. Chimpanzee (Pan troglodytes) spatial problem-solving with the use of mirrors and televised equivalents of mirrors. J. Comp. Psychol. 99, 211–217 10.1037/0735-7036.99.2.211 (doi:10.1037/0735-7036.99.2.211) [DOI] [PubMed] [Google Scholar]
- 39.Itakura S. 1987. Mirror guided behavior in Japanese monkeys (Macaca-fuscata-fuscata). Primates 28, 149–161 10.1007/BF02382568 (doi:10.1007/BF02382568) [DOI] [Google Scholar]
- 40.Iriki A., Tanaka M., Obayashi S., Iwamura Y. 2001. Self-images in the video monitor coded by monkey intraparietal neurons. Neurosci. Res. 40, 163–173 10.1016/S0168-0102(01)00225-5 (doi:10.1016/S0168-0102(01)00225-5) [DOI] [PubMed] [Google Scholar]
- 41.Fourneret P., de Vignemont F., Franck N., Slachevsky A., Dubois B., Jeannerod M. 2002. Perception of self-generated action in schizophrenia. Cogn. Neuropsychiatry 7, 139–156 10.1080/13546800143000212 (doi:10.1080/13546800143000212) [DOI] [PubMed] [Google Scholar]
- 42.Knoblich G., Stottmeister F., Kircher T. 2004. Self-monitoring in patients with schizophrenia. Psychol. Med. 34, 1561–1569 10.1017/S0033291704002454 (doi:10.1017/S0033291704002454) [DOI] [PubMed] [Google Scholar]
- 43.Pacherie E. 2008. The phenomenology of action: a conceptual framework. Cognition 107, 179–217 10.1016/j.cognition.2007.09.003 (doi:10.1016/j.cognition.2007.09.003) [DOI] [PubMed] [Google Scholar]
- 44.Knoblich G., Repp B. H. 2009. Inferring agency from sound. Cognition 111, 248–262 10.1016/j.cognition.2009.02.007 (doi:10.1016/j.cognition.2009.02.007) [DOI] [PubMed] [Google Scholar]
- 45.Sato A. 2009. Both motor prediction and conceptual congruency between preview and action-effect contribute to explicit judgment of agency. Cognition 110, 74–83 10.1016/j.cognition.2008.10.011 (doi:10.1016/j.cognition.2008.10.011) [DOI] [PubMed] [Google Scholar]
- 46.Asai T., Sugimori E., Tanno Y. 2008. Schizotypal personality traits and prediction of one's own movements in motor control: what causes an abnormal sense of agency? Conscious. Cogn. 17, 1131–1142 10.1016/j.concog.2008.04.004 (doi:10.1016/j.concog.2008.04.004) [DOI] [PubMed] [Google Scholar]
- 47.Blakemore S. J., Wolpert D. M., Frith C. D. 1998. Central cancellation of self-produced tickle sensation. Nat. Neurosci. 1, 635–640 10.1038/2870 (doi:10.1038/2870) [DOI] [PubMed] [Google Scholar]
- 48.Haggard P., Clark S., Kalogeras J. 2002. Voluntary action and conscious awareness. Nat. Neurosci. 5, 382–385 10.1038/nn827 (doi:10.1038/nn827) [DOI] [PubMed] [Google Scholar]
- 49.Poulet J. F. A., Hedwig B. 2002. A corollary discharge maintains auditory sensitivity during sound production. Nature 418, 872–876 10.1038/nature00919 (doi:10.1038/nature00919) [DOI] [PubMed] [Google Scholar]
- 50.Eliades S. J., Wang X. 2008. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature 453, 1102–1106 10.1038/nature06910 (doi:10.1038/nature06910) [DOI] [PubMed] [Google Scholar]
- 51.Pepperberg I. M., Garcia S. E., Jackson E. C., Marconi S. 1995. Mirror use by African grey parrots (Psittacus erithacus). J. Comp. Psychol. 109, 182–195 10.1037/0735-7036.109.2.182 (doi:10.1037/0735-7036.109.2.182) [DOI] [Google Scholar]
- 52.Broom D. M., Sena H., Moynihan K. L. 2009. Pigs learn what a mirror image represents and use it to obtain information. Anim. Behav. 78, 1037–1041 10.1016/j.anbehav.2009.07.027 (doi:10.1016/j.anbehav.2009.07.027) [DOI] [Google Scholar]